Abstract
Among various tyrosine kinases, a family of Janus kinases (JAK) has been elucidated as key players in signal transduction from vital cytokine receptors, such as interleukins and interferons. Indeed, recent rapid progress in JAK inhibitors in addition to biological agents provided therapeutic options for various diseases, including immune-mediated inflammatory diseases and hematological disorders. Efforts have culminated in the approval of nine JAK inhibitors in Japan in the recent decade. The safety profiles of JAK inhibitors seem to largely depend on patient populations and drug dosing, rather than on JAK selectivity. Thus, the comparison of various disease indications is pivotal for the understanding and proper use of JAK inhibitors.
Keywords:
Glucocorticoids (GCs) are the mainstay of treatment for a variety of immune-mediated inflammatory diseases (IMIDs), primarily on the basis of their rapid and convincing clinical response in most patients with IMIDs. However, the effects of GCs are inevitably accompanied by frequent and various adverse events, including osteoporosis, hyperglycemia, hyperlipidemia, cardiovascular diseases, cataracts, glaucoma, insomnia, and skin atrophy, in addition to infections [Citation1]. Therefore, recent recommendations/guidelines for many IMIDs suggest the initial combination therapy of GCs and other therapeutic agents, such as immunosuppressants and/or biological agents [Citation2–4]. Furthermore, a trade-off between GCs and immunosuppressants and/or biological agents may improve the long-term outcomes of IMIDs [Citation5].
As a molecular targeted therapy, the tyrosine kinase inhibitor imatinib, which inhibits platelet-derived growth factor receptor, c-Kit, and c-Abl/Bcr-Abl, has been introduced as an epoch-making treatment for chronic myeloid leukemia and gastrointestinal stromal tumors [Citation6]. Among various tyrosine kinases, a family of Janus kinases (JAK) has been elucidated as key players in signal transduction from vital cytokine receptors, such as interleukin (IL)-2, IL-6, IL-12, interferon α/β/γ, granulocyte-monocyte colony stimulating factor, erythropoietin, and thrombopoietin [Citation7,Citation8]. Therefore, the recent rapid progress in JAK inhibitors in addition to biological agents has provided therapeutic options for various diseases, including IMIDs and hematological disorders.
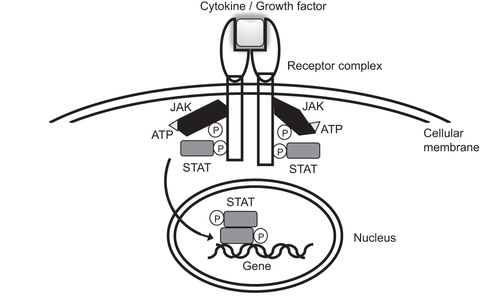
In 1989, Wilks et al. reported two putative protein-tyrosine kinases identified by polymerase chain reaction [Citation9]. Although JAK 1 and 2 have been referred to as ‘just another kinase’ by laboratory members [Citation10], currently, JAK represents ‘Janus kinase’, and the JAK family includes JAK1, JAK2, JAK3, and Tyk2 [Citation7,Citation8,Citation10,Citation11]. JAK3 was first reported by three independent laboratories in 1991 [Citation12–14], while Tyk2 was identified, and its cDNA was cloned, in 1990 [Citation15,Citation16]. All members of the JAK family transduce cytokine signaling through specific receptors via downstream signal transducer and transcription (STAT) molecules [Citation7,Citation8,Citation17] ( and ). Because a pair of JAK proteins is required for signal transduction, selective inhibition of each JAK molecule does not necessarily result in considerably different cellular responses [Citation8,Citation17,Citation18]. However, the knockout of JAK1, as well as that of JAK2, leads to perinatal or embryonic lethal outcomes [Citation19–21], while JAK3 knockout showed severe combined immunodeficiency, and Tyk2 knockout only showed modest viral susceptibility [Citation22–25].
Table 1. Cytokine/growth factor signaling profiles through the JAK-STAT pathway.
The binding of cytokines/growth factors to a specific receptor elicits adenosine triphosphate (ATP)-dependent phosphorylation and activation of JAKs, which in turn phosphorylate and activate STATs. Activated STATs translocate to the nucleus and modulate the transcriptional activity of the target gene.
Thus, it is reasonable that the initial attempt targeted JAK3 inhibition to develop a novel immunosuppressive agent. Tofacitinib (CP-690,550, formerly tasocitinib), currently regarded as a pan-JAK inhibitor, was reported to be effective in the prevention of organ allograft rejection [Citation26]. Subsequently, tofacitinib was developed for IMIDs and approved first for the treatment of rheumatoid arthritis (RA) by the United States Food and Drug Administration (FDA) in 2012, by the Pharmaceuticals and Medical Devices Agency (PMDA) in 2013, and by the European Medicines Agency (EMA) in 2017. The present approval status of JAK inhibitors by the PMDA a regulatory agency in Japan, as of February 2023, is shown in .
Table 2. Characteristics of JAK inhibitors approved by PMDA and their primary doses for each indicated disease.
