Abstract
The short-term effect of tocilizumab (TCZ) on the radiographic progression of rheumatoid arthritis has been reported; however, reports on its long-term effects are scarce. In this study, we aimed to evaluate its long-term effects on joint destruction in patients who had been treated with TCZ for at least two years and for whom X-rays were available. Radiographic progression was evaluated with modified Total Sharp Score (mTSS), and structural remission was defined as the mean annual change in mTSS ≤0.5. Of the 59 patients included in this study (median age, 62 years; female, 81.4%), 34 patients (57.6%) achieved structural remission. Patients who achieved structural remission were relatively younger (59 years vs. 64 years, p = .06), had relatively higher proportion of anti-citrullinated protein antibody positivity (91.2% vs. 72.0%, p = .08), relatively lower C-reactive protein level (0.6 mg/dL vs. 2.2 mg/dL, p = .05), and significantly lower erythrocyte sedimentation rate (ESR) level (28.0 mm/h vs 65.5 mm/h, p = .003) than those who did not. Multivariate logistic regression analysis demonstrated that the baseline ESR level was significantly associated with structural remission (odds ratio, 0.98; 95% confidence interval: 0.96–0.99, p = .049). The baseline ESR level is a critical determinant of the long-term effect of TCZ on joint destruction.
1. Introduction
Rheumatoid arthritis (RA) is characterized by progressive joint destruction, which causes a significant decline in physical function and quality of life (QOL) [Citation1]. Inflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin (IL)-6, released from the inflamed synovium activate osteoclasts, synovial fibroblasts, and other cells, resulting in joint destruction. Therefore, if patients with poor prognostic factors show an inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) such as methotrexate (MTX), blocking inflammatory cytokines using biological DMARDs (bDMARDs) is highly recommended to prevent joint destruction in patients with RA [Citation2]. Targeted synthetic DMARDs (tsDMARDs), such as Janus kinase (JAK) inhibitors, can be used after risk stratification [Citation3,Citation4].
Tocilizumab (TCZ) is an IL-6 receptor antibody endorsed as one of the bDMARDs in the European League Against Rheumatism (EULAR) recommendations for the management of RA [Citation2]. Since IL-6 and JAK inhibitors show efficacy regardless of the concomitant use of MTX, their administration is recommended over other bDMARDs in patients who do not tolerate MTX [Citation2]. Thus, TCZ has been used not only as a second-line bDMARD for patients who failed TNF inhibitors, but also as the first-line bDMARD for patients who failed csDMARD therapy.
The efficacy of TCZ in controlling disease activity has been validated in multiple studies [Citation5–7], and the short-term inhibitory effect of TCZ on joint destruction, such as at 52 weeks, has been verified in several studies [Citation8–10]. However, only a few studies have evaluated this effect over a long period of time. In addition, factors associated with the long-term effects of TCZ on joint destruction remain largely unknown.
In this study, we evaluated joint destruction using the van der Heijde-modified Total Sharp Score (mTSS) in patients who had been treated with TCZ for at least two years. We also aimed to identify predictive factors for TCZ’s long-term inhibition of radiographic progression.
2. Materials and methods
2.1. Study design and patient selection
All patients who fulfilled the 1987 and/or 2010 classification criteria for RA [Citation11,Citation12] at Kyoto University Hospital were registered in a prospective study named the KURAMA cohort. As previously described [Citation13,Citation14], clinical data were recorded at baseline and at every visit in the database. This was a retrospective study using this database. Among the patients who visited Kyoto University Hospital from May 2011 to April 2021 and had been treated with TCZ either intravenously or subcutaneously for more than two years, we enrolled patients for whom X-ray evaluation was possible.
2.2. Clinical characteristics
The medical records of the patients were retrospectively reviewed, including information related to age, sex, disease duration, medication, erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP) level, swollen joint count, tender joint count, physician’s global assessment of RA activity, patient’s global assessment of RA activity, and the titers of rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (ACPAs). RF and ACPA were considered positive if the titers were >15 IU/mL and >4.5 U/mL, respectively. The clinical disease activity index (CDAI) was used to monitor disease activity because patients in this study were treated with TCZ [Citation15].
