Abstract
YKL-40 is implicated in inflammation and tissue repair, but no reports have investigated its involvement in myositis in polymyositis (PM) and dermatomyositis (DM). Therefore, we aimed to investigate the relationship between YKL-40 and PM/DM. We retrospectively enrolled 35 patients diagnosed with PM/DM along with 26 healthy controls (HCs). Both PM and DM were diagnosed according to Bohan and Peter’s criteria. Serum YKL-40 levels were measured, age-corrected to YKL-40 percentile values, and compared to HCs. Patients with myositis without interstitial lung disease were also enrolled and compared to HCs. Immunofluorescence staining was performed to identify YKL-40-positive inflammatory cells in muscle biopsy samples from two patients each with PM and DM. Age-corrected serum YKL-40 levels were significantly higher in patients with PM/DM compared to HCs with and without lung disease; however, these levels decreased significantly after treatment. Immunohistochemical analysis showed infiltration of YKL-40-positive inflammatory cells into the intramuscular sheath and perimuscular membrane. Immunofluorescence staining showed CD68 expression in YKL-40-positive inflammatory cells, suggesting that these cells were macrophages. To the best of our knowledge, this is the first study to demonstrate that YKL-40-positive macrophages are present in PM and DM, indicating that YKL-40 may be involved in PM/DM.
1. Introduction
YKL-40 is a chitinase-like protein; it has a structure similar to that of chitinase and can bind to chitin but is unable to hydrolyse it. The name YKL-40 is derived from the fact that the N-terminal amino acids begin with Y, K and L with a molecular weight of 40 kDa [Citation1,Citation2]. The involvement of YKL-40 in airway inflammation as in conditions such as asthma has been previously reported [Citation2–4]. It is also believed that YKL-40 is involved in inflammation and tissue repair [Citation5]; however, the detailed mechanisms of its involvement have not yet been made clear. Recently, YKL-40 levels have been reported to increase in patients with glioblastoma [Citation6–8], rheumatoid arthritis [Citation9,Citation10], inflammatory bowel disease [Citation11], psoriatic arthritis [Citation12,Citation13], hepatitis [Citation14], idiopathic pulmonary arterial hypertension [Citation15,Citation16], Alzheimer’s disease [Citation17] and malignant disease [Citation18]. It has been reported that YKL-40 is secreted by various cells such as neutrophils [Citation19], macrophages, chondrocytes, synoviocytes [Citation9,Citation10] and tumour cells [Citation18] during inflammation, angiogenesis and tissue fibrosis, and may be involved in the assessment of disease activity and the mechanisms of angiogenesis and tissue remodelling.
Polymyositis (PM) and dermatomyositis (DM) are inflammatory muscle diseases of unknown aetiology characterised primarily by muscle weakness [Citation20,Citation21]. PM is characterised by muscle weakness primarily in the proximal limb, neck and pharyngeal muscles, and elevated levels of myogenic enzymes such as creatine kinase (CK) [Citation21]. In addition, the main histological features of PM include myofibre size irregularities, scattered necrotic and regenerating fibres, and cellular infiltration into the perivascular and fascial areas [Citation22]. The infiltrating cell population is composed of macrophages and activated CD8+ cytotoxic T cells; the invasion of these cells into non-necrotic myofibres has been reported [Citation23]. Like PM, DM is characterised by muscle weakness and is additionally often accompanied by skin rashes such as heliotropes and Gottron’s papules [Citation24]. The main histological features of DM include deposition of the membrane invasion complex around small blood vessels, muscle atrophy around muscle bundles, and capillary loss [Citation25,Citation26]. DM inflammatory cells are found perimuscularly and perivascularly with macrophages, B cells, CD4+ T cells and plasmacytoid dendritic cells [Citation27], which release cytokines and cytotoxic molecules and are thought to cause muscle destruction [Citation28,Citation29]. Thus, although PM and DM are muscle diseases with similar symptoms, different cells are thought to be involved in their pathogenesis. However, there are many unknown factors regarding myositis, such as the fact that some patients have myositis but lack muscle symptoms, and various cells are likely involved in the pathogenesis of the disease.
