Abstract
Smoking is a known risk factor for the development and progression of several autoimmune diseases. Previous studies have pointed out the association of smoking with the development and worsening of symptoms in myasthenia gravis (MG), but further investigation is necessary to confirm this association. Smoking history was investigated in a cross-sectional study of 139 patients with anti-acetylcholine receptor antibody-positive MG, and the association of smoking history with the age at the onset of MG was analyzed. Patients who had been smoking at the onset of MG were significantly younger compared with those who had never smoked or had quit before the onset of MG. A linear regression analysis adjusting for sex and the presence/absence of thymoma showed a significant association between smoking at onset and younger age at onset (regression coefficient −9.05; 95% confidence interval, −17.6, −0.51; p = 0.039). Among patients with smoking exposure within 10 years prior to or at the onset of MG, women were significantly younger at the onset of MG compared with men. Our results suggest that smoking is an independent risk factor for the earlier development of anti-acetylcholine receptor antibody-positive MG and further support the putative link between smoking and MG.
1. Introduction
Myasthenia gravis (MG) is an autoimmune disease of the neuromuscular junction, clinically characterized by early fatigable weakness of the ocular and extraocular muscles [Citation1,Citation2]. The development of MG results from complex interactions between genetic and environmental factors [Citation1]. Smoking is an established risk factor for the development and/or progression of several autoimmune diseases including multiple sclerosis (MS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) [Citation3–6]. In MG, smoking is more prevalent in patients compared with the general population [Citation7,Citation8] and associations between smoking and disease severity or generalization have been reported [Citation9–11], but further investigation is needed. Therefore, to gain further insights into the putative link between smoking and MG, in this study we analyzed the association of smoking with age of onset in patients with anti-acetylcholine receptor (AChR) antibody-positive MG. We planned this study under the assumption that if smoking could trigger MG development, patients with a history of smoking would develop MG at younger ages.
2. Materials and methods
2.1. Settings and participants
This cross-sectional study was conducted at Hokkaido Medical Center, which is a referral hospital for neurology covering a population of approximately 2 million people. Our clinic specializes in neuroimmunological diseases such as MG and follows patients with relatively severe cases. Patient data were collected from 139 consecutive cases of anti-AChR antibody-positive MG in patients aged 16 or older, between August 2019 and November 2019. MG was diagnosed based on medical history and clinical findings, including improvement after intravenous edrophonium, decremental response to repetitive nerve stimulation, and the presence of antibody against AChR in serum [Citation2]. The smoking prevalence of the general population in Hokkaido Prefecture was 22.6% (14.8% in women and 31.7% in men) in 2019 [Citation12]. This study was approved by the ethics review board of Hokkaido Medical Center (2019-8-3) and all participants provided written informed consent.
2.2. Data collection and variables
A questionnaire was used to investigate the smoking history of the MG patients. The questionnaire covered smoking history as well as the ages at which the patient started and quit smoking. Information on patient demographics was collected from medical records.
2.3. Statistical analyses
Descriptive summaries of patient characteristics are reported as the mean and standard deviation for continuous variables, and as the number and percentage of patients for categorical variables. In univariate analyses, the associations between age at onset of MG and variables were assessed using Wilcoxon rank sum test or Kruskal–Wallis test followed by Bonferroni’s multiple comparison test. In multivariate analyses, associations between age at onset of MG and variables were analyzed using linear regression models. Association between the presence of generalized symptoms at MG onset and variables were analyzed using a logistic regression model. Statistical analyses were performed using R software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria), and p values of less than 0.05 were considered statistically significant.
