Abstract
There is an urgent need to stop the coronavirus disease 2019 (COVID-19) pandemic through the development of efficient and safe vaccination methods. Over the short term, plasmid DNA vaccines can be developed as they are molecularly stable, thus facilitating easy transport and storage. pVAX1-SARS-CoV2-co was designed for the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) S protein. The antibodies produced led to immunoreactions against the S protein, an anti-receptor-binding-domain, and a neutralizing action of the pVAX1-SARS-CoV2-co, as previously confirmed. To promote the efficacy of the pVAX1-SARS-CoV2-co vaccine a pyro-drive jet injector (PJI) was used. An intradermally adjusted PJI demonstrated that the pVAX1-SARS-CoV2-co vaccine injection caused a high production of anti-S protein antibodies, triggered immunoreactions, and neutralized the actions against SARS-CoV-2. A high-dose pVAX1-SARS-CoV2-co intradermal injection using PJI did not cause any serious disorders in the rat model. A viral challenge confirmed that intradermally immunized mice were potently protected from COVID-19. A pVAX1-SARS-CoV2-co intradermal injection using PJI is a safe and promising vaccination method for overcoming the COVID-19 pandemic.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has greatly necessitated the development of a safe and effective vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Various vaccine candidates were tested in clinical studies, and several varieties of vaccine platforms have been used. These include protein subunits, replicating viral vectors, non-replicating viral vectors, inactivated viruses, RNA, DNA, live attenuated viruses, and virus-like particles (VLPs). Plasmid DNA [Citation1] and mRNA [Citation2] vaccines have been developed in addition to traditional vaccines because they can be produced quickly by generic manufacturing and are constructed directly from genetic sequence information. Therefore, nucleotide-based vaccines can easily adapt to viral mutations [Citation1]. Furthermore, mass production technology has been established for DNA plasmids. Similarly, new technologies, such as mRNA-stabilizing technology, are being developed for mRNA vaccines [Citation2]. Five mRNA vaccine candidates and four DNA vaccine candidates were involved in the development of a vaccine against SARS-CoV-2. However, a major limitation is the lack of an effective method for introducing these molecules (especially DNA plasmids) into cells for effective protein expression and antibody induction. To resolve this issue, we previously reported the potential of the intradermal jet injection method for DNA vaccination using a model DNA vaccination with an ovalbumin expression plasmid [Citation3]. Further, when developing a new DNA vaccine, it is also important to assess the health effects to evaluate the efficacy.
Nucleic acid-based vaccines can be rapidly developed [Citation4]. In addition, DNA vaccines are thermostable, whereas RNA vaccines require a cold chain system, which can be a bottleneck in the worldwide distribution of anti-COVID-19 vaccines. Recently, novel plasmid DNA vaccines (AG0302-COVID19) against SARS-CoV-2 have been developed and are currently in phase I/II trials in Japan [Citation5]. AG0302-COVID19 has an optimized S protein sequence that accelerates S protein expression in human cells. The S protein expression in AG0302-COVID-transfected cells was confirmed. In a preclinical study, AG0302-COVID19 with an alum adjuvant was injected intramuscularly, which induced both humoral and cellular immune responses. Moreover, AG0302-COVID19-induced antibodies possessed a neutralizing effect on SARS-CoV-2 S protein-binding. The peptide array used to analyze the antibody epitopes revealed that most AG0302-COVID19-induced antibodies recognized the S2 and receptor-binding domain (RBD). The anti-COVID-19 DNA vaccine candidate pVAX1-SARS-CoV2-co has the same nucleotide sequence as AG0302-COVID19. pVAX1-SARS-CoV2-co-triggered antibodies can also neutralize the RBD recombinant protein and the angiotensin-converting enzyme 2 (ACE2), which is a SARS-CoV-2 receptor.
We employed a novel pyro-drive jet injector (PJI) to enhance the pVAX1-SARS-CoV2-co efficacy by dermal injection. Previously, an intramuscular injection was used. One of the limitations of DNA vaccines is their low antibody titers against COVID-19 infections [Citation4]. PJI can inject pVAX1-SARS-CoV2-co into dermal tissues, stimulating various immune cells by S protein expression. In PJI, the combustion of the combined ignition powders leads to the plunger discharging liquid samples into the target tissue at a very high pressure [Citation6]. A notable advantage of PJI is the adjustment of the sample dose and depth of delivery, which can be varied by manipulating the levels of the two types of explosives. The first explosive creates a straight hole in dermal tissue by drug solution. The second explosive makes the drug solution to disperse radially after the solution ceases progressing. The radical radial dispersion transduces pDNA to cells in the dermal tissue. Different plasmid DNAs, luciferase, and ovalbumin (OVA) were successfully delivered to the dermal tissue. An increase in the expression of these proteins was detected in the target tissue when compared to a classic needle syringe injection. Moreover, the antibody titer observed against OVA injection by PJI was more than a hundred times higher than that observed with using a needle syringe injection, even eight weeks after OVA plasmid DNA injection [Citation3]. This study aimed to determine the safety of a new anti-SARS-CoV-2 DNA vaccine candidate administered by intradermal jet injection. The PJI was adjusted to deliver pVAX1-SARS-CoV2-co to the dermal tissue and potent anti-COVID-19 immune reactions were examined.
2. Materials and methods
2.1. Plasmids
pVAX1-SARS-CoV2-co was designed by Takara and is a highly optimized DNA sequence encoding the SARS-CoV-2 spike glycoprotein. Plasmid DNA was amplified and purified using GIGA prep (Qiagen, Hilden, Germany).