2.3. Evaluation of joint destruction by mTSS
Radiographs of each patient’s hands and feet were taken at the time of TCZ introduction and at least two years after TCZ administration; if multiple radiographs were taken after two years, the final radiograph was used for evaluation. Radiographic progression was evaluated by two rheumatologists (RW and KM) who were trained and certified by Prof. van der Heijde (Leiden University) for the mTSS scoring system [Citation16–18]. A dedicated DICOM viewer was provided by the CAC Corporation. The progression of the mTSS per year (ΔmTSS/year) was calculated from the mean progression by the two readers and the duration of TCZ administration. If ΔmTSS/year differed by 10 or more, the two rheumatologists discussed and reached a consensus. Structural remission was defined as ΔmTSS/year ≤0.5 [Citation19], and clinically relevant radiographic progression (CRRP) was defined as ΔmTSS/year >3 [Citation20].
2.4. Ethics approval and consent to participate
This study was performed in accordance with the Helsinki Declaration, and the study protocol was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine (No. R0357). Written informed consent to participate in the study was obtained from all patients.
2.5. Statistical analyses
All statistical analyses were performed using GraphPad Prism 9 software (GraphPad Software, La Jolla, CA, USA). The Mann–Whitney U test was used to analyze continuous variables, while Fisher’s exact test was used for categorical variables. Receiver operating characteristic (ROC) curve analysis was used to determine the cutoff value. A multivariate logistic regression model was used to evaluate the factors associated with long-term structural remission. p-values of less than .05 were considered statistically significant.
3. Results
3.1. Clinical characteristics of patients at the initiation of TCZ
Of the 104 patients who continued TCZ therapy for >2 years, 59 (including 48 women) were available for X-ray evaluation. At the time of TCZ introduction, the participants’ median age was 62 years (interquartile range [IQR], 55–66 years), median disease duration was 7.0 years (IQR 3.0–16.0 years), 75% (42 of 56 patients) were RF-positive, and 83.1% (49 of 59 patients) were ACPA-positive. The median MTX dose was 8.0 mg/week (IQR, 6.0–12.0 mg/week) in 39 patients (66.1%), and the median prednisolone (PSL) dose was 5.0 mg/day (IQR 5.0–7.5 mg/day) in 21 patients (35.6%). The median CDAI score was 13.3 (IQR, 7.3–17.8), and 44 patients (74.6%) had a history of b/tsDMARD use.
3.2. Structural remission rate by long-term administration of TCZ
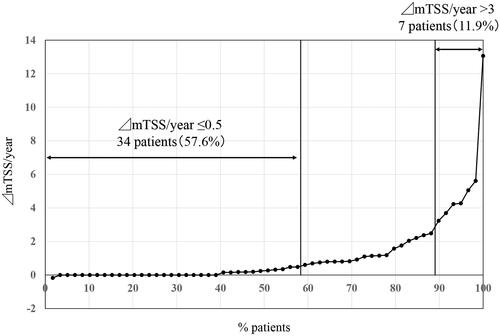
X-rays were assessed at median of 3.1 years (IQR, 3.0–3.4 years) after TCZ introduction. The interobserver reliability (as determined by intraclass correlation coefficient) was 0.66, and only 2 patients (3.3%) required re-evaluation of X-rays. The smallest detectable change in the mTSS in the present study was 1.23. The structural remission rate (ΔmTSS/year ≤0.5) was 57.6% (34 of 59 patients), whereas the proportion of CRRP (ΔmTSS/year >3) was 11.9% (7 of 59 patients) ().
Figure 1. Cumulative probability plots of radiographic progression assessed by the modified Total Sharp Score (mTSS, U/year). Thirty-four patients (57.6%) showed structural remission (ΔmTSS/year ≤0.5), whereas 7 patients showed clinically relevant radiographic progression (ΔmTSS/year >3).
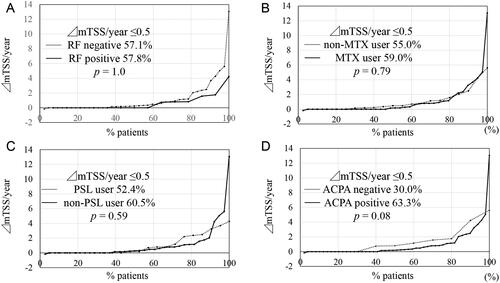
Then, cumulative probability plots were sorted by several common parameters. The structural remission rate was not affected by RF positivity (p = 1.0, ), concomitant use of MTX (p = .79, ), concomitant use of PSL (p = .59, ), or a history of b/tsDMARD use (p = .37, data not shown); however, ACPA-positive patients tended to show a higher structural remission rate than that of ACPA-negative patients (63.3% vs. 30.0%, p = .08, ).