Previous studies on YKL-40 in PM/DM have examined interstitial lung disease (ILD) as a complication of these conditions [Citation30–33]; however, no reports have examined whether YKL-40 is involved in myositis itself in PM/DM. Therefore, this study aimed to investigate the relationship between PM/DM and serum YKL-40 and YKL-40 expression using immunohistochemistry (IHC) and immunofluorescence staining of serum and muscle biopsy specimens from patients with ILD-naive PM and DM.
2. Materials and methods
2.1. Patients
In this retrospective study, we enrolled 35 patients diagnosed with PM/DM and 26 healthy controls (HCs) at Hyogo Medical University Hospital (Nishinomiya, Japan) between 2006 and 2020 to investigate the relationship of PM/DM with YKL-40. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of our institution. Written informed consent was obtained from all patients in accordance with our institutional guidelines (Hyogo Medical University, Nishinomiya, Japan, no. 2434). Patients with definite or probable PM/DM and HCs were included. Both PM and DM were diagnosed according to Bohan and Peter’s criteria [Citation20]. At the time of PM/DM diagnosis, almost all patients underwent systemic examination for malignancy. ILD was diagnosed based on clinical and chest computed tomography findings. Clinical data at the time of diagnosis, including patient characteristics and laboratory data, were obtained from patients’ medical records.
2.2. Data collection
Clinical data were obtained retrospectively from patients’ medical records, including clinical symptoms at diagnosis, presence of ILD, and laboratory findings. Data on age, sex, type of disease, duration of disease, and use of therapeutic agents were also extracted and used for analysis.
2.3. Measurement of serum YKL-40 levels
Baseline serum samples collected at the time of diagnosis were available from the 35 patients with PM/DM. Follow-up serum samples were obtained on the day of discharge after the initiation of PM/DM treatment. Serum samples were also collected from 26 HCs. The samples were stored at −20 °C until further analysis. Serum YKL-40 levels were measured using a YKL-40 enzyme-linked immunosorbent assay (Quantikine ELISA kit, R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions [Citation12,Citation13,Citation16]. Since serum YKL-40 levels have been reported to increase with age, the YKL-40 age percentile was calculated as follows: YKL-40 age percentile = 100/(1 + (serum YKL-40 levels−3) × (1.062age) × 5000) [Citation12,Citation16,Citation34].
2.4. Haematoxylin–eosin staining
To diagnose PM/DM, several centimetres of the patients’ biceps brachii were surgically removed. A 1-cm block was prepared frozen. The frozen blocks were stored at −80 °C until the time of testing. Samples cut 12-µm thick from the block were placed on glass slides. Frozen sections of surgically resected muscle biopsy specimens, prepared as described above (12-µm thick, four samples), were used for haematoxylin–eosin (H&E) staining. The nuclei were stained with haematoxylin solution (Wako, Osaka, Japan) for approximately 10 min. Samples were then rinsed under running water for another 10 min. The cytoplasm was then stained with an eosin solution for 10 min. Samples were dehydrated in a 70–100% ethanol gradient, air-dried, covered with a glass cover, and sealed.
2.5. IHC for YKL-40 antibody
To diagnose PM/DM, immunostaining was performed in accordance with the method described by Furukawa et al. [Citation16]. Muscle biopsy specimens were prepared using the same technique described for H&E staining. The primary antibody against human chitinase 3-like 1 (AF2599, R&D Systems, Inc., Minneapolis, MN) was used, diluted 1/130 in phosphate-buffered saline (PBS). The secondary antibody was anti-goat IgG antibody (sc-2028, Santa Cruz Biotechnology, Inc., Dallas, TX) diluted 1/200 in PBS.