4. Results
In this study, patients were classified into never smoker, ex-smoker, and concurrent smoker groups according to the smoking status at the onset of MG (). One patient was excluded from the analysis because information about the ages of smoking initiation and cessation was not provided. Patients with no smoking history were classified as never smokers. One patient who started smoking after onset of MG was also classified as never smoker. Patients who quit smoking before onset of MG were classified as ex-smokers, while those that had been smoking at the time of onset were classified as concurrent smokers. In total, 62 participants were classified as never smokers, 42 as ex-smokers, and 34 as concurrent smokers (). The concurrent smoking rate of this cohort was 24.6% (15.7% in women and 33.8% in men). The concurrent smoker group was significantly younger at the time of the survey compared with the other groups (). The never-smoker group had a significantly higher proportion of women compared with the other groups. Proportions of patients with thymoma did not differ significantly between the smoking groups. The median interval between smoking cessation and MG onset in ex-smokers was 19 years (range 1–44 years).
Figure 1. A schematic diagram showing the classification of patients according to smoking status at the onset of MG.
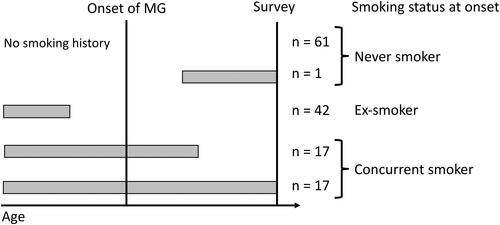
Table 1. Patient characteristics.
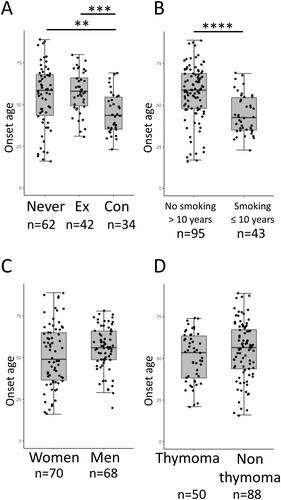
The age at onset of MG was significantly younger in the concurrent smoking group compared with the other two groups (). In RA and SLE, smoking within 5–10 years before development has been shown to affect the risk [Citation5,Citation6]. Accordingly, ex-smokers were separated into two groups depending on the interval between smoking cessation and MG development. In , ex-smokers with more than 10 years of cessation (n = 33) were combined with the never smokers, and those who ceased smoking within 10 years before MG onset (n = 9) were combined with concurrent smokers. As a result, patients with smoking exposure within 10 years prior to or at the onset of MG were significantly younger at onset compared with those without (). Sex and the presence of thymoma were not significantly associated with the age at onset ().
Figure 2. Age at onset of MG between (A) never smokers (Never), ex-smokers (Ex), and concurrent smokers (Con), (B) patients with no smoking history for more than 10 years before MG onset (No smoking > 10 years) and those with smoking exposure within or at the onset of MG (Smoking ≤ 10 years), (C) men and women, and (D) patients with and without thymoma. The number of subjects in each subgroup is shown below figures. **p < 0.01, ***p < 0.001 Kruskal–Wallis test followed by Bonferroni’s multiple comparison test; ****p < 0.0001 Wilcoxon rank sum test.
To assess whether smoking affects the age at the onset of MG preferentially in certain subgroups of patients, the onset age was analyzed in subgroups stratified by sex () or the presence of thymoma (). In women, concurrent smokers were significantly younger at onset compared with never smokers (). In men, the age at onset was significantly younger in concurrent smokers compared with ex-smokers (). As the interval between smoking cessation and onset of MG in ex-smokers significantly differed between women and men ( left panel), the age at onset was compared between those with or without smoking exposure within 10 years. As shown in middle and right panels, those with smoking exposure within 10 years prior to or at the onset of MG were significantly younger at onset compared with those without in both sexes. Among those with smoking exposure within 10 years, age at onset of MG was significantly younger in women compared with men (p < 0.01), while no sex difference was observed in those without smoking exposure within 10 years ( middle and right panels). These observations suggested that the magnitude of the smoking effect on age at onset differs between the sexes.
