Abstract
The World Health Organization stated on 11 March 2020 that a coronavirus illness had been discovered in Wuhan, China in December 2019. Effective vaccinations are eagerly awaited as the global outbreak of COVID-19 continues. The aim is to evaluate the safety, effectiveness, and immunogenicity of Pfizer/AstraZeneca/Modera/Cansino vaccines against COVID-19. An electronic search on different databases yielded 12,907 articles. A total of 20 randomized and non-randomized, published, and ongoing trials were selected. Cochrane RoB version 2.0 was used to assess the authenticity of the studies. Of these 20 trials, three were conducted on Pfizer, three on AstraZeneca, three on Moderna, and two on the Cansino vaccine. These trials have reported promising results for the safety, efficacy, and immunogenicity of the respective vaccines. None of the trials have reported the efficacy and severe adverse outcomes for the Cansino vaccine, hindering its reliability as a safe vaccine against covid-19. Furthermore, the results of these trials have established Pfizer to be the most efficacious vaccine against covid-19, having an efficacy of 94.6%. A few severe adverse events were reported by the included trials. However, further systematic reviews are required to understand the respective vaccine profiles on Immuno-suppressive, organ transplants, and patients with other comorbidities.
1. Introduction
A coronavirus outbreak was discovered in Wuhan, Hubei, China in December of 2019 [Citation1], and the virus quickly spread across the globe [Citation2]. On 11 March 2020, the World Health Organization (WHO) made the terrifying announcement that COVID-19 has spread across the globe [Citation3]. Coronavirus has caused about 15,785,641 confirmed cases and 640,016 fatalities as of July 26th, 2020 [Citation4]. While other coronaviruses, such as SARS and MERS have higher mortality rates, COVID-19 have a lower fatality rate [Citation5–7]. COVID-19 patients present with severe headache, fever, cough, myalgia, and exhaustion as early symptoms [Citation8–10].
1.1. Structure
RNA viruses, such as coronaviruses, are positive sense, single-stranded, and include a nucleocapsid protein (N), an envelope protein (E), and a spike protein (S), as well as numerous access proteins [Citation11]. The angiotensin-converting enzyme 2 (ACE2) is found in the cells of the lower respiratory tract, and S protein helps viruses attach to their hosts [Citation12–14].
1.2. Transmission
SARS-CoV-2 transmits through close-range contact, and air-borne droplets [Citation15]. The common modes of spread are droplet inhalation, cough, sneeze, contact with the eye, nasal, and oral mucous membrane [Citation16]. Saliva, feces, urine, and respiratory tract are the common sources of viral shedding [Citation17–19].
1.3. Immunogenesis
There will be a post-pandemic situation depending on how long SARS-CoV-2 immunity lasts [Citation20]. As a result, it's essential to have a firm grasp of how protective immunity works. Multiple immunological responses, produced by humoral or cell-mediated immunity, are more likely to be responsible for the correlates of protection than just antibody titers, as shown by many studies [Citation21].
1.4. Pfizer vaccine
SARS-CoV-2 prefusion spike glycoprotein vaccine BNT162b2 is a lipid nanoparticle-formulated nucleoside-modified mRNA vaccine. Pfizer-BioNTech COVID-19(BNT162b2) vaccine (Pfizer, Inc; Philadelphia, Pennsylvania) received emergency use authorization (EUA) from the FDA on 11 December 2020 [Citation22]. According to the trial [Citation23], the Pfizer vaccine is 95% effective against the SARS-CoV-19 virus, based on laboratory-confirmed testing on healthy volunteers who had no previous history of infection with COVID-19. Generally, two intramuscular shots are given with a distance of 21 days between the shots. Immunocompromised people are further prescribed a third dose 28 days after the 2nd dose [Citation24].
1.5. AstraZeneca vaccine
An interim review of four ongoing studies has shown that AstraZeneca (ChAdOx1nCoV-19), one of the leading vaccines against SARS-CoV-19, has an overall effectiveness of 70.4% against symptomatic COVID-19. Vaccines made using chimpanzee adenovirus vectors are designed to induce a robust immune response after either one or two intramuscular doses. To make the immune system fight the SARS-CoV-2 virus, the ChAdOx1nCoV-19 vaccine includes the whole SARS-CoV-2 structural surface spike glycoprotein gene. In the UK, the Clinical Biomanufacturing Facility at Oxford University produced AstraZeneca (ChAdOx1nCoV-19) [Citation25].