2.6. Immunofluorescence analysis of muscle biopsy specimens from patients with PM/DM
Immunofluorescent staining was performed on samples prepared using the same method utilized for IHC. The staining method was performed based on the report by Jensen et al. [Citation35]. Primary antibodies against human chitinase-3 like 1 (1/150 diluted with 1% Tween-BSA in PBS, AF2599, R&D Systems, Inc., Minneapolis, MN) and rabbit anti-CD68 monoclonal antibody (1/200 diluted with 1% Tween-BSA in PBS, ab213363, Abcam, Cambridge, UK) were used. The specimens were then incubated with a mixed solution of Alexa Fluor Plus 488 donkey anti-goat IgG (1/200 diluted with 1% BSA in PBS, A11055, Invitrogen, Waltham, MA) and Alexa Fluor Plus 405 donkey anti-rabbit IgG (1/200 diluted 1% BSA in PBS, A48258, Invitrogen, Waltham, MA). The nuclei were counterstained with propidium iodide, mounted, and observed under a confocal microscope (LSM780, Carl ZEISS, Oberkochen, Germany) at ×630 magnification and analysed using ZEN (ZEISS Efficient Navigation, Oberkochen, Germany) software. The images show overlays of YKL-40 (green), CD68 (blue) and nuclei (red).
2.7. Statistical analyses
Statistical analysis was performed using the Steel–Dwass test, the Mann–Whitney U-test and Fisher’s exact test (JMP® 14, SAS Institute Inc., Cary, NC). Statistical significance was set at p < .05 for all tests. All data are expressed as mean ± standard error.
3. Results
3.1. Patient background
This study included 26 HCs. At baseline, the mean age was 49.5 ± 2.9 years for HCs. The sex ratio was 10 male (38%) and 16 female (62%) individuals for HCs. Serological data showed a CK level of 80.7 ± 10.1 IU/L in HCs; the C-reactive protein (CRP) level was 0.07 ± 0.02 mg/dL in HCs. The lactate dehydrogenase (LDH) level was 167.3 ± 8.6 IU/L for HCs.
This study also included 35 patients with myositis. Of the 35 patients with myositis, 20 had ILD complications. The mean age of patients was 55.1 ± 2.7 years for those with ILD complications and 52.5 ± 5.9 years for those without ILD complications (p = .89). The male/female ratio was 4 male and 16 female individuals for patients with ILD complications and 4 male and 11 female individuals for patients without ILD complications (p = .43). The 20 patients with ILD complications included 17 with DM and three with PM, and the 15 patients without ILD complications included 12 with DM and three with PM (p = .69). Serological data showed that the CK level was 839 ± 232 IU/L in patients with ILD and 1781 ± 405 IU/L in those without ILD (p < .05); the CRP level was 0.88 ± 0.25 mg/dL in patients with ILD and 0.45 ± 0.22 mg/dL in those without ILD (p = .08). The LDH level was 414 ± 30 IU/L in patients with ILD and 508 ± 50 IU/L in those without ILD (p = .16). Glucocorticoids (GCs) were administered to 35 (100%) patients with myositis at an initial dose of 36.7 ± 13.9 mg/day. Immunosuppressive drugs were also administered: cyclosporine in five patients with ILD, in three without ILD, tacrolimus in eight with ILD, in eight without ILD, cyclophosphamide in six with ILD, and methotrexate in two without ILD ().
Table 1. Patient background in the presence or absence of interstitial lung disease.