Figure 3. Subgroup analysis stratified by sex. (A) Age at onset of MG between never smokers (Never), ex-smokers (Ex), and concurrent smokers (Con). *p < 0.05, **p < 0.01 Kruskal–Wallis test followed by Bonferroni’s multiple comparison test. (B) The interval between smoking cessation and the onset of MG (left panel). Age at onset of MG in patients with no smoking history for more than 10 years before MG onset (No smoking > 10 years) and those with smoking exposure within the 10 years prior or at the onset of MG (Smoking ≤ 10 years) (middle and right panels). The number of subjects in each subgroup is shown below figures. *p < 0.05, ** p < 0.01, ***p < 0.001 Wilcoxon rank sum test.
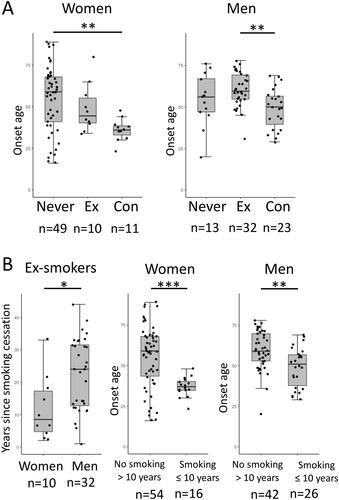
Figure 4. Subgroup analysis stratified by presence or absence of thymoma. (A) Age at onset of MG between never smokers (Never), ex-smokers (Ex), and concurrent smokers (Con). *p < 0.05, **p < 0.01 Kruskal–Wallis test followed by Bonferroni’s multiple comparison test. (B) The interval between smoking cessation and the onset of MG (left panel). Age at onset of MG in patients with no smoking history for more than 10 years before the onset of MG (No smoking > 10 years) and those with smoking exposure within the 10 years prior to or at the onset of MG (Smoking ≤ 10 years) (middle and right panels). The number of subjects in each subgroup is shown below figures. ** p < 0.01, ***p < 0.001 Wilcoxon rank sum test.
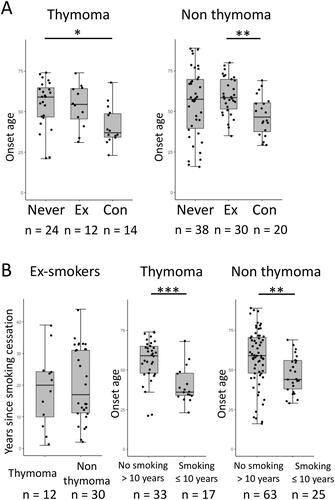
In patients with thymoma, the age at onset of concurrent smokers was significantly younger compared with never smokers (). In non-thymoma patients, the age at onset was significantly younger in concurrent smokers compared with ex-smokers (). No significant difference was observed between patients with or without thymoma with regard to the interval between smoking cessation and onset of MG ( left panel). Patients with smoking exposure within 10 years or at the onset of MG were significantly younger at onset compared with those without in both thymoma and non-thymoma subgroups ( middle and right panels). Among those with smoking exposure within 10 years, the age at onset of MG in thymoma patients showed a tendency toward younger age compared with non-thymoma patients ( middle and right panels; p > 0.05).
Next, the association of concurrent smoking and age at onset was assessed in a linear regression model adjusting for sex and presence of thymoma. As the subgroup analyses suggested effect modification by sex and thymoma, interaction terms between concurrent smoking and female sex or presence of thymoma were included in the model. As shown in , concurrent smoking was significantly associated with younger age at onset. However, no significant interactions were noted between smoking and female sex or the presence of thymoma. A similar result was obtained when those with smoking exposure within 10 years prior to or at the onset of MG were regarded as smokers (Supplementary Table).
Table 2. Linear regression model for factors associated with age at onset of MG.
Finally, the association of concurrent smoking and the presence of generalized symptoms at MG onset were assessed in a logistic regression model adjusting for sex and presence of thymoma. As shown in , concurrent smoking was not associated with the presence of generalized symptoms at MG onset.