1.6. Moderna vaccine
Pre-fusion stabilized version of SARS-CoV-2 spike protein encoded by mRNA-1273 is an LNP encapsulated vaccine with a 94.1% efficacy against Covid-19 disease, according to the phase-III study findings. Two mRNA1273 (100 g IM) injections are usually given 28 days apart [Citation26]. NIAID-the National Institute of Allergy and Infectious Diseases-, and Moderna worked together to create the vaccine [Citation27].
1.7. Cansino vaccine
Vaccine Cansino (Ad5-nCoV) encodes the full-length spike protein of SARS-CoV-2 and is genetically designed to have replication-defective human adenovirus type 5-based COVID-19 replication [Citation28]. When given as a single intramuscular dosage containing 5 × 1010 virus particles per ml, this vaccine has an acceptable safety profile. Developed by CanSino Biologics and the Beijing Institute of Biotechnology, the Cansino (Ad5-nCoV) vaccine is a collaboration between the two organizations. The vaccination is only available to those who are at least 18 years old. Patients with secondary comorbidities have an increased risk of severe covid-19 [Citation29], therefore this vaccination is advised for them in particular.
All relevant published and unpublished data on various databases were reviewed to evaluate the safety, effectiveness, and immunogenicity of COVID-19 vaccines from Pfizer, AstraZeneca, and Moderna and Cansino in this systematic review.
2. Methods
When we carried out our study using PRISMA's recommendations, we were able to give a comprehensive overview of the main results from the studies performed to evaluate the effectiveness, safety, and immunogenicity of covid-19 vaccines.
2.1. Literature searches
We performed an electronic search on PubMed, EMBASE, and International Clinical Trials Registry (ICTRP) to identify the studies that were published from the inception of the databases. A broad search strategy was deployed, consisting of the combination of the MeSH terms (‘SARS-CoV-2’ OR ‘Covid-19’ OR ‘coronavirus-19’ AND ‘Vaccines’ OR ‘Immunization’ OR ‘Pfizer’ OR ‘BNT162b2’ OR ‘Moderna’ OR ‘mRNA-1273’ OR ‘AstraZeneca’ OR ‘ChAdOx1nCoV-19’ OR ‘AZD1222’ OR ‘Cansino’ OR ‘Ad-5 nCoV-19’). The same search strategy was used on all the databases.
2.2. Inclusion and exclusion criteria
We extracted all the phase-I/II/III randomized and non-randomized clinical trials, recruiting either healthy participants or participants with COVID history and no other comorbidities. Only those trials that assessed the efficacy, safety, or/and immunogenicity of Pfizer/AstraZeneca/Moderna/Cansino, were included. Case reports, editorials, animal studies, grey literature, and all studies published in languages other than English were excluded.
2.3. Data extraction
Initially, the search results were screened according to the study title and abstracts and then according to the full texts. The selected studies were then scanned according to the inclusion criteria, and the finalized articles were included in this review. Reference lists of relevant articles were also scanned to identify further relevant studies. All the duplicates were removed, however, if more than one published study were found on the same ongoing trial, all of them were included given that different outcomes were assessed by each of the published studies. Two authors independently screened and extracted the relevant data from the finalized articles. Microsoft Excel was used for the data extraction purpose and tables were made in Microsoft Word.
2.4. Risk of bias assessment
Cochrane's risk of bias assessment tool (RoB 2.0, version 2019, accessible at www.riskofbias.info) was utilised by two researchers to perform an authenticity test and evaluate the overall methodology and results of trials [Citation30]. These five domains have been included in the tool's latest iteration to help researchers determine whether or not their results are truly unbiased: the randomization process, deviations from intended interventions, missing outcomes data, outcomes measurement, and selecting which findings to report. Trials were classified as ‘high risk’ if any one of its domains was deemed ‘high risk’ overall. Conversely, the trials had to have at least three domains with ‘some concern’ to be considered as ‘some concerns’ overall.
3. Results
3.1. Systemic search
A literature search on different databases (1st January 2020–27th September 2021) yielded a total of 12,907 articles. Forty-four articles were sought after reviewing the titles and abstracts of the articles. Further 11 articles were removed after a full-length review. Finally, 20 randomized and non-randomized trials were selected to be included in the review ().