2.2. Animals
The study on rat model was performed using 7-week-old male Crl:CD (SD) rats (Charles River Laboratories Japan, Inc., Kanagawa, Japan). Studies on mouse model were performed using 8–10-week old C57BL/6NCrSlc mice (C57BL/6N) (Japan SLC, Inc.). All animals were maintained in controlled conditions (temperature: 21.0–24.5 °C, humidity: 45 ± 15%, ventilation: 8–15 times/h, light/dark cycle: 12 h) in a pathogen-free room. The animals accessed food and water ad libitum and were handled while strictly following the approved protocols of the Animal Committee of Osaka University (Suita, Osaka, Japan) (No. 28-021-028), the Ethics Committee for Animal Experiments of the Safety Research Institute for Chemical Compounds Co. Ltd. (Sapporo, Hokkaido, Japan) (No. AN20200617-03), and the Animal Committee of KAC Co. Ltd. (Kusatsu, Shiga, Japan) (No. 20–0508). All experiments were conducted with approval from the Animal Research Committee of the Research Institute for Microbial Disease at Osaka University (Suita, Osaka, Japan).
2.3. Immunogenicity test
Two groups comprising six animals each received pVAX1-SARS-CoV2-co injections on both sides of their flank regions every two weeks for a total of three times. The animals received an injection of 30 μL of plasmid solution at two sites on each side. The injection areas varied for each injection to avoid repeated injections at the same site. Specimens from the first and second group were anesthetized before sera samples were collected every two weeks for 12 weeks for the ELISpot assay.
2.4. Safety evaluation
Animals were divided into three groups of six animals each. Every two weeks, the animals received three pVAX1-SARS-CoV2-co injections on their flank regions on both sides. For each injection, the first group received 50 μL of PBS solution at four sites, the second group received 50 μL of plasmid solution at one site, and the third group received 50 μL of plasmid solution at four sites. Therefore, each rat received 0, 100, or 400 μg of DNA vaccination. The injected areas varied between each injection to prevent repeated injections at the same skin site. Over the course of six weeks, specimens were anesthetized and sera were collected every two weeks to assess the immune response. All specimens were euthanized on day 43. In both experiments, the PJI Actranza™ supplied by Daicel Corporation (Osaka, Japan) was used for the intradermal injection.
During the treatment period, animals were observed daily for general signs and skin reactions in the injected region. Body weights and food consumption were measured weekly. The necropsy, hematology, biochemistry, and immune response assays were performed under isoflurane anesthesia. Urine samples were collected for urinalysis. After euthanasia, specimens were visually inspected. Organs were sampled for microscopic examination including all the blood, the skin region, inguinal lymph nodes, reproductive organs, and any other organ that appeared abnormal after the visual inspection. All injected skin sites were excised to assess the recovery of the injection site. The organ samples were fixed in 10% neutral-buffered formalin solution. Thin paraffin sections were prepared, stained with hematoxylin and eosin, and subjected to histopathological analysis.
2.5. SARS-CoV-2 expression detection via Western blot analysis
Twenty-four hours after the pVAX-SARS-CoV-2-co plasmid injection, the tissue from the injection site was humanely removed from the specimen. The tissue was hemolyzed using a radioimmunoprecipitation assay buffer (Nacalai Tesque, Japan). The lysed samples were treated with sample buffer (Bio-Rad, USA) and subjected to Bis-Tris-PAGE gel electrophoresis before being transferred onto PVDF membranes and incubated with the following antibodies: primary anti-SARS-CoV-2 (COVID-19) spike antibody (GTX135356; GeneTex, Inc., USA); secondary anti-rabbit IgG, and HRP-linked antibody (7074S; Cell Signaling Technology, USA) using the iBind system (Thermo, USA). The samples were then developed using HRP Substrate (Wako, Japan). The protein quantification, imaging, and analysis were performed using an iBright FL1500 Imaging System (Thermo, USA).
2.6. ELISA
Briefly, 1 μg/mL of recombinant 2019-nCoV spike protein (S1 + S2) (ECD, His tag) (BLPSN-0986P, BETA Life Sciences, Fairfield, NJ, USA) was immobilized in a 96-well plate (442,404, MAXISORP F96 NUNC Immuno-plate, Thermo Fisher Scientific, Roskilde, Denmark) at 4 °C for 16 h. After discarding the recombinant protein solution, the plate was incubated with 5% skimmed milk in PBS (blocking buffer) for 2 h at room temperature (21.0–24.5 °C). After blocking, the diluted rat sera (50-, 250-, 1250-, 6250-, 3250-, and 156,250-fold dilutions for S1 + S2; 10-, 50-, 250-, 1250-, 6250-, 31,250-, and 156,250-fold dilutions for RBD) or diluted mouse sera (50-, 250-, 1250-, 6250-, 3250-, and 156,250-fold dilutions for S1 + S2; 50-, 250-, 1250-, 6250-, and 31,250-fold dilutions for RBD) were incubated at 4 °C overnight. All wells were washed seven times with PBST (200 μL/well) and incubated with 1:1000 diluted Amersham ECL anti-rat IgG horseradish peroxidase-linked species-specific whole antibody (NA935, GE Healthcare UK Limited, UK) or Amersham ECL anti-mouse IgG horseradish peroxidase-linked species-specific whole antibody (NA931, GE Healthcare UK Limited, UK) in blocking buffer for 3 h at room temperature. After washing four times with 50 μL/well of PBST, a 50 μL/well of 3,3′-5,5′-tetramethylbenzidine (TMB) Liquid Substrate System for ELISA (T040, Sigma-Aldrich Co. LLC., St. Louis, MO) was added to the plate. After 30 min of incubation, 50 μL/well of 0.9 N H2SO4 solution was added and OD450 nm was measured using an iMark Microplate Reader (1,681,135 J, Bio Rad, Hercules, CA). The half-maximal antibody titers of the serum samples were calculated from the highest absorbance in the dilution range using Prism 8 (Graph Pad Software Inc. San Diego, CA 92018, USA).