Table 3. Linear regression model for factors associated with the presence of generalized symptoms at MG onset.
5. Discussion
In this study, we found that smoking is an independent risk factor for earlier onset in patients with anti-AChR antibody-positive MG. Smoking is a well-recognized risk factor for the development of several autoimmune diseases, including MS, RA, and SLE [Citation3–6]. In addition, recent studies have pointed out that smoking is more prevalent in MG patients compared with the general population [Citation7,Citation8], suggesting a similar association. In this study, we analyzed the association between smoking and age at onset of MG, under the assumption that if smoking could trigger MG development, patients with a history of smoking would develop MG at younger ages. Indeed, the association of smoking and younger age at onset has been observed in patients with RA and MS [Citation13–15]. Furthermore, passive smoking in childhood has been associated with earlier onset of RA [Citation16] and MS [Citation17]. Our present findings support the putative link between smoking and the risk of developing MG.
Although the mechanism through which smoking affects age at onset of MG cannot be clarified from the results of this study, we speculate that the detrimental effect of smoking on the humoral immune system and neuromuscular transmission might lead to earlier onset of MG. Smoking is not only associated with the risk of development, but has also been associated with the worsening of MS, RA, and SLE [Citation3,Citation4,Citation18]. In addition, a few studies have shown the association of smoking after MG onset and severity of symptoms or the presence of generalized manifestations in MG [Citation9–11]. Multiple pathways, including alterations of cytokine levels, modulation of cellular and humoral immunity, induction of tissue damage, and an anti-estrogenic effect, have been proposed as underlying mechanisms [Citation4]. Interestingly, smoking was shown to be associated with the positivity of autoantibodies in RA and SLE [Citation4]. Likewise, smoking might augment humoral immunity and lead to the earlier development of MG in genetically predisposed patients. Another possible underlying mechanism is the pharmacological effect of nicotine on AChR. It has been suggested that prolonged exposure to nicotine causes desensitization or even permanent inactivation of AChR [Citation19]. Interestingly, an exacerbation of MG by transdermal application of nicotine has been reported [Citation20]. Thus, it is reasonable to speculate that systemic exposure to nicotine by smoking might augment abnormal neuromuscular transmission and result in the manifestation of symptoms at younger ages in those with subclinical MG pathology. In these contexts, it is interesting to note that no association was found between smoking at MG onset and the presence of generalized symptoms at presentation in our study, suggesting that smoking facilitates the development of MG but not the extent of muscular involvement at the prodromal phase of the disease.
As in other autoimmune diseases, sex affects multiple aspects of MG. Compared with men, women with MG have been reported to develop MG at earlier ages, present generalized symptoms and thymic hyperplasia more frequently, exhibit higher serum levels of anti-AChR antibody, and respond better to thymectomy [Citation11,Citation21–23]. In the present study, we found significantly younger onset age of MG in women compared with men in subjects with smoking exposure within 10 years prior to or at the onset of MG, but not in those without. It is possible that smoking affects onset age of MG more robustly in women, although we could not confirm a significant interaction between smoking and sex in our multivariable analysis. It would be interesting to pursue the possible gender-specific effect of smoking on MG in a larger cohort of patients with adjustment for other possible confounders such as alcohol and diet, which we could not consider in the present study.
Although one study showed smoking as a risk factor for the development of thymoma [Citation24], in our study we could not find a difference in the proportion of patients with thymoma between those with and without a history of smoking. Our finding suggests that at least with regard to the onset of MG, smoking acts at a pathological phase downstream of thymoma development, and in a pathway common to patients with and without thymoma.