Figure 1. PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses [Citation51].
![Figure 1. PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses [Citation51].](/cms/asset/a80f2cdf-993d-4974-952c-3ddb7106c107/timm_a_2068331_f0001_c.jpg)
3.2. Baseline details
Out of these 20 articles, nine are ongoing trials and 11 are complete phase-I, II, and III studies. Among the 11 articles, three trials reported for Pfizer vaccine, three for AstraZeneca vaccine, three for Moderna vaccine, and two trials documented the results for Cansino vaccine. Baseline details of these trials are shown in . Further details of the ongoing trials are listed in .
Table 1. Demographic details of included trials.
Table 2. Demographic details of ongoing trials.
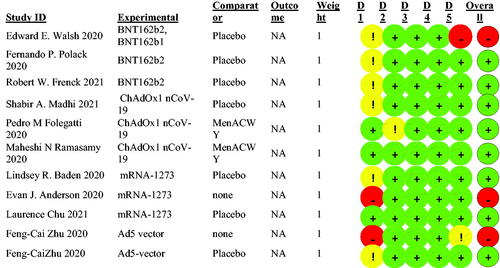
3.3. Risk of bias
A revised tool to assess the risk of bias in randomized trials (RoB) was used and all the questions for each domain were answered using the published studies in this review. Trials protocols were also used upon necessity. With the aim of intention-to-treatment, the assessment highlighted three studies to be at high risk overall, while the remaining studies were considered to have low risks of bias as shown in .
3.4. Efficacy of the vaccines
Primarily, the trials documented the efficacy of the vaccines against confirmed SARS-Cov-2 with onset at least 7 days after the second dose in participants who had been without serologic evidence of Covid-19 infection. Secondarily, efficacy in participants with and without evidence of prior infection was reported.
The trial [Citation40] on Pfizer vaccine reported that out of 36,523 participants with no history of confirmed Covid-19 infection, eight cases were examined to have contracted the infection at least 7 days after the 2nd dose of the vaccine, while 162 cases of Covid-19 onset were observed in the placebo group. Among individuals with and without evidence of prior Coronavirus infection, nine and 169 cases were observed with the onset of infection at least 7 days after the 2nd dose in the BNT162b2 and placebo group, respectively. The vaccine efficacy was reported as 94.6%(95% CI,68.7–99.9) after the 2nd dose. Vaccine efficacy was dropped to 52%(95%CI,29.5–68.4) during the interval between 1st and 2nd dose due to an increase in new viral cases in the vaccine group. Another phase III trial [Citation41] compared the efficacy of the interventional and placebo group. Among 1983 participants with no history of COVID-19 cases, no cases of virus contraction were observed after 7 or more days of the 2nd dose. Comparatively, the placebo group showed 16 such cases, this result corresponded to 100% efficacy of the observed vaccine.
Out of all the trials conducted on AstraZeneca, only one trial assessed its efficacy against the B.1.351 variant [Citation42]. The incidence of covid-19 infection two weeks post-second dose was 2.5% in the vaccine group vs. 3.2% in the placebo group in volunteers who were initially seronegative. The vaccine efficacy was found to be 10.4%. in volunteers who were seronegative at baseline and 10.6% in volunteers who were either seronegative or seropositive at baseline [Citation42].
The efficacy of the mRNA-1273 vaccine was assessed in the trial [Citation43] that evaluated a total of 185 positive covid-19 cases two weeks after the second dose in the placebo group, as compared to only 11 cases in the vaccine group in participants who were non-serological at baseline. Furthermore, this vaccine was found to be 94.1% (95%CI 89.3–96.8) efficacious, and this efficacy was consistent across different subgroups categorized according to baseline characteristics. 30 participants were found to have severe covid-19 in the placebo group, indicating the 100% efficacy of this vaccine in preventing severe illness. Additionally, no case of covid-19 was seen in the group of participants who were seropositive at baseline after receiving the mRNA-1273 vaccine [Citation43].
Efficacy for the Cansino vaccine was not reported in any of the recruited articles due to the lack of published phase-III trials.
3.5. Safety profiles of the vaccines
All the included trials have reported the safety profiles of the respective vaccines. The safety endpoints are divided further into three sub-headings for ease of understanding.