2.7. ELISpot assays
The splenocyte preparation involved the humane harvest of rat spleens from the vaccinated rats after six weeks. The splenocytes were passed through a 70 μm cell strainer in RPMI 1640 medium (Nacalai Tesque Inc., Kyoto, Japan) supplemented with 0.1 mg/mL penicillin-streptomycin (Nacalai Tesque Inc.). The residual erythrocytes were lysed using a hemolysis buffer (Immuno-Biological Laboratories Co., Ltd., Gunma, Japan) for 5 min and washed with PBS. The splenocytes were cultured in a complete RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; GE Healthcare Life Sciences, Logan, UT), 0.1 mg/mL penicillin-streptomycin, and 50 μM β-mercaptoethanol (Nacalai Tesque Inc.) and incubated at 37 °C in a humidified atmosphere of 5% CO2. Rat IFN-γ and IL-4 ELISPOT assays (CT079 and CT081, U-CyTech Biosciences, Utrecht, Netherlands) were performed following the manufacturer’s instructions. Rat splenocytes (2 × 105) were plated in duplicate on 96-well PVDF plates (Millipore, Billerica, MA, USA) and stimulated with a 5 μg/mL recombinant 2019-nCoV spike protein (S1 + S2 ECD, His tag; Beta Lifescience, Fairfield, NJ) for 48 h. The resultant stained well membranes were scanned and the spot-forming cells were counted.
2.8. IgG subclass analysis
To analyze the immunoglobulin subclass profile of the sera in vaccinated rats, standardized ELISA protocols were performed. Briefly, 96-well plates were coated with 10 µg/mL SARS-CoV-2 SV2 recombinant protein and incubated overnight at 4 °C. The serum samples were also incubated in the appropriate wells at 4 °C overnight. After incubation, the serum samples were removed and the IgG subtypes were detected over three hours at room temperature using the following HRP-conjugated antibodies: anti-IgG1 H&L (ab106753; Abcam), anti-IgG2a H&L (ab106783; Abcam). anti-IgG2b H&L (ab106750; Abcam), and anti-IgG2c (3075–05, Southern Biotech). After the HRP-conjugated antibody detection, the plates were developed using POD (T0440; Sigma) with a stopping buffer (9,562,606; Nacalai Tesque) and the absorbance was detected at 450 nm (iMark; Bio-Rad).
2.9. ACE2 binding inhibition assay
To analyze the binding inhibition of S1 + S2 and ACE2 by the neutralizing antibodies in the immunized rat and mouse sera, 96-well plates were coated with human ACE2 recombinant protein (1 μg/mL, mFc tag; #83986, Cell Signaling Technology) and then blocked with PBS containing 5% skim milk for 2 h at room temperature. After blocking, the pre-incubated samples of diluted rat or mouse serum with recombinant S1 + S2 protein (2 μg/mL, His tag; Beta Lifescience) were added to the coated wells and incubated overnight at 4 °C. The wells were washed with PBST and incubated with anti-His antibody conjugated with HRP (GTX21187, GeneTex, Inc., Irvine, CA) for 2 h at room temperature. After washing with PBST, the peroxidase chromogenic substrate TMB (Sigma-Aldrich) was added to the wells and incubated for 30 min at room temperature. The reaction was stopped by the addition of sulfuric acid (0.5 N). The absorbance was measured at 450 nm using a microplate reader (Bio-Rad). At various time points, the percentage inhibition of the immunized blood serum was calculated and plotted using GraphPad Prism software. The neutralization titer at ID75 was also derived.
2.10. Pseudovirus neutralization assay for SARS-CoV-2
The neutralizing activity of the vaccine-induced antibodies were analyzed using pseudotyped vesicular stomatitis virus (VSVs) as previously described [Citation7]. Briefly, Vero E6 cells stably expressing TMPRSS2 were seeded in 96-well plates and incubated at 37 °C for 24 h. Pseudoviruses were incubated using a series of dilutions of inactivated rat serum for 1 h at 37 °C and then added to the Vero E6 cells. Precisely 24 h after infection, the cells were lysed with cell culture lysis reagent (Promega), and the luciferase activity was measured using Centro XS3 LB 960 (Berthold).
2.11. SARS-CoV-2 infection in mice that received pVAX1-SARS-CoV2-co plasmids
Female mice received pVAX1-SARS-CoV-2-co plasmids intradermally (ID). The ID injected specimens received 160 µg of plasmid at two sites on each flank using PJI (Actranza™). The animals received 20 μL (40 μg) pVAX-SARS-CoV-2-co plasmid at two sites on each side, totaling 160 μg of plasmid in each mouse, using PJI. The pVAX1-SARS-CoV-2-co plasmids were administered on days 0 and 14. Sixteen weeks after the first administration, sera were collected from immunized mice and assessed for antibody induction against the 2019-nCoV spike protein (S1 + S2), the RBD region of the spike protein, and for any binding inhibition of the recombinant hACE2 protein.