Figure 2. Cumulative probability plots of radiographic progression assessed by the modified Total Sharp Score (mTSS, U/year) sorted by clinical parameters. (A) Sorted by rheumatoid factor (RF) positivity. (B) Sorted by methotrexate (MTX) use. (C) Sorted by prednisolone (PSL) use. (D) Sorted by anti-cyclic citrullinated peptide antibodies (ACPA) positivity. The structural remission rate (ΔmTSS/year ≤0.5) was not affected by RF positivity (p = 1.0), concomitant use of MTX (p = .79), concomitant use of PSL (p = .59), but ACPA-positive patients tended to show higher structural remission rate than ACPA-negative patients (p = .08).
3.3. Comparison between patients who achieved structural remission and those who did not
Patients who achieved long-term structural remission were compared with those who did not (). Those who achieved structural remission were relatively younger (59 years vs. 64 years, p = .06), had relatively higher proportion of ACPA positivity (91.2% vs. 72.0%, p = .08), and significantly lower ESR level (28.0 mm/h vs. 65.5 mm/h, p = .003) than those who did not; CRP level also tended to be lower (0.6 mg/dL vs. 2.2 mg/dL, p = .05). Other clinical characteristics, including a history of b/tsDMARDs, did not differ significantly with the introduction of TCZ. The proportion meeting the definition of difficult-to-treat RA [Citation21] also did not differ between the two groups. Notably, disease activity measured by the CDAI did not differ between the groups during the study period ().
Table 1. Comparison between patients who achieved structural remission and those who did not.
3.4. Multivariate logistic analysis for long-term structural remission by TCZ
Multivariate logistic regression analysis was performed to identify the factors associated with long-term structural remission (). In addition to the variables that have clinical significance (age, sex, and ACPA), we have also included the parameter (ESR) that were significantly different between the groups as explanatory variables. In model 1, age, sex, ACPA positivity, and baseline ESR level were employed. The results showed that ACPA positivity (odds ratio: 6.5, 95% CI: 1.18–36, p = .032) and the baseline ESR level (odds ratio, 0.97; 95% CI: 0.95–0.99, p = .032) were significantly associated with structural remission. When age, sex, ACPA titers, and baseline ESR level were employed in model 2, only the baseline ESR levels were significant (odds ratio, 0.98; 95% CI: 0.96–0.99, p = .049).
Table 2. Multivariate logistic analysis for long-term structural remission by tocilizumab.
3.5. ROC analysis to determine the cut-off value of ESR
Finally, we examined the cutoff value of the baseline ESR level using ROC analysis. As a result, the cutoff value of the baseline ESR level was determined to be 73 mm/h (area under the curve: 0.71, 95% CI: 0.56–0.86, p = .008, ). Accordingly, the structural remission rate was significantly higher in patients with a baseline ESR <73 mm/h than that in those with a baseline ESR ≥73 mm/h (p = .006, ). Annual progression of erosion and joint space narrowing were both reduced in the lower ESR group than in the higher ESR group (p = .0036, p = .0021, respectively, ).
Figure 3. Baseline erythrocyte sedimentation rate (ESR) predicts long-term structural remission by tocilizumab. (A) Receiver operating characteristics (ROC) analysis was performed to determine the cutoff value of baseline ESR. The cutoff value of baseline ESR was determined to be 73 mm/h (area under the curve [AUC]: 0.71, 95%CI: 0.56–0.86, p = .008). (B) The structural remission rate was significantly higher in patients with baseline ESR level <73 mm/h than that in those with a baseline ESR level of ≥73 mm/h (p = .006). Annual progression of erosion (C) and joint space narrowing (D) were both reduced in the lower ESR group than in the higher ESR group (p = .0036, p = .0021, respectively). The data are presented as mean + SD.
