Abstract
Programmed cell death 1 (PD-1) is an immune checkpoint and has been reported to be associated with several autoimmune diseases. We aimed to investigate the association between human PD-1 gene (PDCD1) polymorphisms and multiple sclerosis (MS). This case-control study was conducted on 229 MS patients and 246 healthy controls. Genotyping of rs36084323 (PD-1.1 G/A), rs11568821 (PD-1.3 G/A) and rs2227981 (PD-1.5 C/T) polymorphisms was performed by PCR-RFLP technique. The frequency difference of PD-1.1 genotypes and alleles (−536 G/A) between patients and healthy controls was not significant. Regarding PD-1.3, the AA + AG genotype was found to be relatively higher in the control group. Concerning PD-1.5 (+7785 C/T), the frequency of T allele carriers (TT + CT) was relatively higher in MS patients, which was marginally insignificant (p = .07). PD-1 gene polymorphisms may be associated with MS; however, accurate conclusions require further studies with a larger number of samples.
1. Introduction
Multiple sclerosis (MS) is the most common neurological disease in young adults, which is mainly diagnosed in people between the ages of 20 and 40 and is more common in women [Citation1]. This disabling disease of the central nervous system is a chronic inflammatory disease with an autoimmune origin. Numerous studies have shown that environmental and genetic factors can influence the occurrence and progression of MS [Citation2]. The function of self-reactive T cells in the pathogenesis of the disease has been well identified. Molecules present on the cell surface are involved in regulating the activity of T lymphocytes and thus the immune response [Citation3]. The main pathways for T cell activity include the interaction of CD28 molecules on T cell surfaces with B7-1 (CD80) and B7-2 (CD86) costimulatory molecules on the surface of antigen-presenting cells (APCs) [Citation4].
Some members of B7-CD28 family have inhibitory activities in the adaptive immunity and play crucial roles through the regulation of T cell responses under different physiological and pathological conditions [Citation5]. Consequently, study on the function and genetic variations of B7-CD28 family members have confirmed the important role of this family in susceptibility to various autoimmune diseases, including MS [Citation6].
Programmed death 1 (PD-1) is a well-known member of the large family of CD28 molecules with a molecular weight of 55 kDa, which is expressed on the surface of the active B and T cells and myeloid dendritic cells [Citation7]. This surface protein seems to play an important role in immunological tolerance. Interaction of PD-1 with two ligands, PDL-1 (B7-H1) and PDL-2 (B7-DC), reduces its activity and proliferation, and diminishes cytokine secretion, while inducing programmed cell death in T lymphocytes [Citation8]. The role of PD-1 in immune tolerance and lymphocyte homeostasis has been confirmed using mice with defective PD-1 expression. These mice develop an innate autoimmune disease with a set of clinical manifestations similar to systemic lupus erythematosus and human rheumatoid arthritis [Citation9,Citation10]. The Human PD-1 gene (PDCD1) is located at position 2q37.3 on chromosome 2, where numerous genetic variations, including single nucleotide polymorphisms (SNPs), have been identified [Citation11–14]. So far, the association of SNPs in PDCD1 gene with several diseases caused by immune system disorders, including systemic lupus erythematosus, rheumatoid arthritis and type 1 diabetes, has been investigated [Citation12,Citation15–17]. In the present study, the association of three SNPs in PDCD1 gene, i.e., PD-1.1 (−536 G/A), PD-1.3 (+7146 G/A) and PD-1.5 (+7785 C/T), with susceptibility to MS was investigated.
2. Materials and methods
2.1. Sample preparation
In this case-control study, a total of 229 patients with MS, including 175 women and 54 men, were recruited. Furthermore, 246 healthy controls with no history of autoimmune disease in family members and relatives, including 186 women and 60 men, were included in the study process. The patients were selected from those referred to the Neurology Department at Shahid Chamran Hospital in Shiraz, Iran, during 2019. Patients’ eligibility was evaluated based on definitive MS diagnosis, which was obtained by medical interviews, clinical signs and diagnostic tests, such as MRI and confirmed according to the 2017 McDonald criteria [Citation18]. The ethics committee approved the study protocols of Immunology Research Center at Shiraz University of Medical Sciences. Before starting, the subject and aims of the research was explained to the patients and the controls and, thereafter, a questionnaire and a written informed consent was filled out by all participants. Age, sex, age of onset, disease progression index, patient disability rate (Expanded Disability Status Scale) and the disease type (relapsing-remitting (RR), primary progressive (PP), secondary progressive (SS), progressive relapsing (PR) was determined for each patient. After that, 5 mL of peripheral blood was taken from the subjects and added to falcons containing EDTA anticoagulant. Samples were stored at −20 °C until DNA extraction.