3.5.1. Local reactions
A phase-I [Citation44] and a phase-II/III trial [Citation40] of Pfizer vaccine, have reported that the younger patient cohort had experienced more local reactions than the older patient cohort. Pain at the injection site of mild to moderate severity, the most frequently complained reaction, was reported mostly by the younger participants. No patients in both cohorts had complained of severe pain after both doses. Few younger patients had reported redness, and swelling after the 1st dose, no cases of these reactions were reported after the 2nd dose. Grade 4 local reaction was not reported by any participant. Another phase-III trial [Citation41] reported similar local reactions with injection-site pain being the most common reaction. The pain was reported majorly by the intervention group compared to the placebo group after the 2nd dose. The severity of pain was mild to moderate, however, a few young adults in the vaccine group reported severe reactions. The results for this candidate vaccine are shown in .
Table 3. Safety profile of included trials.
Tenderness and pain at the site of injection in the first 2 days after receiving AstraZeneca vaccine, were the most frequently reported localized reactions according to the study [Citation45]. This finding was corroborated by the other two randomized trials [Citation42,Citation46]. Mild bruising, itching, and warmth at the injection site were some of the other frequently reported localized reactions [Citation42,Citation46]. These events have been summarized in .
All the trials conducted on the Moderna vaccine have reported pain (at the injection site) of mild to moderate severity as the most frequently occurring local event after both doses [Citation43,Citation47,Citation48]. Moreover, swelling or induration, particularly after the second dose were also reported in all three trials [Citation43,Citation47,Citation48]. Induration of moderate intensity after the second dose was reported in all the subgroups of one trial [Citation47]. Erythema and lymphadenopathy of mild to moderate intensity were some of the other localized reactions reported by the trials [Citation43,Citation48]. The results for this candidate vaccine are shown in .
In the trial [Citation49], conducted to assess the safety of the Cansino, pain at the injection site, within 7 days after vaccination, was the most frequently reported localized reaction. Induration, redness, swelling, and itch were some of the other localized reactions reported in these trials [Citation49,Citation50], as mentioned in .
3.5.2. Systemic reactions
Out of the trials conducted on Pfizer, one trial [Citation44] reported that fatigue was the most reported systemic event. Younger patients of 18–55 years reported more fatigue events than older patients of 65–85 years after the 2nd dose specifically. Severe systemic reactions, for instance, fatigue, chills, headache, and muscle pain, were reported by an insignificant number of younger participants. None of the patients reported any grade 4 systemic events. A study [Citation40] reported fatigue to be the dominant systemic reaction generally. It was examined that fatigue was complained more frequently by the younger age group and more often after the 2nd dose. Temperature (≥38 °C) was examined to be experienced more by the vaccine group compared to the placebo group. High fever (≥40 °C) was reported by two patients, each in the vaccine and placebo group. A trial [Citation41] reported more frequent events after 2nd dose. Headache and fatigue were the two most reported reactions among the participants. Fever was more dominant among the age group 12–15 years than the age group 16–25 years after 2nd dose. High fever(≥40 °C) was reported among a single patient of the formerly mentioned age group. Overall, all the studies mentioned the increased use of antipyretic agents after 2nd dose relative to 1st dose. The systemic events of this vaccine are summarized in .
In the phase-I/II trial [Citation46], fatigue and headache were the most frequently reported systemic adverse events. The incidence of fatigue and headache in the AstraZeneca group was higher than in the MenACWY group. Feverishness, headache, muscle pain, and weakness were some of the commonly reported systemic events in one trial [Citation42]. Another trial [Citation45] illustrated that after the first dose, feverishness, chills, and joint pain were most commonly reported by participants in the age group 18–55 years, whereas fatigue, headache, and muscle ache were reported by participants of all age groups. The results further explained that after the boost vaccination the incidence of reported adverse reactions declined as compared to the prime vaccination. These results have been summarized in .