![Figure 3. Baseline erythrocyte sedimentation rate (ESR) predicts long-term structural remission by tocilizumab. (A) Receiver operating characteristics (ROC) analysis was performed to determine the cutoff value of baseline ESR. The cutoff value of baseline ESR was determined to be 73 mm/h (area under the curve [AUC]: 0.71, 95%CI: 0.56–0.86, p = .008). (B) The structural remission rate was significantly higher in patients with baseline ESR level <73 mm/h than that in those with a baseline ESR level of ≥73 mm/h (p = .006). Annual progression of erosion (C) and joint space narrowing (D) were both reduced in the lower ESR group than in the higher ESR group (p = .0036, p = .0021, respectively). The data are presented as mean + SD.](/cms/asset/b5bcb36e-729e-44bc-abaf-b12175cdc334/timm_a_2170384_f0003_b.jpg)
4. Discussion
In the present study, the long-term structural remission rate for patients who had been treated with TCZ for at least two years, with a median of 3.1 years, was 57.6%. Long-term structural remission was more likely to be achieved in ACPA-positive younger patients with a low ESR level at the time of TCZ introduction. This study showed for the first time that long-term structural remission induced by TCZ could be predicted using the baseline ESR level. Therefore, the results of this study may help identify patients who would most benefit from TCZ in daily clinical practice.
Few studies have reported the long-term inhibitory effects of TCZ on radiographic progression. Kaneko et al. conducted a randomized controlled trial to determine whether an add-on or switch to TCZ is effective in patients with inadequate response to MTX (SURPRISE study) [Citation9]. In this study, the 52-week structural remission rate was 66% in the add-on group and 64% in the switch group from MTX to TCZ, with no significant difference (p = .92), whereas CRRP was 7% in the add-on group and 15% in the switch group, with borderline significance (p = .07) [Citation9]. The two-year structural remission rate of this extension study was 83.7% in the add-on group and 72.9% in the switch group (p = .23) [Citation22]. Using real-world data, Hirabayashi et al. showed that the structural remission rate was 66% (33 of 50 patients) in patients receiving TCZ for three years [Citation23]. The sample size, study duration, and structural remission rate of this study were similar to those of our study. They also demonstrated that three-year structural remission was associated with the number of swollen joint counts being less than or equal to 1 within six months [Citation23]. Thus, their study predicted long-term structural remission from the clinical response after initiation of TCZ, while our study predicted it from laboratory data at the initiation of TCZ. As suggested by Kaneko et al. the inhibitory effect of TCZ on joint destruction is maximized when TCZ is added on to MTX therapy [Citation9]. The recently published consensus statement on IL-6 inhibitors also recommends that TCZ be administered in combination with MTX, at least in the early treatment phase [Citation24]. However, in our study, the structural remission rate in patients who had concomitant use of MTX was not different from that of patients who did not use MTX (p = .79), possibly due to the small sample size ().
Since IL-6 is the major regulator of the acute phase response and increases ESR and CRP levels [Citation25,Citation26], it is assumed that patients with low baseline ESR levels, who were likely to achieve long-term structural remission with TCZ in our study, had low serum IL-6 levels. Although it has been reported that IL-6 inhibitors tend to have a better response than TNF inhibitors when patients have high IL-6 or CRP levels, when patients have moderate or low serum IL-6 or CRP levels, IL-6 inhibitors show a similar efficacy and/or drug retention rate to that of TNF inhibitors [Citation27,Citation28]. With regard to joint destruction, it has been reported that IL-6 inhibitors are more effective in suppressing joint destruction in patients with moderate or low serum IL-6 levels than in those with high serum IL-6 levels [Citation27], which is compatible with our study results.
If IL-6 increases both ESR and CRP, why was ESR, but not CRP, a predictive factor for long-term structural remission? Since ESR levels are known to be affected not only by IL-6 but also by immunoglobulin levels and other factors, we compared total IgG levels between groups achieving and not achieving structural remission, which resulted in no difference (p = .78, ). However, it is possible that the ESR levels were affected by something other than IgG, which may have made the ESR a predictive factor for structural remission.