3.2. Serum YKL-40
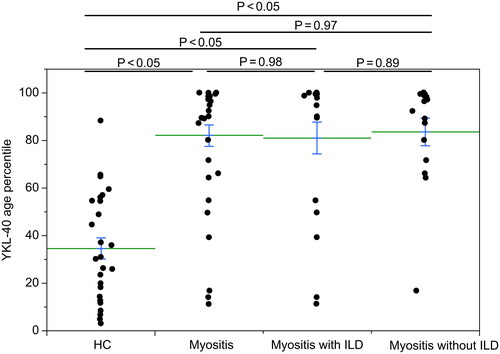
Serum YKL-40 age percentile values were compared before treatment in HCs, in all patients with myositis (including ILD), and in patients with myositis complicated with and without ILD. Patients with myositis were assigned into groups 1–3, while HCs were assigned to group 0. Group 1 included all patients with myositis. Group 2 included patients with myositis with ILD, and group 3 included myositis patients without ILD. Serum YKL-40 age percentile was calculated by percentile = 100/(1 + (YKL-40)−3 × 1.062age × 5000). YKL-40 age percentile was significantly higher in group 1 (81.3 ± 4.5), group 2 (80.0 ± 6.7) and group 3 (83.2 ± 5.8) than in group 0 (34.6± 4.5). The YKL-40 age percentile had no significant difference between groups 1 and 2 (p = .98), groups 2 and 3 (p = .89) and groups 1 and 3 (p = .97) (). Compared with HCs, serum YKL-40 age percentile levels were significantly elevated in patients with myositis with and without ILD.
Figure 1. YKL-40 age percentile in patients with myositis (groups 1–3) and healthy controls (group 0). Serum YKL-40 age percentile was calculated by percentile = 100/(1 + (YKL-40)−3 × 1.062age × 5000). Values are expressed as means (horizontal lines) and standard errors (top and bottom of each bar). YKL-40 age percentile was significantly higher in group 1 (81.3 ± 4.5), group 2 (80.0 ± 6.7) and group 3 (83.2 ± 5.8) than in group 0 (34.6 ± 4.5) (p < .05, the Steel–Dwass test). The YKL-40 age percentile has no significant difference between groups 1 and 2 (p = .98, the Mann–Whitney U-test), groups 2 and 3 (p = .89, the Mann–Whitney U-test) and groups 1 and 3 (p = .97, the Mann–Whitney U-test). HC: healthy controls; ILD: interstitial lung disease.
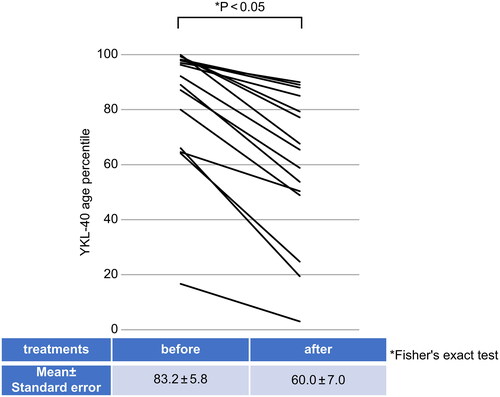
In addition, serum YKL-40 age percentile values measured before the start of treatment were compared with serum YKL-40 age percentile values assessed approximately 2 months after the start of treatment in patients with myositis without ILD complications. The serum YKL-40 age percentile value before the start of treatment was 83.2 ± 5.8. The treatment consisted of GCs and immunosuppressive agents. The serum YKL-40 age percentile value approximately 2 months after the start of treatment was 60.0 ± 7.0, indicating a significant decrease after treatment (p < .05) ().
Figure 2. Serum YKL-40 age percentile values before and after treatment in myositis (uncomplicated ILD). Serum YKL-40 age percentile values were measured in patients with myositis without ILD complications (n = 15) before and 2 months after treatment (p < .05, Fisher’s exact test). Vertical axis: serum YKL-40 age percentile value. Horizontal axis: test points. Table: (mean ± standard error) serum YKL-40 age percentile values before and 2 months after treatment. ILD: interstitial lung disease.
3.3. Immunohistochemical staining
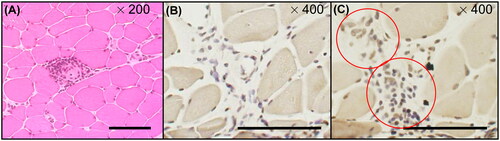
Immunohistochemical staining of muscle biopsy samples from patients with PM/DM (two cases each of PM and DM, total n = 4) was performed. shows H&E staining of the samples from patients with DM, showing small and large myofibres and inflammatory cell infiltration around myofibres. shows YKL-40-positive inflammatory cell infiltrate in the myofibre interstitium (red circles), which was not stained in the control ().