2.2. Genetic analysis
Genomic DNA was extracted from the blood samples using the phenol-chloroform method. The quality and quantity of the extracted DNA samples were confirmed using agarose 1% gel electrophoresis and NanoDrop-1000 device, respectively. DNA fragments containing rs36084323 (PD-1.1 G/A), rs11568821 (PD-1.3 G/A) and rs2227981 (PD-1.5 C/T) polymorphisms were amplified using the polymerase chain reaction (PCR) technique with the primers which are shown in . The reactions were prepared in a total volume of 30 µL containing 300 ng template DNA, 10 mM Tris–HCl,0.2 mM dNTPs, 2.4 mM MgCl2, 10 pM of forward and reverse primers and 1 unit Taq DNA polymerase. Thermal cycling conditions for the amplification of each SNPs are shown in . The amplified fragments were run on 1.5% agarose gel electrophoresis to confirm the accuracy of the fragments. Thereafter, we carried out a restriction fragment length polymorphism (RFLP) technique to digest the PCR products. For this purpose, 10 µL of each amplified sample was mixed with 1 unit of the restriction enzymes () for 5 min at 65 °C to allow digestion. Then the products were run on 1.5% agarose gel electrophoresis to evaluate the genotypes according to the digestion pattern. illustrates an example for PD-1.3.
Figure 1. Amplified fragments of PD1.3, which were digested by PstI. The first lane is 100 bp DNA marker. The PCR product size was 180 bp. If the product was digested, the allele was identified as A; otherwise, it was identified as G.
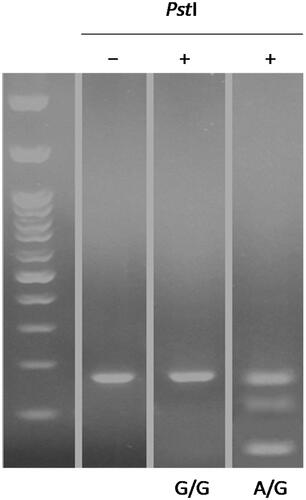
Table 1. Oligonucleotide primers sequences used in PCR-RFLP.
Table 2. Thermal cycling conditions for PCR amplification of PD-1.1, PD-1.3 and PD-1.5 polymorphisms.
2.3. Statistical analysis
In this study, the Mann-Whitney U test was used to compare the differences between the clinical and biochemical findings in two groups. Demographic and biochemical variables were also compared across the polymorphisms using Pearson’s χ2 tests. Quantitative variables were stated as mean ± SD and p < .05 was considered as statistically significant.
3. Results
3.1. Allele and genotype frequencies of PD-1.1 polymorphism in MS and control groups
Despite the higher frequency of G allele in the patient group compared to the control group (98.3% in patients vs. 97.4% in the control group), shown in the difference was not statistically significant (p = .4). Comparison of the frequency of genotypes showed that GG genotype in patients is more than the control group (96.5% in patients vs. 94.7% in controls), but this difference was also not significant (p = .34). There was also no association between genotypes frequencies and susceptibility or resistance to the disease (p = .21 in women) and (p = .27 in men). Also, no significant difference was observed between the frequency of GG genotype and allele carriers A (AA + AG) in patients and controls in the total population, in men, and in women (p = .3, p = .21 and p = .47, respectively). Obtaining the abundance of AA genotype with allele carrier (GG + AG) was not possible due to the absence of AA genotype.
Table 3. Comparison of genotypes and alleles from PD-1.1 polymorphism in patient and control groups.
3.2. Allele and genotype frequencies of PD-1.3 polymorphism in MS and control groups
There was a statistically significant difference between the frequency of genotypes resulting from rs11568821 polymorphism in patient group and controls (p < .001) shown in . After classification by sex, there was a significant difference in the frequency of PD-1.3 polymorphism genotypes between patients and controls only in women (p < .0001). This difference was due to a significant increase in AA genotype in controls compared to the patient group. In fact, comparison of AA genotype with G allele carriers (AG + GG) between patients and controls in the whole population (p < .00001) and in females (p < .001) showed a higher frequency of AA genotype in the control group.