In addition to serum IL-6, ESR, and CRP levels, biomarkers that have been reported to predict the efficacy of TCZ include serum IL-1 levels [Citation29], baseline hemoglobin levels [Citation30], RF positivity [Citation31], serum TCZ concentrations [Citation32], and others [Citation33]. Recently, we reported that neutrophil count reduction after TCZ initiation could be a marker of its efficacy [Citation34]. As IL-6 affects the expression of adhesion molecules on neutrophils, such as CD162 [Citation35], IL-6 inhibitor treatment results in neutropenia [Citation36]. Our previous study demonstrated that patients who showed a reduction in neutrophil counts within one month of TCZ therapy were likely to achieve clinical remission within one year without increasing serious infections [Citation34]. Based on these results, we hypothesized that the reduction in neutrophil count may reflect serum TCZ concentrations. Whether the reduction in neutrophil counts within one month of TCZ therapy is correlated with long-term structural remission requires further study.
ACPA positivity is a poor prognostic factor in the treatment of RA [Citation2] and is strongly correlated with joint destruction [Citation37]. However, in the present study, patients who achieved long-term structural remission had a relatively higher proportion of ACPA positivity than those who did not (p = .08). In addition, ACPA-positive patients tended to show a higher structural remission rate than ACPA-negative patients (p = .08; ). These results apparently seem inconsistent. However, it has been reported that ACPA-positive patients are prone to joint destruction as well as joint repair [Citation38], indicating that they may have higher bone turnover or remodeling potential than ACPA-negative patients. Although the small sample size precludes definitive conclusions, our data demonstrate that TCZ can achieve long-term structural remission, even in ACPA-positive patients.
This study had several limitations. First, this was a single-center retrospective study with a small sample size. In addition, this study did not include patients who discontinued TCZ or for whom X-rays were not available, which biased the results. Because treatment after discontinuation of TCZ varied widely from patient to patient, this study was limited to only those who continued TCZ. Second, annual radiographic evaluations using mTSS were not performed. Radiographs taken at TCZ initiation and after at least two years were evaluated. Third, this study did not measure the serum IL-6 levels, TCZ concentrations, or other markers.
Despite these limitations, we evaluated long-term joint destruction in patients who had been treated with TCZ and found that baseline ESR levels could be a predictor of long-term structural remission. The results of this study may be informative for shared decision making with patients.
Author contribution
RW, KMurakami, HI, KMurata, TFujii, HO, AO, MT, AM, MH provided patients’ care. TFujisaki supported evaluation of X-rays. WY supported data collection. RW drafted the initial manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the patients and medical staff for their contribution to this study. We also thank Editage for language review (https://www.editage.jp/).
Disclosure statement
Department of Advanced Medicine for Rheumatic Diseases is supported by Nagahama City, Shiga, Japan, Toyooka City, Hyogo, Japan and five pharmaceutical companies (Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co. Ltd, UCB Japan Co. Ltd, AYUMI Pharmaceutical Co., and Asahi Kasei Pharma Corp.). It is also supported by grant from Daiichi Sankyo Co. Ltd. Above-mentioned pharmaceutical companies were not involved in the study design, data collection and analysis, manuscript writing, and manuscript submission. RW has received research grant and/or speaker’s fee from AbbVie, Asahi Kasei, Eli Lilly, and Sanofi. KMurakami has received speaking fees, and/or consulting fees from Eisai Co. Ltd, Chugai Pharmaceutical Co. Ltd., Pfizer Inc., Bristol-Myers Squibb, Mitsubishi Tanabe Pharma Corporation, UCB Japan Co. Ltd, Daiichi Sankyo Co. Ltd. and Astellas Pharma Inc. HI has received a research grant and/or speaker fee from Bristol-Myers Squibb. KMurata received a speaking fee and/or consulting fees from AbbVie GK; Eisai Co., Ltd., Chugai Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Corporation; Pfizer Inc.; Bristol-Myers Squibb; Asahi Kasei Pharma Corp. TFujii received speaker fees from Abvie, Asahi Kasei, Jansen, Tanabe Mitsubishi, and Eisai. HO has received research grants and/or speaker fees from AbbVie, Asahi Kasei, Astellas Pharma Inc., Eisai Co. Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, and Daiichi Sankyo Co. Ltd. AO has received research grants and/or speaker fees from Pfizer Inc., Bristol-Myers Squibb., Advantest, Asahi Kasei Pharma Corp., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K, Ono Pharmaceutical Co., UCB Japan Co., Mitsubishi Tanabe Pharma Co., Eisai Co. Ltd., Abbvie Inc., Takeda Pharmaceutical Co. Ltd., and Daiichi Sankyo Co. Ltd. MT has received research grants and/or speaker fees from AbbVie GK, Asahi Kasei Pharma Corporation, Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly and Company, Pfizer Inc., UCB Japan Co., Ltd., Janssen Pharmaceutical K.K., Kyowa Kirin Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Taisho Pharma Co., Ltd, and Teijin Pharma, Ltd. AM has received honorarium from AbbVie G.K., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Eisai Co. Ltd., Pfizer Inc., Bristol-Myers Squibb., Mitsubishi Tanabe Pharma Co., Astellas Pharma Inc., and Gilead Sciences Japan., and has received research grants from AbbVie G.K., Asahi Kasei Pharma Corp., Chugai Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Co. and Eisai Co. Ltd. outside the work. MH received research grants and/or speaker fee from Abbvie, Asahi Kasei, Astellas, Ayumi, Brystol Meyers, Chugai, EA Pharma, Eisai, Daiichi Sankyo, Eli Lilly, Nihon Shinyaku, Novartis Pharma, Tanabe Mitsubishi.