Figure 3. H&E and immunochemical tissue staining of muscle biopsies from patients with PM/DM H&E and immunohistochemical staining of muscle biopsies from patients with myositis (PM, n = 2; DM, n = 2). The muscle biopsies from patients with DM. (A) H&E staining. (B, C) Immunohistochemical staining of inflammatory cell infiltration sites. (B) Normal goat IgG was used as the control. Inflammatory cell infiltration was observed in the interstitium but was not stained in slice (B). (C) Immunohistochemical staining of the inflammatory cell infiltration sites. Slices at the same level as (B) were stained with goat anti-human YKL-40 antibody. YKL-40-positive inflammatory cell infiltrates were observed in the stroma (red circles). Magnification: (A) ×200, (B) ×400 and (C) ×400. Bar, 100 μm. DM: dermatomyositis; H&E: haematoxylin–eosin; PM: polymyositis.
3.4. Immunofluorescence staining
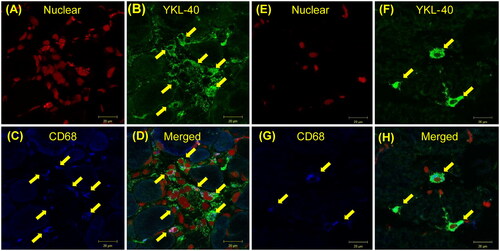
Immunofluorescence staining was performed on muscle biopsy specimens from two patients each of PM and DM (four patients in total); shows the results of fluorescence staining of biopsy samples from patients with PM/DM; shows stained biopsy samples from patients with PM and shows stained biopsy samples from those with DM. In , the nuclei are indicated in red. Nuclei derived from inflammatory cells and myocytes are uniformly shown in red; shows cells positive for YKL-40 in green. Several cells were observed in the cytoplasm, as shown in green in the figure. shows cells positive for CD68 in blue. shows merged images, where cells positive for both YKL-40 and CD68 in one cell are shown in light blue as indicated by the yellow arrows.
Figure 4. Fluorescence staining of muscle biopsy from patients with PM/DM. Fluorescence staining of PM/DM muscle biopsies (PM, n = 2; DM, n = 2). (A–D) PM; (E)–(H) DM. (A, E) nuclei in red; (B, F) YKL-40 in green; (C, G) CD-68 in blue; (D, H) merged cells in light blue. The yellow arrows indicate the macrophages. The cells containing CD-68 and YKL-40 and the merged cells are all the same cells. Magnification is ×630. Bar, 20 μm. DM: dermatomyositis; PM: polymyositis.
4. Discussion
We measured serum YKL-40 levels in HCs and patients with myositis without ILD and found that the levels in patients with myositis without ILD were significantly higher than those in HCs (). Reports on serum YKL-40 levels in patients with myositis have used serum YKL-40 levels to evaluate ILD complicated by myositis [Citation30–33,Citation36]. In a report of elevated serum YKL-40 levels in patients with antisynthetase syndrome [Citation36], ILD was a complication in approximately 89% of eligible patients. As the present study was limited to patients without ILD complications, eliminating the influence of ILD in myositis, we believe that serum YKL-40 levels were elevated in myositis itself.
This study used age-corrected percentile values for serum YKL-40 levels. Serum YKL-40 values have been reported to be affected by age, and the older the patient, the higher the value [Citation34]. Previous studies on myositis and YKL-40 have used actual serum YKL-40 values, and it is possible that the results could change depending on the age of the individual from whom samples were collected. This study also showed a significant increase in age-corrected serum YKL-40 percentile values, and we believe that myositis itself increased serum YKL-40.