The mouse-adapted SARS-CoV-2 virus (HuDPKng19-020 strain-Q498Y/P499T) was generated in vitro using reverse genetics [Citation8,Citation9]. Infectious titers in the culture supernatants were determined by analyzing the 50% tissue culture infective doses (TCID50). Mice were intranasally infected with 2.0 × 105 TCID50 mouse-adapted SARS-CoV-2 virus. Two days post-infection, specimens were euthanized and the lungs were isolated. Approximately 0.02 g of lung tissue was homogenized using 100 µL of PBS. The homogenate was serially diluted ten-fold in DMEM containing 2% FBS and loaded onto VeroE6/TMPRSS2 cells for analysis.
2.12. Statistics
Specimen body weight, food consumption, hematology, blood biochemistry, urinalysis, and organ weight were analyzed using a Mitsui Toxicological Data Processing System (Mitsui E&S Systems Research Inc., Tokyo, Japan). The statistical analysis of the antibody titers and ELISpot was performed using BellCurve in Excel (Social Survey Research Information Ltd., Tokyo, Japan). Statistical significance was confirmed if p < .05.
3. Results
3.1. pVAX-SARS-CoV2-co vaccine injected into mouse and rat dermal tissue via PJI
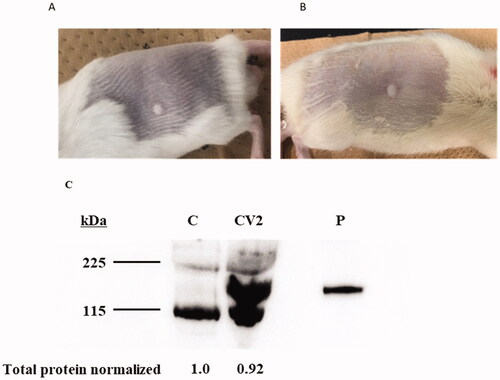
A novel injection device, PJI, was used to transport pVAX1-SARS-CoV2-co into dermal tissue. Briefly, 20 or 50 μL of the pVAX1-SARS-CoV2-co solution was injected into the backs of mice or rats using PJI. A wheal of approximately 5 mm diameter was formed on the back of the mouse/rat and no bleeding or inflammation was observed (). After 24 h, dermal tissue samples from the pVAX1-SARS-CoV2-co-injected sites were collected and the spike glycoprotein expression was analyzed. Spike glycoprotein was detected by a specific anti-spike glycoprotein antibody (). Therefore, pVAX1-SARS-CoV2-co could be efficiently introduced into rat dermal tissue via PJI to initiate spike glycoprotein expression.
Figure 1. Skin conditions and S protein expression after pVAX-SARS-CoV2-co injection using Pyro-drive jet injector. (A) A wheal on the back of the rat immediately after a 20 μL injection using PJI. (B) A wheal on a rat back immediately after a 50 μL injection by PJI. (C) The protein expression of SARS-CoV-2 spike glycoprotein in a rat. C: control, CV2: pVAX-SARS-CoV-2-co plasmid injection by Pyro-drive Jet Injector, P: recombinant S1 + S2 protein positive control (0.5 μg/lane) (the predicted molecular weight was 134.36 kDa.).
3.2. The increase and retention of anti-COVID19 spike protein antibodies in the rat serum
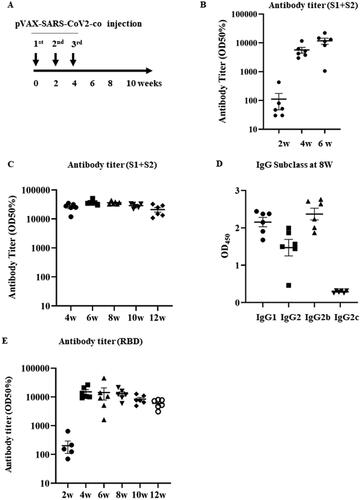
The pVAX1-SARS-CoV2-co was injected into the backs of the rats three times every second week using a PJI (). Blood samples were collected every two weeks until 12 weeks after the first injection and the sera were purified from the blood samples. Anti-COVID19 spike protein (S1 + S2) antibody titers at 2, 4, and 6 weeks after the first injection were measured using enzyme-linked immunosorbent assay (ELISA). After two weeks, the antibody titer started to rise and reached its highest value at six weeks (). The antibody titer was measured until after 12 weeks to confirm antibody retention upon pVAX1-SARS-CoV2-co injection. The antibody titer peaked after 6 weeks and gradually decreased until week 12. However, all titers remained very high until 12 weeks (). To determine the polarization of the immunoglobulin subclass profile of the rat sera following the vaccine treatment, we analyzed the variation in the SARS-CoV2-specific IgG1, IgG2a, IgG2b, and IgG2c in rat sera harvested eight weeks after the vaccine treatment. Our results showed that overall, SARS-CoV2-specific IgG1, IgG2a, and IgG2b levels were high, whereas IgG2c levels were low (). Importantly, the SARS-CoV2-specific IgG2b subclass was more elevated than the IgG1. This result indicates Th1-mediated subclass polarization. Additionally, the antibody titers of RBD spike glycoprotein in rat serum were also measured. The RBD titers were elevated at 4 and 12 weeks after the first vaccination (). The production of both anti-S1 + S2 glycoprotein and anti-RBD antibodies indicates that intradermal pVAX1-SARS-CoV2-co injection successfully induced an immune response.