Table 4. Comparison of genotypes and alleles from PD-1.3 polymorphism in patient and control groups.
3.3. Allele and genotype frequencies of PD-1.5 polymorphism in MS and control groups
We compared the genotypes in patients and controls to unravel the relationship between rs2227981 polymorphism and susceptibility to MS. As shown in , the differences between C and T alleles were not statistically significant. Also, we did not observe a considerable difference between CC genotype and T allele carriers (TT + CT) in patients and controls in the whole population (p = .07).
Table 5. Comparison of genotypes and alleles from PD-1.5 polymorphism in patient and control groups.
3.4. Association of the PD-1.5 polymorphism with different forms of MS
Regarding the relationship between genotypes resulting from +7785 C/T polymorphism (PD-1.5) with the type of disease in the whole population, women and men, p values were .75, .85 and .9, respectively. Therefore, the association between PD-1.5 polymorphism and disease types was not statistically significant. Comparison of genotypes showed that the frequency of CC genotype in SP MS is higher than in PP and RR types and the frequency of CT genotype was more elevated in RR type. PP MS, was more common than RR and SP, but this difference was not statistically significant. Information on the frequency of rs2227981 alleles and genotypes with disease type is shown in . The two other studied polymorphisms did not show any significant relationship with the different forms of MS. No significant relationship was observed between the genotypes of the studied polymorphisms and the age of onset of the disease (p = .2).
Table 6. Frequency of genotypes, alleles and allele carriers of PD-1.5 polymorphism in subjects with different courses of MS disease.
3.5. The association between EDSS score and PD-1 polymorphisms in MS patients
Expanded Disability Status Scale (EDSS) is an indicator to quantify disability in patients with MS. This index is classified from zero to ten, zero being equivalent to normal neurological examinations and ten equivalents to deaths due to MS. Regarding the relationship between genotypes resulting from PD-1.5 C/T polymorphism and EDSS score in the whole patient population, a p value of .86 was obtained, which was not statistically significant. On the other hand, the progression index of the disease was also calculated. This coefficient is obtained by dividing the EDSS score by the time elapsed after the onset of the disease. According to the results, there was no significant association between the disease progression coefficient and the polymorphisms’ genotypes.
4. Discussion
MS is an inflammatory disease of the central nervous system which has an autoimmune origin. Numerous studies have shown that environmental and genetic factors can influence its occurrence and progression [Citation19]. PD-1 is a member of the large CD28/B7 family, which is expressed on the surface of active B and T cells and myeloid dendritic cells. Interaction of this receptor with two ligands, PDL-1 (B7-H1) and PDL-2 (B7-DC) results in a diminishment in the activity and proliferation, as well as decreased cytokine secretion and induced programmed death in T lymphocytes [Citation8]. Pieces of evidence about the importance of CD28 family molecules in controlling cellular immunity are increasing, in which the PD-1/PD-L signaling pathway plays a decisive role in the mechanisms of tolerance and inhibition of the immune response [Citation20]. Also, the study of the function and genetic differences in the B7-CD28 family confirms the important roles of this family in autoimmune diseases, including MS. Therefore, any factor that affects the expression of PD-1 molecule can lead to changes in susceptibility to autoimmune diseases [Citation21]. An important example of these factors includes polymorphisms in the PD-1 encoding gene (PDCD1). One of the selected polymorphisms that have been reported to reduce the inhibitory effect of PD-1 and enhance T lymphocyte activity is PD-1.3 (+7146 G/A). Although no detailed research has been published on the association of allele A or G with the production of PD-1 at protein level, it is generally believed that a change from allele G to A in the intron of the PD-1 quadriceps disrupts the binding of transcription factor Vector 1 RunX to the amplifying region and possibly results in diminished PD-1 gene expression, which in turn can lead to susceptibility to autoimmune diseases [Citation22].