The serum YKL-40 age percentile values taken after 2 months of treatment in this study were significantly lower than those taken before treatment (). This suggests that YKL-40 responds to steroid and immunosuppressive treatments and may be altered by the severity of myositis. We performed IHC and immunofluorescence staining of muscle biopsies of patients with PM/DM ( and ) and found that YKL-40-expressing macrophages are common in both diseases (). Although YKL-40-positive macrophages have been reported in alveolar epithelium in ILD by Hozumi et al. [Citation30], this was the first time they were reported in muscle tissue in PM/DM patients. It appears that a YKL-40-positive, CD68-negative cell was observed in our results, but this is unlikely to be just one different cell, since the surrounding cells are YKL-40-positive, CD68-positive, and it is possible that the CD68 blue was weak and difficult to see, or that the CD68 antigen did not enter due to the way the cells were cut.
It was previously thought that in PM, CD8+ cytotoxic T cells directly injure myocytes [Citation23], and in DM, cytokines and cytotoxic molecules involving CD4+ T cells cause muscle injury [Citation27,Citation29]. Recently, however, the involvement of macrophages in PM/DM has also been studied [Citation37–42], and macrophage infiltration may be related to myositis [Citation40]. In this study, we found that YKL-40-positive macrophages were common in patients with both PM and DM. Using H&E and IHC staining, we hypothesised that YKL-40-positive macrophages may be involved in myositis because these cells infiltrate the intramuscular sheaths and interstitial spaces of patients with myositis, consistent with the site of the disease. The mechanism by which YKL-40-positive macrophages contribute to myositis will be our next topic of study.
The present study has several limitations. Because its purpose was to examine the relationship between myositis itself and YKL-40, the number of cases was small and limited to patients without ILD complications to exclude YKL-40 modified by ILD. Therefore, we did not examine whether there were differences in autoantibodies. Furthermore, the number of muscle biopsies was small. This was due to the small sample size, as the muscle biopsy is a highly invasive test; therefore, this is an issue for future research, and further study is needed to increase the number of patients.
In conclusion, YKL-40 positive macrophages were found to be present in both PM/DM and may be involved in the pathogenesis of PM/DM. The manner in which these cells function in both diseases is a subject for future research.
Author contributions
KN and TF contributed to study design, data acquisition, data interpretation and manuscript preparation; KM analyzed the data and revised the manuscript; TK and SK provided patient data; YT contributed to muscle biopsy and immunostaining; SH contributed to the interpretation of pathology data; TY, TH and NA contributed to the interpretation of data and revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank our technical assistant, Mrs. Sachie Kitano, for her assistance. We would also like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
KM received research grants from Chugai and Asahi Kasei. NA received lecture fees and/or honoraria from Eisai Co., Ltd. and Janssen Pharmaceutical K.K.
Additional information
Funding
References
- Johansen JS, Williamson MK, Rice JS, et al. Identification of proteins secreted by human osteoblastic cells in culture. J Bone Miner Res. 1992;7(5):501–512. doi: 10.1002/jbmr.5650070506.
- Shuhui L, Mok YK, Wong WS. Role of mammalian chitinases in asthma. Int Arch Allergy Immunol. 2009;149(4):369–377. doi: 10.1159/000205583.
- Chupp GL, Lee CG, Jarjour N, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357(20):2016–2027. doi: 10.1056/NEJMoa073600.
- Tang H, Fang Z, Sun Y, et al. YKL-40 in asthmatic patients, and its correlations with exacerbation, eosinophils and immunoglobulin E. Eur Respir J. 2010;35(4):757–760. doi: 10.1183/09031936.00034409.
- Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53(2):172–209.
- Schultz NA, Johansen JS. YKL-40—a protein in the field of translational medicine: a role as a biomarker in cancer patients? Cancers. 2010;2(3):1453–1491. doi: 10.3390/cancers2031453.
- Shao R, Francescone R, Ngernyuang N, et al. Anti-YKL-40 antibody and ionizing irradiation synergistically inhibit tumor vascularization and malignancy in glioblastoma. Carcinogenesis. 2014;35(2):373–382. doi: 10.1093/carcin/bgt380.