It should be noted that deucravacitinib is a novel class of potent allosteric inhibitors that act by binding to and stabilizing the pseudokinase Janus Homology 2 (JH2) domain of Tyk 2, while other JAK inhibitors are active site-directed inhibitors that bind to the adenosine triphosphate (ATP) site of the catalytic JH1 domain of the JAK protein. This prevents catalytic activity of the kinase by blocking ATP, downstream phosphorylation, and resulting pathway signal transduction () [Citation27]. Comparative studies of safety profiles across the various indications of JAK inhibitors are crucial because of the differences in patient backgrounds, including age and comorbidities, and the dosing regimen among the indicated diseases [Citation28]. Moreover, the dose of JAK inhibitors seems to be critical for the potency of JAK-STAT inhibition, in addition to the selectivity of each JAK inhibitor [Citation29].
A topic of recent interest related to JAK inhibitors is the safety concern raised by clinical trial (ORAL Surveillance) data, which suggested that tofacitinib increases the risk of major cardiovascular problems, cancer, venous thromboembolism, serious infections, and death due to any cause when compared with medicines belonging to the class of tumor necrosis factor (TNF) inhibitors [Citation30–32]. However, these risks largely depend on patient profiles, including age, obesity, smoking status, and comorbidities, which clearly differ among the diseases for which JAK inhibitors have been approved. In addition, it should be noted that the increased risks in patients receiving JAK inhibitors compared with those receiving TNF inhibitors do not necessarily indicate the harm caused by JAK inhibitors, and instead, some of the data may simply represent the beneficial effects of TNF inhibitors.
Accordingly, a special themed issue of the safety, efficacy, and effectiveness of JAK inhibitors across various clinical immunology fields has been planned and issued in Immunological Medicine. The following review from the fields of dermatology, gastroenterology, hematology, and rheumatology should be useful and informative.
Disclosure statement
The author is a member of the advisory board for Asahi-Kasei, Astellas, Aurinia, Eisai, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Sanofi, and UCB; has received speaker fees/honoraria from AbbVie, Asahi-Kasei, Astellas, Boehringer Ingelheim, Bristol-Myers, Chugai, Eisai, Eli Lilly, Gilead, Janssen, Mitsubishi Tanabe, Novartis, Pfizer, and UCB; and has received a research grant from AbbVie, Asahi-Kasei, Boehringer Ingelheim, Chugai, Eisai, Mitsubishi Tanabe, and Taisho.
Additional information
Funding
References
- Stone JH, McDowell PJ, Jayne DRW, et al. The glucocorticoid toxicity index: measuring change in glucocorticoid toxicity over time. Semin Arthritis Rheum. 2022;55:152010.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–745.
- Chung SA, Langford CA, Maz M, et al. American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Care Res (Hoboken). 2021;73(8):1088–1105.
- Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3–18.
- Imaizumi C, Ogura T, Inoue Y, et al. Reduced rate of disease flares in Japanese patients with systemic lupus erythematosus: an altered balance between the use of glucocorticoids and immunosuppressants in recent decades. Intern Med. 2022;61:3189–3196.
- Kameda H, Suzuki M, Takeuchi T. Platelet-derived growth factor as a therapeutic target for systemic autoimmune diseases. Drug Target Insight. 2007;2:239–247.
- Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27:1984–2009.
- Morinobu A. JAK inhibitors for the treatment of rheumatoid arthritis. Immunol Med. 2020;43:148–155.
- Wilks AF. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989;86(5):1603–1607.
- Wilks, AF. The JAK kinases: not just another kinase drug discovery target. Semin Cell Dev Biol. 2008;19:319–328.
- Wilks AF, Harpur AG, Kurban RR, et al. Two novel protein-tyrosine kinases, each with a second phsphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991;11(4):2057–2065.
- Cance WG, Craven RJ, Weiner TM, et al. Novel protein kinases expressed in human breast cancer. Int J Cancer. 1993;54(4):571–577.
- Takahashi T, Shirasawa T. Molecular cloning of rat JAK3, a novel member of the JAK family of protein tyrosine kinases. FEBS Lett. 1994;342(2):124–128.
- Kawamura M, McVicar DW, Johnston JA, et al. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci U S A. 1994;91(14):6374–6378.
- Krolewski JJ, Lee R, Eddy R, et al. Identification and chromosomal mapping of new human tyrosine kinase genes. Oncogene. 1990;5:277–282.
- Firmbach-Kraft I, Byers M, Shows T, et al. tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene. 1990;5:1329–1336.
- Schindler CW. JAK-STAT signaling in human disease. J Clin Invest. 2002;109:1133–1137.
- Tanaka Y, Luo Y, O’Shea JJ, et al. Janus kinase-targeting therapies in rheumatology: a mechanism-based approach. Nat Rev Rheumatol. 2022;18:133–145.
- Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383.
- Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93(3):385–395.
- Neubauer H, Cumano A, Müller M, et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93(3):397–409.
- Macchi P, Villa A, Giliani S, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature. 1995;377(6544):65–68.
- Park SY, Saijo K, Takahashi T, et al. Development defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–782.
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–560.
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFNα signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561–571.
- Changelian PS, Flanagan ME, Ball DJ, et al. Prevention of argan allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973–8995.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71–81.
- Traves PG, Murray B, Campigotto F, et al. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signaling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. 2021;80:865–875.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–326.
- U.S. Food & Drug Administration. Drug Safety Communication. Tofacitinib, baribitinib, and upadacitinib. 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death.
- European Medicines Agency. Issued on 11 November 2022 (EMA/860610/2022). 2022. https://www.ema.europa.eu/en/documents/referral/janus-kinase-inhibitors-jaki-article-20-referral-ema-confirms-measures-minimise-risk-serious-side_en-0.pdf.