The PD-1.1 polymorphism is located in the promoter region (−536 from transcription start site). It is known that mutations in the promoter region (5′-flank) might be concerned with the transcription factor binding sites (TFBS) and motifs, as well as interrupting the activation of gene and the start of transcription. Hence, when a polymorphism is located in the promoter region of the PDCD1 gene, it can likewise influence the transcription and activation of the PD-1 gene, affecting the development of cancer and progression of human diseases. The PD-1.3 polymorphism is located in intron 4 (+7146 A/G). This SNP is a guanine (G) to adenine (A) polymorphism in the PD-1 intron was described as an enhancer-like due to the existence of four tandem repeats that contain multiple putative binding sequences of transcription factors. Recent studies have shown that the PD-1.3 polymorphism in this region is a regulatory SNP and indicated to be associated in vulnerability to cancers, likewise can alter the binding of the runt-related transcription factor 1 (RUNX1) and modify the transcriptional regulation and the proficiency of the PD-1 gene [Citation22,Citation23]. Moreover, investigations show that the presence of the A allele of the PD-1.3 polymorphism disturbed the binding site for RUNX1 transcription factors and cause ruined impairing PD-1 inhibitory influence, which leads to greater lymphocyte activity. Particularly, it has been shown that the PD-1.3 (A/G) polymorphism is associated with MS in German population [Citation24]. The PD-1.5 polymorphism is located in exon 5 (+7785 C/T) and is a synonymous polymorphism that does not modify the final amino acid structure of the protein. Significant associations between PD-1.5 and MS probably roots in the PD-1.5 variation linkage disequilibrium with other PD-1 gene polymorphisms that may lead to alteration of the PD-1 expression level.
Interestingly, in the present study, comparing the frequency of different genotypes and alleles of PD-1.3 (+7146 G/A) in the patient and control groups, showed a higher frequency of allele A and genotype AA in the control group. This result is inconsistent with the above-mentioned assumption that allele A is associated with decreased expression of the PD-1 gene. Several studies on other autoimmune diseases, e.g., systemic lupus erythematosus have indicated a higher frequency of allele A in normal individuals [Citation25,Citation26]. Based on these results, allele A may be associated with increased PD-1 production and resistance to systemic lupus erythematosus. However, the results of more similar studies in other autoimmune diseases are inconsistent with the results of the present study, although the reason for the inconsistency is not apparent. Additionally, a previous study on the association of PD-1.3 polymorphism with MS risk in an Iranian population did not show significant results [Citation27]. Such differences might stem from the sample size, power of study, MS diagnosis criteria. On the other hand, a paper published by Proconia on the association of PD-1 gene polymorphisms with susceptibility to systemic lupus erythematosus [Citation22], researchers did not clearly show that A allele was associated with a reduction in PD-1 incidence, and only by examining Allele A for transcription factor Vector 1 RunX this conclusion was reached. To achieve more conclusive results regarding the relationship between rs11568821A and PD-1 production, the amount of PD-1 produced at the protein level should be compared using immunohistochemistry and flow cytometry in individuals with AA genotype and individuals with GG genotype. Therefore, based on our results, it is possible that allele A is associated with increased production of PD-1 and leads to disease resistance.
In the present study, two other polymorphisms of the PD-1 gene were also studied. PD-1.5 (+7785 C/T) which is a silent polymorphism in exon 5 and, therefore, does not alter the final amino acid sequence of PD-1 protein [Citation21]. The molecular mechanism of action of PD-1.5 is also not well understood. Previous studies have examined the association of this polymorphism with several different cancers, including breast [Citation28] and colon cancers [Citation29]. In addition, the association of this polymorphism with other autoimmune diseases such as rheumatoid arthritis [Citation21] and type 1 diabetes [Citation30] has hardly been investigated from the results of the above studies on the effect of C or T alleles on detection of PD-1 molecule [Citation31]. However, according to research by Hau et al., the C allele is associated with a higher risk of breast cancer, and for this reason, the occurrence of this allele may be associated with greater activity of the PD-1 gene, as it reduces T lymphocyte activity [Citation32]. Studies on autoimmune diseases have almost shown no significant relationship between PD-1.5 (+7785 C/T) and type 1 diabetes. However, in a study by Lin et al., the association between T allele or CT genotype and the risk of rheumatoid arthritis has been reported. Based on this finding, it can be suggested that T allele of PD-1.5 is associated with a decrease in the expression of the PD-1 gene and an increase in the activity of self-reactive lymphocytes. Therefore, T allele may increase the chance of developing autoimmune diseases such as rheumatoid arthritis. In the present study, the relationship between PD1.5 polymorphism and the risk of MS was investigated. According to the information obtained, the CC genotype was relatively higher in the control group, although our results were not statistically significant (p = .07). In fact, if the number of samples increases, there will be a possibility that this result is statistically significant.