- Shao R, Taylor SL, Oh DS, et al. Vascular heterogeneity and targeting: the role of YKL-40 in glioblastoma vascularization. Oncotarget. 2015;6(38):40507–40518. doi: 10.18632/oncotarget.5943.
- De Ceuninck F, Gaufillier S, Bonnaud A, et al. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem Biophys Res Commun. 2001;285(4):926–931. doi: 10.1006/bbrc.2001.5253.
- Turkyilmaz AK, Devrimsel G, Kirbas A, et al. Relationship between pulse wave velocity and serum YKL-40 level in patients with early rheumatoid arthritis. Rheumatol Int. 2013;33(11):2751–2756. doi: 10.1007/s00296-013-2810-4.
- Vind I, Johansen JS, Price PA, et al. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol. 2003;38:599–605.
- Imai Y, Aochi S, Iwatsuki K, et al. YKL-40 (chitinase 3-like 1) as a biomarker for psoriasis vulgaris and pustular psoriasis. J Dermatol Sci. 2011;64(1):75–77. doi: 10.1016/j.jdermsci.2011.06.012.
- Imai Y, Aochi S, Iwatsuki K, et al. YKL-40 is a serum biomarker reflecting the severity of cutaneous lesions in psoriatic arthritis. J Dermatol. 2013;40(4):294–296. doi: 10.1111/1346-8138.12061.
- Johansen JS, Christoffersen P, Møller S, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32(6):911–920. doi: 10.1016/s0168-8278(00)80095-1.
- Chen G, Yang T, Gu Q, et al. Elevated plasma YKL-40 as a prognostic indicator in patients with idiopathic pulmonary arterial hypertension. Respirology. 2014;19(4):608–615. doi: 10.1111/resp.12283.
- Furukawa T, Matsui K, Kitano M, et al. Relationship between YKL-40 and pulmonary arterial hypertension in systemic sclerosis. Mod Rheumatol. 2019;29(3):476–483. doi: 10.1080/14397595.2018.1480256.
- Melah KE, Lu SY, Hoscheidt SM, et al. Cerebrospinal fluid markers of Alzheimer’s disease pathology and microglial activation are associated with altered white matter microstructure in asymptomatic adults at risk for Alzheimer’s disease. J Alzheimers Dis. 2016;50(3):873–886. doi: 10.3233/JAD-150897.
- Yeo IJ, Lee CK, Han SB, et al. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol Ther. 2019;203:107394. doi: 10.1016/j.pharmthera.2019.107394.
- Deutschmann C, Sowa M, Murugaiyan J, et al. Identification of chitinase-3-like protein 1 as a novel neutrophil antigenic target in Crohn’s disease. J Crohns Colitis. 2019;13(7):894–904. doi: 10.1093/ecco-jcc/jjz012.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292(8):403–407. doi: 10.1056/NEJM197502202920807.
- Findlay AR, Goyal NA, Mozaffar T. An overview of polymyositis and dermatomyositis. Muscle Nerve. 2015;51(5):638–656. doi: 10.1002/mus.24566.
- Dalakas M. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991;325(21):1487–1498. doi: 10.1056/NEJM199111213252107.
- Dalakas M, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971–982. doi: 10.1016/S0140-6736(03)14368-1.
- Euwer RL, Sontheimer RD. Amyopathic dermatomyositis: a review. J Invest Dermatol. 1993;100(1):124S–127S. doi: 10.1111/1523-1747.ep12356896.
- Kissel J, Mendell J, Rammohan K. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med. 1986;314(6):329–334. doi: 10.1056/NEJM198602063140601.
- Emslie-Smith A, Engel A. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990;27(4):343–356. doi: 10.1002/ana.410270402.
- Arahata K, Engel A. Monoclonal antibody analysis of mononuclear cells in myopathies. I: quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol. 1984;16(2):193–208. doi: 10.1002/ana.410160206.