Regarding mRNA-1273 vaccine, fatigue, headache, and myalgia were the most commonly reported systemic events in the vaccination group of all trials. In the trial [Citation47], the headache was the most frequently reported systemic particularly in the younger participants. Fever of mild to severe intensity was observed in the participants of all three trials after the second vaccination. The results of these trials demonstrated that the incidence of severe systemic events increased after the second dose. Arthralgias, chills, and nausea were the other systemic events reported in these trials. Furthermore, the results of the trial [Citation43] illustrated that the severity of systemic events particularly grade 2 events, increased after the second dose. Another key finding of the trial [Citation43] was that both local and systemic events were more frequently reported in the younger participants as compared to the older participants. This finding was further confirmed by the trial [Citation48], according to which, pain at the injection site was majorly reported by younger participants as compared to older participants. These systemic reactions are summarized in .
Regarding Cansino vaccine, fever (46%), fatigue (44%), headache (39%), and muscle ache (17%) were the frequently reported systemic reactions in the trial [Citation49]. In both the trials [Citation49,Citation50] the incidence of these reactions was significantly higher in the high dose group as compared to the low and middle dose group. For illustration, in the trial [Citation50], the incidence of fever in the 5 × 1010 viral particles group was 16%, whereas that in the 1 × 1011 viral particles group was 32%. Similarly, the incidence of headache was lower in the middle dose group, relative to the high dose group [Citation49]. The results of these trials illustrated that high pre-existing Ad5 immunity, older age, and male sex was associated with a lower incidence of post-vaccination fever. The results for this candidate vaccine are shown in .
3.5.3. Adverse reactions
The adverse events were shown to be more common in younger individuals in the phase-II/III trial [Citation40] conducted by Pfizer. Patients in the vaccination group had almost thirteen times the number of cases of lymphadenopathy as those in the placebo group. BNT162b2 patients had four severe adverse events as a result of the intervention. The enrolled individuals did not experience any vaccine- or COVID-related fatalities. A phase-III trial [Citation41] found that vaccination recipients had more side effects than those who received a placebo. Lymphadenopathy, for example, was shown to be more common in teenagers than in young adults. In the study, no one died as a result of the vaccination. Adverse reactions reported in trials conducted on Pfizer are summarized in .
There were 13 serious side effects recorded in the AstraZeneca [Citation45] study. However, none of these issues were linked to the vaccination in any way. After 9 days of immunization, one person in the MenACWY group suffered from hemolytic anemia, which was documented in the trial [Citation32]. These adverse events are included in .
In the Moderna [Citation43] study, the vaccination group and the placebo group both had a 0.6% incidence of serious side effects. There were two vaccine-related fatalities and three placebo-related deaths in this study. Other side effects that were seen in this study were hypersensitivity and Bell's palsy. One instance of reduced appetite was observed in the low dosage group and one case of severe hypoglycemia was recorded in the high dose group; both cases were moderate in severity. A participant in the trial's low dosage group complained of paronychia [Citation47]. Last but not least, the trial [Citation48] found a greater frequency of unintentional AEs in the 100 g group compared to the 50 g or the placebo group [Citation50]. The low-dose group in the trial [Citation48] also had community-acquired pneumonia, according to the results. However, the vaccination was not shown to be responsible for cases of pneumonia or paronychia. The results for the serious adverse events of this candidate vaccine are shown in .
No serious adverse reactions were reported for the Cansino vaccine in the included trials.
3.6. Immunogenicity of the vaccines
A total of two clinical studies confirmed the Pfizer vaccine's immunogenicity. After the second dosage, all patients had enhanced Antigen-binding IgG and virus-neutralizing responses, according to a study [Citation44]. However, the two reactions were shown to be lower in older individuals. Researchers also found that 7 and 14 days following the second treatment, neutralizing titers had dropped significantly in the animals. Younger individuals had larger differences in neutralizing Geometric Mean Titers (GMTs) between the two days than older patients, according to the study. Another trial [Citation41] found that teenage participants had a comparable immune response to young adults. When comparing the first and second age cohorts, the Geometric Mean Ratio (GMR) of neutralizing GMTs satisfied the non-inferiority criteria (i.e., a lower limit of the 95% confidence interval of >0.67). The GMR showed a larger response in adolescents than young adults when the lower limit of the 95% confidence interval was >1. One month following the second dosage, the serum-neutralizing GMT was found to be greater in adolescents than in younger adult patients. Both cohorts' placebo groups had similar outcomes. In the teenage group, significant increases in 50% neutralizing GMTs were seen from baseline to 1 month after the second dosage, with significantly higher GMFRs. The placebo groups in both cohorts had similar GMFRs, indicating that the differences were insignificant.