Figure 2. Humoral immune response against pVAX-SARS-CoV2-co vaccination. (A) Vaccination protocol. The pVAX1-SARS-CoV2-co was injected intradermally using PJI fortnightly for three times. Blood samples were collected every two weeks for antibody titer and subclass analysis. (B) Early (2–6 weeks after the first injection) antibody titer (half-maximum) for the recombinant glycoprotein S1 + S2 assessed using ELISA. C) Antibody titer (half-maximum) for the recombinant glycoprotein S1 + S2 (4–12 weeks) D) Immunoglobulin subclasses of the rat serum 8 weeks after the vaccine injection. The IgG subclasses in the rat sera (IgG1, IgG2a, IgG2b, and IgG2c) were analyzed using ELISA. The results were assessed at 450 nm. E) Antibody titer (half-maximum) for the recombinant spike glycoprotein RBD assessed using ELISA (2–12 weeks).
3.3. The cellular immune response of vaccinated rats
To examine the Th1/Th2 cellular immune responses to the vaccine, we isolated solenocytes from vaccinated rats 2 weeks after the final vaccination and analyzed the S1 + S2-specific IFN- and IL-4-secreting T cells using ELISpot assays. The splenocytes harvested from the vaccinated rats showed a significant increase in the IFN-γ-secreting S1 + S2-specific T cells (), whereas the IL-4-secreting S1 + S2-specific T cells were detected in much lower numbers (data not shown). The results suggest the intradermal injection of pVAX1-SARS-CoV2-co induced a strong Th1-type cellular immune response.
Figure 3. Cellular immune response and neutralizing antibodies against pVAX-SARS-CoV2-co vaccination. (A) IFN-γ and IL-4 ELISpot responses in the splenocytes of rats vaccinated with 240 μg pVAX1-SARS-CoV2-co, with or without re-stimulation with recombinant S1 + S2. The p-value calculated using Welch’s t-test comparing IFN-γ secreting cell number between the antigen-stimulated group and the un-stimulated group was 0.0059 (n = 6). (B) ACE2 (and S1 + S2) binding inhibition assay using serum of rat immunized for 2–12 weeks normalized to pre-serum, at reciprocals 10- to 31,250-fold dilutions. (C) Neutralization titers (ID75) of serum of rat immunized for 2–12 weeks by the dilution of serum required for 75% inhibition of ACE2-S1 + S2 binding as shown in (B). (D) The inhibition rate targeting the pseudovirus. The immunized rat serum at 8 weeks was used in the reciprocal 20- to 12,500-fold dilutions. (E) Neutralization activity (ID75; 75% inhibition). The values indicate the inhibitory dose of the serum has a 75% inhibition rate against pseudovirus binding as provided in (D).
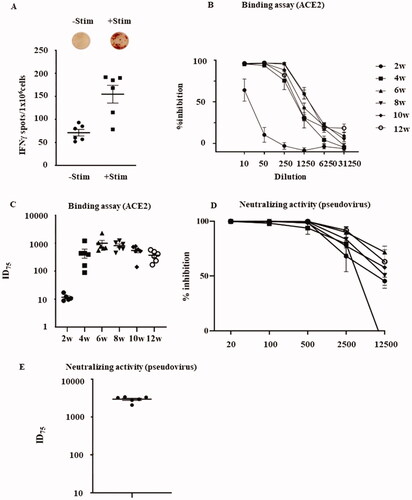
3.4. ACE2 binding inhibition and pseudovirus neutralization assays
To further investigate the neutralizing ability of the vaccine-induced antibodies against SARS-CoV-2, we analyzed the binding inhibition of human ACE2 (a receptor of the SARS-Cov-2 spike glycoprotein) to the S1 + S2 recombinant protein in immunized rat serum using ELISA [Citation10]. Rat serum collected two weeks after the first vaccination showed some inhibition (62%) of hACE2 binding to the S1 + S2 recombinant protein at a 10-fold dilution (). In comparison, rat serum collected between weeks 4 and 12 had a high neutralizing ability (>90%) against the binding of hACE2 to the S1 + S2 recombinant protein at an up to 50 folds dilution and maintained high neutralization activity for 6–8 weeks at an up to 250 folds dilution. Correspondingly, neutralization titers against hACE2-S1 + S2 binding (at a 75% inhibitory/neutralization dose (ID75)) showed a significant spike between weeks 2 and 4 after the first vaccination, reaching a peak between weeks 6 and 8, and then decreasing gradually after week 8 (). Moreover, a pseudovirus neutralization assay using pseudotyped VSVs with the luciferase gene and Vero E6 cells stably expressing TMPRSS2 [Citation7,Citation11] was performed to examine the neutralizing activity. Eight weeks after the first vaccination, a series of rat serum dilutions showed strong neutralizing activity against the pseudovirus infection (). Neutralizing titers (ID75) exhibited the highest titer after six weeks, which was approximately 30 times higher than that achieved with intramuscular injection ().