As an observational study, this study is not free from limitations. First, as a study performed in a referral hospital, our cohort may not be representative of MG patients in general because it contains not a small number of intractable cases, thymoma-associated MG, and patients with comorbidities. Second, because the smoking history was obtained from patients via questionnaire, the information might be subject to some recall bias. In particular, uncertainty about the ages at smoking initiation and cessation might have affected our results. Finally, because we could not obtain reliable information about the amount of tobacco consumption for each patient, dose responsiveness could not be evaluated, and therefore this point needs to be assessed in future studies.
In conclusion, smoking is an independent risk factor for the earlier development of anti-AChR antibody-positive MG, and our result further supports the putative link between smoking and the risk of MG.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Berrih-Aknin S, Panse RL. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun. 2014;52(8):90–100.
- Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570–2581.
- Belbasis L, Bellou V, Evangelou E, et al. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263–273.
- Harel-Meir M, Sherer Y, Shoenfeld Y. Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3(12):707–715.
- Barbhaiya M, Tedeschi SK, Lu B, et al. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the nurses’ health study cohorts. Ann Rheum Dis. 2018;77(2):196–202.
- Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa women’s health study. Am J Med. 2002;112(6):465–471.
- Maniaol AH, Boldingh M, Brunborg C, et al. Smoking and socio‐economic status may affect myasthenia gravis. Eur J Neurol. 2013;20(3):453–460.
- Westerberg E, Landtblom A, Punga AR. Lifestyle factors and disease‐specific differences in subgroups of Swedish myasthenia gravis. Acta Neurol Scand. 2018;138(6):557–565.
- Gratton SM, Herro AM, Feuer WJ, et al. Cigarette smoking and activities of daily living in ocular myasthenia gravis. J Neuroophthalmol. 2016;36(1):37–40.
- Apinyawasisuk S, Chongpison Y, Thitisaksakul C, et al. Actors affecting generalization of ocular myasthenia gravis in patients with positive acetylcholine receptor antibody. Am J Ophthalmol. 2020;209(1):10–17.
- Miyazaki Y, Niino M, Sakushima K, et al. Association of smoking and generalized manifestations of myasthenia gravis. Intern Med. 2022;61(11):1693–1698.
- Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. 2021. https://ganjoho.jp/reg_stat/statistics/dl/index.html#smoking
- Hutchinson D, Lynch MP, Moots RJ, et al. The influence of current cigarette smoking on the age of onset of rheumatoid arthritis (RA) in individuals with sporadic and familial RA. Rheumatology (Oxford). 2001;40(9):1068–1070.
- Papadopoulos N, Alamanos Y, Voulgari P, et al. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients? Clin Exp Rheumatol. 2005;23(6):861–866.
- Briggs FBS, Yu JC, Davis MF, et al. Multiple sclerosis risk factors contribute to onset heterogeneity. Mult Scler Relat Disord. 2019;28(2):11–16.
- Seror R, Henry J, Gusto G, et al. Passive smoking in childhood increases the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2019;58(7):1154–1162.
- Mikaeloff Y, Caridade G, Tardieu M, KIDSEP study group, et al. Parental smoking at home and the risk of childhood-onset multiple sclerosis in children. Brain. 2007;130(Pt 10):2589–2595.
- Hernán MA, Jick SS, Logroscino G, et al. Cigarette smoking and the progression of multiple sclerosis. Brain. 2005;128(Pt 6):1461–1465.
- Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol. 1997;15(2):193–222.
- Moreau T, Depierre P, Brudon F, et al. Nicotine-sensitive myasthenia gravis. Lancet. 1994;344(8921):548–549.
- Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141–149.
- Wolfe GI, Kaminski HJ, Aban IB, MGTX Study Group, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375(6):511–522.
- Berrih-Aknin S. Role of the thymus in autoimmune myasthenia gravis. Clin Exp Neuroimmunol. 2016;7(3):226–237.
- Eriksson M, Kaerlev L, Johansen P, et al. Tobacco smoking and alcohol consumption as risk factors for thymoma: A European case-control study. Cancer Epidemiol. 2019;61(8):133–138.