AstraZeneca's immunogenicity was examined in three separate studies using anti-SARS-CoV-2 spike protein neutralizing IgG antibodies. A maximum Median Elisa unit (EU) of 157 EU was achieved in the trial [Citation46] 28 days after the primary immunization, and this level remained high (119 EU) for 56 days following vaccination in individuals who had only received a single dose of vaccine. Anti-spike IgG levels peaked at 639 EU in individuals who received a booster dose on day 56, the researchers found. Measuring IgG antibodies against both spike and RBD, similar increases were seen in the MIA findings. Twenty-eight days following the initial immunization, anti-spike IgG titers were comparable across individuals who got a normal dose and those who received a low dose in the trial [Citation45]. Anti-spike IgG responses, however, diminished with age (28 days after the initial immunization). All individuals who got a booster vaccine, regardless of age or vaccination dosage, had comparable antibody responses 28 days following the second vaccination. Researchers found that GMTs of anti-SARS-CoV-2 D614G neutralizing antibodies were 132 (IQR 20–404) at 28 days after the first vaccination and rose to 277(IQR 124–526) after the second vaccination, according to a study [Citation42].
The Moderna vaccine's immunogenicity was studied in two separate studies. Researchers found that the levels of serum binding antibodies (bAbs) against the S-2P protein rose substantially 28 days after the first vaccination and peaked 14 days after the second immunization in a study (which included only seronegative individuals at baseline) [Citation48]. Comparing these findings to those of COVID-19 sera, it was discovered that they were considerably higher. The trial's findings [Citation47] were quite similar. This study also found that 100-g doses resulted in substantially greater neutralization reactions than 25-g doses or convalescent serum in terms of neutralization. Also, 4 weeks after the second dosage, the neutralizing activity was still high. The trial [Citation47] also documented the vaccine's effect on T cells. Type 1 helper T (Th1) cells were seen in the CD4 cytokine responses produced by the vaccination in both the older and younger age groups of individuals who received 100 and 25 g, respectively.
Using the GMTs of anti-RBD and anti-spike antibodies (measured by ELISA) and neutralizing antibody responses against live virus or pseudovirus, two studies looked at the immunogenicity of Cansino. The GMTs of anti-RBD antibodies in the trial [Citation50] were lower in the high dosage group than in the low dose group on day 14 (after immunization). Nevertheless, on day 28, the high dosage group's levels reached 656.5, whereas the low dose group's values reached 571. The antibody responses in the control group, on the other hand, did not change from day 14 to day 28. From 14 to 28 days after starting the trial [Citation49], GMTs of antibodies specific to RBD in the low and high dosage groups rose 4-fold. There were also no negative GMTs for neutralizing antibodies against the live virus on day 0 and the GMTs rose to 32.0 (high dosage group), 16.2 (middle dose group), and 14.5 (low dose group) (low dose group). On the 28th day following the immunization, these numbers had significantly risen. The trial's findings backed up these conclusions [Citation50]. These studies' findings show that individuals with higher levels of prior anti-Ad5 immunity had superior antibody responses than those with lower levels of previous anti-Ad5 immunity.
3.7. Strengths and limitations
A total of three most frequently-used databases were employed for the recruitment of the trials. The review included the trials on the four most efficacious vaccines. However, it does not include the studies which elucidated the potential therapeutic drugs for the treatment of Covid-19 nor does it include those studies which have highlighted those covid-19 treatment therapies that were under investigation before the emergence of these vaccines. This review includes all phases trials(I/II/III) of the respective vaccines except for the phase-III trial for the vaccine Cansino due to the lack of published data on this vaccine. Some ongoing trials related to the topic are also recruited in this review so that the readers may have easy access to the compiled list of these trials for future research.
4. Conclusion
The trials included in this review demonstrated promising results for the efficacy, safety, adverse events, and immunogenicity of Pfizer, AstraZeneca, and Moderna vaccines. A few severe adverse events and no vaccine-related deaths were reported even in those trials which experimented on large-scale populations. This confirms the safety and acceptance of vaccines by the people. Phase-III trials, however, need to be done on the Cansino vaccine to bring the respective vaccine to the market. Further systematic reviews are required to understand the respective vaccine profiles on Immuno-suppressive, organ transplants, and patients with other comorbidities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Huang P, Liu T, Huang L, et al. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;295(1):22–23.