Additional information
Funding
References
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001.
- Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699.
- Kragstrup TW, Glintborg B, Svensson AL, et al. Waiting for JAK inhibitor safety data. RMD Open. 2022;8(1):e002236.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326.
- Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–1550.
- Humby F, Durez P, Buch MH, et al. Rituximab versus tocilizumab in anti-TNF inadequate responder patients with rheumatoid arthritis (R4RA): 16-week outcomes of a stratified, biopsy-driven, multicentre, open-label, phase 4 randomised controlled trial. Lancet. 2021;397(10271):305–317.
- Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–997.
- Hirabayashi Y. Tocilizumab can efficiently prevent bone destruction in patients with recent-onset rheumatoid arthritis. Mod Rheumatol. 2021;31(5):966–971.
- Kaneko Y, Atsumi T, Tanaka Y, et al. Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study). Ann Rheum Dis. 2016;75(11):1917–1923.
- Nakashima Y, Kondo M, Shono E, et al. Suppression of joint destruction with subcutaneous tocilizumab for Japanese patients with rheumatoid arthritis in clinical practice. Mod Rheumatol. 2020;30(5):807–815.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–1588.
- Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324.
- Katsushima M, Minamino H, Shirakashi M, et al. High plasma homocysteine level is associated with increased prevalence of non-remission state in rheumatoid arthritis: findings from the KURAMA cohort. Mod Rheumatol. 2022;2022:roac106.
- Watanabe R, Hashimoto M, Murata K, et al. Prevalence and predictive factors of difficult-to-treat rheumatoid arthritis: the KURAMA cohort. Immunol Med. 2022;45(1):35–44.
- Kawashiri SY, Kawakami A, Iwamoto N, et al. Disease activity score 28 may overestimate the remission induction of rheumatoid arthritis patients treated with tocilizumab: comparison with the remission by the clinical disease activity index. Mod Rheumatol. 2011;21(4):365–369.
- Koga T, Okada A, Fukuda T, et al. Prognostic factors toward clinically relevant radiographic progression in patients with rheumatoid arthritis in clinical practice: a Japanese multicenter, prospective longitudinal cohort study for achieving a treat-to-target strategy. Medicine. 2016;95(17):e3476.
- Murakami K, Sekiguchi M, Hirata S, et al. Predictive factors for structural remission using abatacept: results from the ABROAD study. Mod Rheumatol. 2019;29(3):406–412.
- van der Heijde DM, van Riel PL, Nuver-Zwart IH, et al. Effects of hydroxychloroquine and sulphasalazine on progression of joint damage in rheumatoid arthritis. Lancet. 1989;1(8646):1036–1038.
- Takeuchi T, Tanaka Y, Amano K, et al. Clinical, radiographic and functional effectiveness of tocilizumab for rheumatoid arthritis patients–REACTION 52-week study. Rheumatology. 2011;50(10):1908–1915.
- Bruynesteyn K, Van Der Heijde D, Boers M, et al. Detecting radiological changes in rheumatoid arthritis that are considered important by clinical experts: influence of reading with or without known sequence. J Rheumatol. 2002;29(11):2306–2312.