PD-1.1 (−536 G/A) is another polymorphism in the PDCD1 gene, which is located at the site of transcription initiation or in the gene promoter [Citation33]. Transcription initiation is an important part in regulating gene expression. Polymorphisms in the promoter can affect the natural process of gene activation and transcription initiation, thus, increasing or decreasing the amount of mRNA and protein [Citation34]. There is lack of studies on the relation between this polymorphism and susceptibility to MS. In a study by Li et al., the association of PD-1.1 polymorphism and ovarian cancer outcomes was evaluated and the authors reported no significant difference between the patient and control groups [Citation21]. In two other studies, the association of this SNP with rheumatoid arthritis was investigated and contradictory results were obtained [Citation33,Citation35]. In the study of Tahoori et al., the relationship between PD-1 gene polymorphisms and rheumatoid arthritis in the Iranian population was investigated. The results of these researchers only showed the association of PD-1.1 A allele with an increased risk of rheumatoid arthritis [Citation35]. However, Kong et al., showed the association of PD-1.1 A allele and prevention of rheumatoid arthritis in Chinese patients [Citation33]. In the present study, the genotypes of PD-1.1 polymorphism, which is located in the promoter of PD-1 gene, were determined for the first time in patients with MS and control individuals. The results of the present study indicate that PD-1.1 is not associated with MS. As a result, according to our research and a series of similar studies in other diseases, it appears that these SNPs or other genes involved in linkage disequilibrium do not play a role in susceptibility to MS or related clinical symptoms.
5. Conclusion
In the present study, the frequency of PD-1.1 genotypes and alleles in people with MS was investigated for the first time. The results did not show a significant association of PD-1.1 with MS susceptiblity. Regarding PD-1.5, the frequency of T allele carriers (TT + CT) was relatively higher in patients (p = .07). Therefore, it seems that with increasing the number of patients, there may be a possibility of observing a statistically significant difference. In addition, an association was observed between PD-1.3 polymorphism and the risk of MS. According to the information obtained, genotype AA and allele A were relatively higher in the control group. These connections may be due to linkage disequilibrium with other genes. Also, in the present study, no association was detected between PD-1.5, PD-1.3, and PD-1.1 SNPs and clinical symptoms and MS disease types.
Acknowledgement
The authors thank all individuals who participated in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- McElroy JP, Oksenberg JR. Multiple sclerosis genetics 2010. Neurol Clin. 2011;29(2):219–231.
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747.
- Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23(9):445–449.
- Francisco LM, Sage PT, Sharpe AH. The PD‐1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236(1):219–242.
- Markovic-Plese S, Cortese I, Wandinger K-P, et al. CD4+ CD28–costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108(8):1185–1194.
- Anderson DE, Bieganowska KD, Bar-Or A, et al. Paradoxical inhibition of T-cell function in response to CTLA-4 blockade; heterogeneity within the human T-cell population. Nat Med. 2000;6(2):211–214.
- Watanabe T, Bertoletti A, Tanoto T. PD‐1/PD‐L1 pathway and T‐cell exhaustion in chronic hepatitis virus infection. J Viral Hepat. 2010;17(7):453–458.
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034.
- Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151.
- Zamani MR, Aslani S, Salmaninejad A, et al. PD-1/PD-L and autoimmunity: a growing relationship. Cell Immunol. 2016;310:27–41.
- Lv F, Gao Y-F, Zhang Z-H, et al. Polymorphisms in programmed death-1 gene are not associated with chronic HBV infection in Chinese patients. World J Hepatol. 2011;3(3):72–78.
- Salmaninejad A, Valilou SF, Shabgah AG, et al. PD‐1/PD‐L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234(10):16824–16837.
- Zamani MR, Asbagh FA, Massoud AH, et al. Association between a PD-1 gene polymorphism and antisperm antibody-related infertility in Iranian men. J Assist Reprod Genet. 2015;32(1):103–106.