3.5. Increasing injection doses
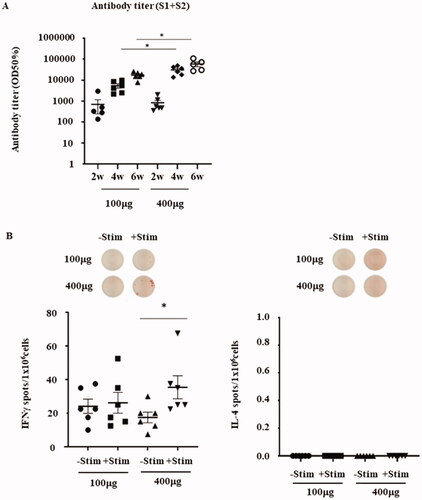
To assess whether vaccine dosage affects antibody production and cellular immune responses, we assessed different pVAX1-SARS-CoV2-co dosages from 100 µg (low-dose) to 400 µg (high-dose). When 100 or 400 µg of the DNA vaccine was injected into the rat skin, antibody production increased from weeks 2 to 6 after each injection. After six weeks, the maximum antibody titers of the 100 and 400 µg DNA vaccination groups were 17,056 ± 6331 and 59,068 ± 33,578, respectively. In addition, there was a statistically significant difference between the antibody titers of the 100 µg and 400 µg injected groups after both 4 and 6 weeks (). To assess whether the vaccine dosage affects Th1/Th2 cellular immune responses, we compared the S1 + S2-specific IFN- and IL-4 ELISpot assays between rats vaccinated with 100 or 400 μg of pVAX1-SARS-CoV2-co. The splenocytes harvested from the 400 μg/high-dose vaccinated rats showed a significant increase in S1 + S2-specific IFN- secretion when re-stimulated with the antigen. Conversely, the low-dose vaccinated rats exhibited only a modest increase when re-stimulated (). The IL-4-secreting T cells were barely detectable in either group. These results suggest that a high-dose vaccine could induce a stronger IFN-γ response compared to the lower dose vaccine, potentially causing a more predominant Th1-driven cellular immune response.
Figure 4. Effects on antibody production and cellular immune response by higher vaccine dosage. (A) Antibody titer (half-maximum) for the recombinant glycoprotein S1 + S2 (2–6 weeks) of the 100 μg and 400 μg pVAX1-SARS-CoV2-co-injected groups. The exact p-values calculated using Welch’s t-test between the 100 μg and 400 µg doses were 0.0060 or 0.0496 after 4 and 6 weeks, respectively. Both p-values were less than .05. Therefore, a statistically significant difference was observed between the 100 μg and 400 µg doses. (B) IFN-γ and IL-4 ELISpot responses in the splenocytes of rats vaccinated with 100 μg or 400 μg of pVAX1-SARS-CoV2-co-injected groups, with or without re-stimulation with recombinant S1 + S2. The p-value calculated using Student’s t-test between -Stim and + Stim was p = .0394.
3.6. DNA vaccine prevents a viral infection challenge in mice
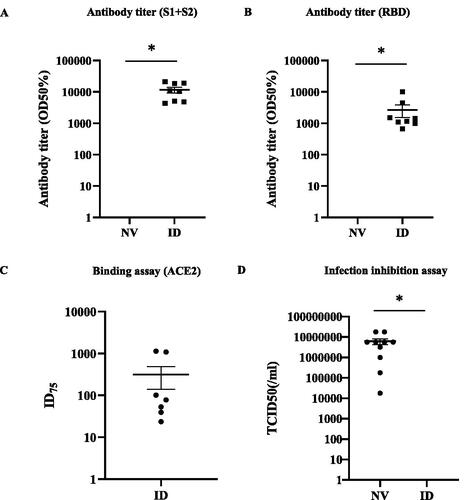
To assess whether the DNA vaccination could prevent viral infection, we intradermally injected mice with the mouse-adapted SARS-CoV-2 virus (HuDPKng19-020 strain – Q498Y/P499T) [Citation8] intradermally (ID; PJI). Prior to the infection challenge and 16 weeks post-immunization, the antibody titers for S1 + S2 in the ID injection group were significantly higher than those in the non-vaccinated (NV) group (, Supplemental Figure 1(A)). Similarly, the antibody titers of RBD in the ID injection group were higher than those in the NV group (, Supplemental Figure 1(B)). The ID injection group also displayed a strong inhibitory ability and high neutralization titers restricting hACE2-S1 + S2 binding (). After the viral infection challenge, no detectable viral load was found in the lungs of any of the eight mice in the ID injection group (, Supplemental Figure 1(D)). Taken together, these results suggested that pVAX1-SARS-CoV2-co immunized mice (especially mice vaccinated with PJI) could effectively achieve upregulated antibody responses against the SARS-CoV-2 spike protein.
Figure 5. Prevention of a viral infection challenge by the pVAX-SARS-CoV2-co immunized mice. (A) Pre-viral challenge of mice immunized with 160 μg of pVAX1-SARS-CoV2-co plasmid intradermally (ID; n = 8) or not-vaccinated (NV: n = 10) two times at 2-week intervals. The antibody titer (half-maximum) for the recombinant S1 + S2 in the blood serum 16 weeks post initial vaccination and assessed using ELISA (p < .001; Student’s t-test). (B) Antibody titer (half-maximum) for the recombinant RBD in the blood serum in ID and NV mice (p = .018; Student’s t-test). (C) Neutralization titer (ID75) for the recombinant ACE2-S1 + S2 binding inhibition in the blood serum of ID mice. (D) ID and NV (n = 10) mice were intranasally infected with the mouse-adapted SARS-CoV-2 virus. The 50% tissue culture infective doses (TCID50) in the lung tissues of each animal are shown (p = .016; Student’s t-test). All individual values are provided in Supplemental Figure 1.
3.7. Safety assessment of pVAX1-SARS-CoV2-co intradermal injection and the immune response
No animals died at the time of necropsy or showed any abnormalities, except for initial skin reactions. There were no significant differences in body weight, food consumption, or hematology between the PBS-injected and DNA vaccination groups (). In addition, the urinalysis data showed no signs of toxicity (data not shown). As provided in , the blood biochemistry revealed significantly lower levels of triglycerides and β-globulin in the 400 µg group than in the PBS group. After the DNA vaccination, erythema and scab formations were observed at the injection site in rats injected with the vaccine, whereas no erythema or scab formation was observed in the PBS-injected group (). In the 100 µg DNA vaccine-injected group, scab formation occurred 5, 4, or 3 days after the first, second, or third vaccinations, respectively. In contrast, in the 400 μg DNA vaccine-injected group, scab formation occurred 5, 3, or 2 days after the first, second, or third vaccination, respectively. The scab did not last as long in the high-dose group when compared to the low-dose group. In addition, recovery from the scab varied with vaccination time. After the first, second, and third injections, the scab disappeared after 3, 5, 7, and 11 d, respectively. However, all scabs disappeared and the skin recovered 14 days after injection ().