- Nagaraju K, Lundberg IE. Polymyositis and dermatomyositis: pathophysiology. Rheum Dis Clin North Am. 2011;37(2):159–171, v. doi: 10.1016/j.rdc.2011.01.002.
- Venalis P, Lundberg IE. Immune mechanisms in polymyositis and dermatomyositis and potential targets for therapy. Rheumatology. 2014;53(3):397–405. doi: 10.1093/rheumatology/ket279.
- Hozumi H, Fujisawa T, Enomoto N, et al. Clinical utility of YKL-40 in polymyositis/dermatomyositis-associated interstitial lung disease. J Rheumatol. 2017;44(9):1394–1401. doi: 10.3899/jrheum.170373.
- Jiang L, Wang Y, Peng Q, et al. Serum YKL-40 level is associated with severity of interstitial lung disease and poor prognosis in dermatomyositis with anti-MDA5 antibody. Clin Rheumatol. 2019;38(6):1655–1663. doi: 10.1007/s10067-019-04457-w.
- Gao MZ, Wei YY, Xu QW, et al. Elevated serum YKL-40 correlates with clinical characteristics in patients with polymyositis or dermatomyositis. Ann Clin Biochem. 2019;56(1):95–99. doi: 10.1177/0004563218786979.
- Zhang PL, Yang HX, Zhang LN, et al. Value of serum YKL-40 in the diagnosis of anti-MDA5-positive patients with dermatomyositis complicated with severe pulmonary injury. Beijing Da Xue Xue Bao Yi Xue Ban. 2021;53:1055–1060.
- Bojesen SE, Johansen JS, Nordestgaard BG. Plasma YKL-40 levels in healthy subjects from the general population. Clin Chim Acta. 2011;412(9–10):709–712. doi: 10.1016/j.cca.2011.01.022.
- Jensen KY, Jacobsen M, Schrøder HD, et al. The immune system in sporadic inclusion body myositis patients is not compromised by blood-flow restricted exercise training. Arthritis Res Ther. 2019;21(1):293. doi: 10.1186/s13075-019-2036-2.
- Carboni RCS, Behrens Pinto GL, Shinjo SK. High YKL-40 serum levels and its expression in the muscle tissues of patients with antisynthetase syndrome. Adv Rheumatol. 2021;61(1):44. doi: 10.1186/s42358-021-00199-z.
- Rostasy KM, Piepkorn M, Goebel HH, et al. Monocyte/macrophage differentiation in dermatomyositis and polymyositis. Muscle Nerve. 2004;30(2):225–230. doi: 10.1002/mus.20088.
- Peng QL, Zhang YL, Shu XM, et al. Elevated serum levels of soluble CD163 in polymyositis and dermatomyositis: associated with macrophage infiltration in muscle tissue. J Rheumatol. 2015;42(6):979–987. doi: 10.3899/jrheum.141307.
- Horiike Y, Suzuki Y, Fujisawa T, et al. Successful classification of macrophage-mannose receptor CD206 in severity of anti-MDA5 antibody positive dermatomyositis associated ILD. Rheumatology. 2019;58(12):2143–2152. doi: 10.1093/rheumatology/kez185.
- Zuo Y, Ye L, Liu M, et al. Clinical significance of radiological patterns of HRCT and their association with macrophage activation in dermatomyositis. Rheumatology. 2020;59(10):2829–2837. doi: 10.1093/rheumatology/keaa034.
- Yin R, Wang G, Zhang L, et al. Dermatomyositis: immunological landscape, biomarkers, and potential candidate drugs. Clin Rheumatol. 2021;40(6):2301–2310. doi: 10.1007/s10067-020-05568-5.
- Chen S, Li H, Zhan H, et al. Identification of hub biomarkers and immune cell infiltration in polymyositis and dermatomyositis. Aging. 2022;14(10):4530–4555. doi: 10.18632/aging.204098.