- Nagy G, Roodenrijs NMT, Welsing PM, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80(1):31–35.
- Kaneko Y, Kato M, Tanaka Y, et al. Tocilizumab discontinuation after attaining remission in patients with rheumatoid arthritis who were treated with tocilizumab alone or in combination with methotrexate: results from a prospective randomised controlled study (the second year of the SURPRISE study). Ann Rheum Dis. 2018;77(9):1268–1275.
- Hirabayashi Y, Munakata Y, Miyata Met al. Clinical and structural remission rates increased annually and radiographic progression was continuously inhibited during a 3-year administration of tocilizumab in patients with rheumatoid arthritis: a multi-center, prospective cohort study by the. Mod Rheumatol. 2016;26(6):828–835.
- Aletaha D, Kerschbaumer A, Kastrati K, et al. Consensus statement on blocking interleukin-6 receptor and interleukin-6 in inflammatory conditions: an update. Ann Rheum Dis. 2022;2022:annrheumdis-2022-222784.
- Castell JV, Gomez-Lechon MJ, David M, et al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242(2):237–239.
- Kishimoto T, Kang S. IL-6 revisited: from rheumatoid arthritis to CAR T cell therapy and COVID-19. Annu Rev Immunol. 2022;40:323–348.
- Boyapati A, Schwartzman S, Msihid J, et al. Association of high serum interleukin-6 levels with severe progression of rheumatoid arthritis and increased treatment response differentiating Sarilumab from adalimumab or methotrexate in a post hoc analysis. Arthritis Rheumatol. 2020;72(9):1456–1466.
- Nakayama Y, Hashimoto M, Watanabe R, et al. Favorable clinical response and drug retention of anti-IL-6 receptor inhibitor in rheumatoid arthritis with high CRP levels: the ANSWER cohort study. Scand J Rheumatol. 2021;2021:1–10.
- Okano T, Inui K, Tada M, et al. Levels of interleukin-1 beta can predict response to tocilizumab therapy in rheumatoid arthritis: the PETITE (predictors of effectiveness of tocilizumab therapy) study. Rheumatol Int. 2016;36(3):349–357.
- Narvaez J, Magallares B, Diaz Torne C, et al. Predictive factors for induction of remission in patients with active rheumatoid arthritis treated with tocilizumab in clinical practice. Semin Arthritis Rheum. 2016;45(4):386–390.
- Maneiro RJ, Salgado E, Carmona L, et al. Rheumatoid factor as predictor of response to abatacept, rituximab and tocilizumab in rheumatoid arthritis: systematic review and meta-analysis. Semin Arthritis Rheum. 2013;43(1):9–17.
- Arad U, Elkayam O. Association of serum tocilizumab trough concentrations with clinical disease activity index scores in adult patients with rheumatoid arthritis. J Rheumatol. 2019;46(12):1577–1581.
- Nouri B, Nair N, Barton A. Predicting treatment response to IL6R blockers in rheumatoid arthritis. Rheumatology (Oxford). 2020;59(12):3603–3610.
- Nakajima T, Watanabe R, Hashimoto M, et al. Neutrophil count reduction 1 month after initiating tocilizumab can predict clinical remission within 1 year in rheumatoid arthritis patients. Rheumatol Int. 2021;42(11):1983–1991.
- Hashizume M, Higuchi Y, Uchiyama Y, et al. IL-6 plays an essential role in neutrophilia under inflammation. Cytokine. 2011;54(1):92–99.
- Ogata A, Kato Y, Higa S, et al. IL-6 inhibitor for the treatment of rheumatoid arthritis: a comprehensive review. Mod Rheumatol. 2019;29(2):258–267.
- Koga T, Okada A, Fukuda T, et al. Anti-citrullinated peptide antibodies are the strongest predictor of clinically relevant radiographic progression in rheumatoid arthritis patients achieving remission or low disease activity: a post hoc analysis of a nationwide cohort in Japan. PLoS One. 2017;12(5):e0175281.
- van der Linden MP, Boja R, Klarenbeek NB, et al. Repair of joint erosions in rheumatoid arthritis: prevalence and patient characteristics in a large inception cohort. Ann Rheum Dis. 2010;69(4):727–729.