Table 1. Effect of DNA vaccination on body weight in rats. Body weight average of 0, 100 and 400 μg/body DNA vaccinated rat group from day 1 to 42.
Table 2. Effect of DNA vaccination on food consumption in rats. Food consumption average of 0, 100 and 400 μg/body DNA vaccinated rat group from day 1 to 42.
Table 3. Effect of DNA vaccination on hematology in rats. Hematology analysis average of 0, 100 and 400 μg/body DNA vaccinated rat group.
Table 4. Effect of DNA vaccination on blood biochemistry in rats. Blood analysis average of 0, 100 and 400 μg/body DNA vaccinated rat group.
Table 5. Clinical signs of DNA vaccination in rats. Skin conditions (erythema, scab formation or none) observation after DNA vaccination.
A histopathological analysis of the lymph nodes revealed slight to mild hyperplasia of the follicular lymph nodes at the inguinal lymph node in five of the six animals in the 400 µg group. Also, in the 400 µg group, slight hyperplasia of follicular was observed at the axillary lymph node of one of six animals. Furthermore, hyperplasia of the plasma cells was observed only in the 400 µg group. Conversely, only slight hyperplasia of the follicular was observed in one of the six animals in the 100 µg group. None of these abnormalities were observed in the PBS-injected group. Therefore, the hyperplasia might be an immune response to the pVAX1-SARS-CoV2-co injection. However, sperm reduction in the epididymis, interstitial inflammation of the prostate, and sperm granuloma were observed in all groups, including the PBS-injected group ().
Table 6. Histopathological results of 0, 100 and 400 µg/body DNA vaccinated rat organs and tissues.
4. Discussion
During the recent COVID-19 pandemic, efficient and safe DNA vaccine development has become an important issue. We tested the immunogenicity and safety of a combination of new human codon-optimized COVID-19 DNA vaccine candidates (pVAX1-SARS-CoV2-co) [Citation3] and PJI [Citation6]. The development of vaccines against SARS-CoV-2 is a priority for many pharmaceutical companies and academic organizations worldwide. A variety of SARS-CoV-2 vaccines, such as protein subunits [Citation12], replicating or non-replicating viral vectors [Citation13–15], inactivated viruses [Citation16], live attenuated viruses [Citation17,Citation18], virus-like particles (VLPs) [Citation19,Citation20], and RNA- [Citation21,Citation22] and DNA-based [Citation5,Citation23,Citation24] vaccines are currently under development. DNA vaccines have several advantages over traditional vaccines such as protein subunits, peptides, and living/inactivated pathogens. When compared to protein- or peptide-based vaccines or living/inactivated virus vaccines, DNA vaccines do not require cultivating the target pathogen and can be produced on an industrial scale [Citation25]. Furthermore, DNA vaccines can be rapidly adapted when mutations occur in the target DNA/RNA sequences.
Vaccine administration is an important factor that enhances the efficacy. In recent years, the main vaccination method has been intramuscular administration using a needle syringe. Intradermal administration is a promising vaccination method because dermal tissue is an easily accessible, immune-rich environment that can induce both humoral and cellular immunity [Citation26]. Tebas et al. reported that intradermal Ebola GP DNA vaccine administration demonstrated humoral and cellular immunogenicity advantages when compared to intramuscular administration [Citation27].
When 100 or 400 µg of pVAX1-SARS-CoV2-co was injected into rats, the SARS-CoV-2 spike protein (S1 + S2) antibody was detected (). Statistically significant differences were observed between the 100 and 400 µg pVAX1-SARS-CoV2-co-injected groups after 4 and 6 weeks (p < .05). Therefore, the dose-dependent anti-SARS-CoV-2 spike protein (S1 + S2) antibody production was induced. This finding supports previous research using OVA expression plasmid DNA [Citation3]. Interestingly, the antibody was detected in the fortnightly sample at both doses. Therefore, the antibody release was induced quickly after the first injection. The potential for rapid antibody production is likely to be due to the vaccination method. In addition, antibody titers increased eight times in the low-dose group and 37 times in the high-dose group after the second injection, and 2–3 times after the third injection. This indicates the importance of a second injection as an immune booster. In addition, PJI intradermal injection is expected to be an effective administration method. Previous study shows that antibody titers (S1 + S2 and RBD) and neutralizing antibody (pseudovirus) data in which COVID-19 DNA vaccine was administrated intramuscularly [Citation6]. PJI intradermal injection of DNA vaccine shows approximately ten times higher values of the antibody titers and neutralizing activity than the intramuscular administration. Moreover, the number of IFN-γ-producing cells increased in the pVAX1-SARS-CoV2-co-injected group, indicating cellular immunity was elicited by the pVAX1-SARS-CoV2-co intradermal injection. Cellular immunity is important for preventing and curing viral infections. Thus, the combination of pVAX1-SARS-CoV2-co with intradermal injection via PJI is a promising method for the treatment of SARS-CoV-2.