- Mahmoudi M, Rezaiemanesh A, Harsini S, et al. PDCD1 single nucleotide polymorphisms in Iranian patients with juvenile idiopathic arthritis. Acta Med Iran. 2017;55:676–682.
- Shadmehri AA, Nicknam MH, Shokrgozar MA, et al. Assessment of PD-1 gene variation in patients with multiple sclerosis. Tehran Univ Med J. 2010;68(2):87–93.
- Iwamoto T, Ikari K, Inoue E, et al. Failure to confirm association between PDCD1 polymorphisms and rheumatoid arthritis in a Japanese population. J Hum Genet. 2007;52(6):557–560.
- Mahmoudi M, Rezaiemanesh A, Salmaninejad A, et al. PDCD1 single nucleotide genes polymorphisms confer susceptibility to juvenile-onset systemic lupus erythematosus. Autoimmunity. 2015;48(7):488–493.
- Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173.
- Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004;3(12):709–718.
- Braun-Prado K, Petzl-Erler ML. Programmed cell death 1 gene (PDCD1) polymorphism and Pemphigus foliaceus (fogo selvagem) disease susceptibility. Genet Mol Biol. 2007;30(2):314–321.
- Lin SC, Yen JH, Tsai JJ, et al. Association of a programmed death 1 gene polymorphism with the development of rheumatoid arthritis, but not systemic lupus erythematosus. Arthritis Rheum. 2004;50(3):770–775.
- Prokunina L, Castillejo-López C, Öberg F, et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet. 2002;32(4):666–669.
- Pawlak-Adamska E, Nowak O, Karabon L, et al. PD-1 gene polymorphic variation is linked with first symptom of disease and severity of relapsing-remitting form of MS. J Neuroimmunol. 2017;305:115–127.
- Kroner A, Mehling M, Hemmer B, et al. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol. 2005;58(1):50–57.
- Ferreiros‐Vidal I, Gomez‐Reino JJ, Barros F, et al. Association of PDCD1 with susceptibility to systemic lupus erythematosus: evidence of population‐specific effects. Arthritis Rheum. 2004;50(8):2590–2597.
- Fathi F, Sadeghi E, Lotfi N, et al. Effects of the programmed cell death 1 (PDCD1) polymorphisms in susceptibility to systemic lupus erythematosus. Int J Immunogenet. 2020;47(1):57–64.
- Shadmehri AA, Nicknam MH, Shokrgozar MA, et al. Assessment of PD-1 gene variation in patients with multiple sclerosis. Tehran Univ Med J. 2010;68(2):87–93.
- Haghshenas MR, Naeimi S, Talei A, et al. Program death 1 (PD1) haplotyping in patients with breast carcinoma. Mol Biol Rep. 2011;38(6):4205–4210.
- Mojtahedi Z, Mohmedi M, Rahimifar S, et al. Programmed death-1 gene polymorphism (PD-1.5 C/T) is associated with colon cancer. Gene. 2012;508(2):229–232.
- Cooper JD, Smyth DJ, Bailey R, et al. The candidate genes TAF5L, TCF7, PDCD1, IL6 and ICAM1 cannot be excluded from having effects in type 1 diabetes. BMC Med Genet. 2007;8(1):71.
- Salmaninejad A, Khoramshahi V, Azani A, et al. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics. 2018;70(2):73–86.
- Hua Z, Li D, Xiang G, et al. PD-1 polymorphisms are associated with sporadic breast cancer in Chinese Han population of Northeast China. Breast Cancer Res Treat. 2011;129(1):195–201.
- Kong EKP, Prokunina‐Olsson L, Wong WHS, et al. A new haplotype of PDCD1 is associated with rheumatoid arthritis in Hong Kong Chinese. Arthritis Rheum. 2005;52(4):1058–1062.
- De Vooght KM, Van Wijk R, Van Solinge WW. Management of gene promoter mutations in molecular diagnostics. Clin Chem. 2009;55(4):698–708.
- Tahoori MT, Pourfathollah AA, Akhlaghi M, et al. Association of programmed cell death-1 (PDCD-1) gene polymorphisms with rheumatoid arthritis in Iranian patients. Clin Exp Rheumatol. 2011;29(5):763.