- Phan L, Nguyen T, Luong Q, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872–874.
- Organization WH. COVID-19 pandemic; 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19–-11-march-2020
- WHO coronavirus disease (COVID-19) dashboard. Available from: https://covid19.who.int/
- Guarner J. Three emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19. Clin Infect Dis. 2020;153(4):420–421.
- Rajgor D, Lee M, Archuleta S, et al. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;395:497.
- Sun Q, Qiu H, Huang M, et al. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu province. Ann Intensive Care. 2020;10(1):1–4.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815.
- Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281–286.
- Tian S, Hu N, Lou J, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–404.
- Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355.
- Guo Y-R, Cao Q-D, Hong Z-S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military Med Res. 2020;7(1):1–10.
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses. New York (NY): Springer; 2015. p. 1–23.
- Walls AC, Park Y-J, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;183(6):1735.
- Jayaweera M, Perera H, Gunawardana B, et al. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188:109819.
- Umakanthan S, Sahu P, Ranade AV, et al. Origin, transmission, diagnosis, and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96(1142):753–758.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207.
- Tang B, Bragazzi NL, Li Q, et al. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov). Infect Dis Model. 2020;5:248–255.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513.
- Abebe EC, Dejenie TA, Shiferaw MY, et al. The newly emerged COVID-19 disease: a systemic review. Virol J. 2020;17(1):96.
- Ong E, Wong MU, Huffman A, et al. COVID-19 coronavirus vaccine design using reverse vaccinology and machine learning. Front Immunol. 2020;11:1581.
- Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine emergency use authorization. Silver Spring (MD): US Department of Health and Human Services, Food and Drug Administration; 2020. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
- Oliver SE, Gargano JW, Marin M, et al. The advisory committee on immunization practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–1924.
- Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html
- Voysey M, Clemens SA, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111.
- Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines are currently authorized in the United States; 2021.
- Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567–571.
- Hernández-Bello J, Morales-Núñez JJ, Machado-Sulbarán AC, et al. Neutralizing antibodies against SARS-CoV-2, anti-Ad5 antibodies, and reactogenicity in response to Ad5-nCoV (CanSino biologics) vaccine in individuals with and without prior SARS-CoV-2. Vaccines. 2021;9(9):1047.
- Available from: https://ncoc.gov.pk/sop/vac/20210503%20Guidelines%20for%20Cansino%20Bio%20Vaccine-5902.pdf
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
- BioNTech SE. Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals. ClinicalTrials.gov: NCT04368728; 2020.
- Available from: https://clinicaltrials.gov/ct2/show/record/NCT04324606
- AstraZeneca, Iqvia Pty Ltd. Phase III double-blind, placebo-controlled study of AZD1222 for the prevention of COVID-19 in adults. 2020.
- Pollard AJ. A randomized controlled, phase III study to determine the safety, efficacy, and immunogenicity of the non-replicating ChAdOx1 nCoV-19 vaccine. 2020.
- ModernaTX A. A study to evaluate the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine in adults aged 18 years and older to prevent COVID-19. ClinicalTrials. Gov; 2020.
- ClinicalTrials.gov. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19). 2020.
- Available from: https://clinicaltrials.gov/ct2/show/NCT04813796?term=safety±and±immunogenicity±study±of±2019-nCoV±vaccine±%28mRNA-1273%29±to±prevent±SARS-CoV-2±infection&draw=2&rank=3
- Petrovax NP. Clinical trial of recombinant novel coronavirus vaccine (adenovirus type 5 vector) against COVID-19. 2020.
- Available from: https://clinicaltrials.gov/ct2/show/NCT04526990
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615.
- Frenck RW Jr., Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250.
- Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 covid-19 vaccine against the B. 1.351 variant. N Engl J Med. 2021;384(20):1885–1898.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416.
- Walsh EE, Frenck RW Jr., Falsey AR, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450.
- Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomized, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993.
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet. 2020;396(10249):467–478.
- Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438.
- Chu L, McPhee R, Huang W, et al. mRNA-1273 study group. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39(20):2791–2799.
- Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomized, first-in-human trial. Lancet. 2020;395(10240):1845–1854.
- Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488.
- Available from: http://www.prisma-statement.org/PRISMAStatement/FlowDiagram