In the safety test, no animals died at the time of necropsy, and there were no significant variations in the body weight, food consumption, urinalysis, organ weight, or hematology between the PBS- and pVAX1-SARS-CoV2-co-injected groups. As demonstrated in , the blood biochemistry revealed significantly lower levels of triglycerides and β-globulin in the 400 µg group than in the PBS group. However, the historical data suggests these changes were within normal variation patterns among animals. These results suggest a pVAX1-SARS-CoV2-co injection at all doses tested did not cause systemic toxicity in rats. Scab formulation was observed in the injected region, and the duration between injection and scab formation decreased with each vaccination. In addition, the duration of scab in the high-dose group was shorter than the low-dose group (). These results suggest that scab formation is related to the immune response caused by the pVAX1-SARS-CoV2-co injection. However, all scabs disappeared within 14 d of injection. However, in the high dose group, slight to mild hyperplasia of the follicular or plasma cells was observed in the inguinal lymph nodes of five of the six animals and the axillary lymph nodes of one in six animals. Conversely, slight to mild hyperplasia occurred in the inguinal lymph nodes of one in six animals in the low-dose group (). The immune response in the high-dose group was stronger than in the low-dose group. Thus, hyperplasia is thought to be an immune response against antigen invasion, which is non-toxic. Sperm reduction in the epididymis, interstitial inflammation of the prostate, and sperm granuloma were observed in all groups, including the PBS-injected group. These observations did not indicate any signs of toxicity.
5. Conclusion
We evaluated the safety of intradermal vaccination using a human codon-optimized COVID-19 DNA vaccine candidate (pVAX1-SARS-CoV2-co). Intradermal PJI injection of pVAX1-SARS-CoV2-co did not result in any systemic toxicity. Erythema and scab formation were observed at the skin injection sites. However, visual observations and histopathological analyses indicated that these abnormalities were not permanent and were expected to fully recover over time. No other signs of toxicity were noted. These results indicate an intradermal administration of pVAX1-SARS-CoV2-co is a safe and promising method for inducing potent antibodies against SARS-CoV-2.
Author contributions
TN wrote the manuscript. T.N., C.C., J.A.T., H.H., J.S., C.O., Y.M., R.I., M.S., and K.Y. performed the experiments and analyzed the data. T.N., C.C., J.A.T., H.H., C.O., Y.M., R.I., J.M., M.S., M.Y., H.N., and K.Y. designed the study and revised the manuscript.
Supplemental Figure
Download MS Word (76.9 KB)Disclosure statement
The Department of Device Application for Molecular Therapeutics is a joint research department that is supported by Daicel Co. The Department of Health Development and Medicine, Graduate School of Medicine, is an endowed department that is supported by Anges, Daicel, and FunPep. R. I., J.M., and K.Y. were employees of FunPep Co., Takara Bio Inc., and Daicel Co., respectively. All other authors declare no competing interests.
Additional information
Funding
References
- Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239(1):62–84.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279.
- Chang C, Sun J, Hayashi H, et al. Stable immune response induced by intradermal DNA vaccination by a novel needleless pyro-drive jet injector. AAPS PharmSciTech. 2020;21(1):19.
- Kaur SP, Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288:198114.
- Hayashi H, sun j, Yanagida Y, et al. Preclinical study of DNA vaccines targeting SARS-CoV-2. Curr Res Transl Med. 2022;70(4):103348.
- Miyazaki H, Atobe S, Suzuki T, et al. Development of pyro-drive jet injector with controllable jet pressure. J Pharm Sci. 2019;108(7):2415–2420.
- Matsuyama S, Nao N, Shirato K, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117(13):7001–7003.
- Torii S, Ono C, Suzuki R, et al. Establishment of a reverse genetics system for SARS-CoV-2 using circular polymerase extension reaction. Cell Rep. 2021;35(3):109014.
- Dinnon KH, 3rd, Leist SR, Schäfer A, et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586(7830):560–566.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8.
- Bukowska A, Spiller L, Wolke C, et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med. 2017;242(14):1412–1423.
- Dai L, Zheng T, Xu K, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182(3):722–733.e11.
- Dieterle ME, Haslwanter D, Bortz RH, 3rd, et al. A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition. Cell Host Microbe. 2020;28(3):486–496.e6.
- Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854.
- Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488.
- Palacios R, Patiño EG, de Oliveira Piorelli R, et al. Double-Blind, randomized, Placebo-Controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (inactivated) vaccine manufactured by Sinovac - PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):853.
- Armengaud J, Delaunay-Moisan A, Thuret JY, et al. The importance of naturally attenuated SARS-CoV-2in the fight against COVID-19. Environ Microbiol. 2020;22(6):1997–2000.
- Fidel PL, Jr., Noverr MC. Could an unrelated live attenuated vaccine serve as a preventive measure to dampen septic inflammation associated with COVID-19 infection? mBio. 2020;11(3):e00907-20.
- Ghorbani A, Zare F, Sazegari S, et al. Development of a novel platform of virus-like particle (VLP)-based vaccine against COVID-19 by exposing epitopes: an immunoinformatics approach. New Microbes New Infect. 2020;38:100786.
- Pushko P, Tretyakova I. Influenza virus like particles (VLPs): opportunities for H7N9 vaccine development. Viruses. 2020;12(5):518.
- Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - Preliminary report. N Engl J Med. 2020;383(20):1920–1931.
- Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438.
- Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11(1):2601.
- Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369(6505):806–811.
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9(10):776–788.
- Hettinga J, Carlisle R. Vaccination into the dermal compartment: techniques, challenges, and prospects. Vaccines. 2020;8(3):534.
- Tebas P, Kraynyak KA, Patel A, et al. Intradermal SynCon® ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J Infect Dis. 2019;220(3):400–410.
