Abstract
The complement component C5a contributes to the recruitment of immune cells to inflamed tissues and local inflammation. The proinflammatory cytokine interleukin (IL)-1β is also related to inflammatory disorders through inflammasome activation. However, the association between inflammasome activation and C5a is unclear. Human peripheral blood mononuclear cells (PBMCs) were stimulated with C5a and measured for IL-1β secretion by enzyme-linked immunosorbent assay (ELISA). The pro-IL-1β expression in cell lysates was also examined by Western blot analysis. Similarly, magnetic bead-isolated CD14+ monocyte-depleted and lymphocyte-depleted PBMCs were stimulated with C5a, and immunoblot analysis was performed using an anti-cleaved-IL-1β (p17) antibody. FACS was performed to detect caspase-1-activated cells. C5a-stimulated PBMCs produced IL-1β in C5a concentration-dependent manner. The protein levels of pro-IL-1β in the cell lysates were significantly increased. Furthermore, the cleaved-IL-1β (p17) was faintly detected in the same lysates. Active caspase-1 was demonstrated in C5a-simulated CD14+ monocytes by FACS. Cleaved-IL-1β (p17) was demonstrated in the supernatant of C5a-stimulated PBMCs. Lymphocyte-depleted PBMCs stimulated with C5a but monocyte-depleted PBMCs produced cleaved-IL-1β (p17). C5a induced the production of mature IL-1β in PBMCs. The IL-1β production is mediated mainly by caspase-1 activation in CD14+ monocytes. These results suggest that C5a alone potentiates mature IL-1β production mainly in monocytes.
Keywords:
1. Introduction
The complement system plays an important role in immunity by promoting inflammation and protecting against pathogens [Citation1]. However, the dysregulation of the complement system is critical to several autoimmune and inflammatory syndromes [Citation2]. Previous studies have indicated that the complement system is activated during autoimmune or inflammatory diseases [Citation3,Citation4]. Complement-activation products possess powerful biological features that induce inflammation [Citation5]. C5a, one of the complement-activation products, is the most potent chemotactic factor for immune cells [Citation6]. C5a is a potent regulator of acute inflammatory responses and has been implicated in various inflammatory diseases [Citation7].
The elevated expression of the proinflammatory cytokine interleukin (IL)-1β is also linked to inflammatory disorders [Citation8]. IL-1β activates the release of other proinflammatory cytokines such as tumor necrosis factor (TNF)-α and IL-6, and induces a T helper 17 (Th17) bias in the cellular adaptive responses [Citation9]. IL-1 cytokine family including IL-1β is a crucial component of the host defense against infections [Citation10]. IL-1β is secreted following intracellular caspase-1 activation by inflammasomes [Citation11]. The accumulation of pro-IL-1β and caspase-1-mediated cleavage of pro-IL-1β to form mature IL-1β is critical for IL-1β secretion [Citation12]. These IL-1β maturation processes require caspase-1 activation via inflammasome complex formation [Citation11]. Pro-IL-1β activation process including caspase-1 activation and complement system is associated with the development of inflammation. However, the interaction between complement-activation products and inflammasome as well as pro-IL-1β processing had not been clarified. We hypothesized that complement-activation products released upon complement activation can affect IL-1β maturation process. To investigate this hypothesis, we focused on C5a because of its proinflammatory property. Accordingly, we aimed to investigate the link between C5a and IL-1β maturation using human peripheral blood mononuclear cells (PBMCs). The study demonstrates that C5a elicits the secretion of mature IL-1β from human PBMCs.
2. Materials and methods
2.1. Isolation of PBMCs
Venous peripheral blood was collected from healthy volunteers between October 2021 and September 2022. The blood was layered on a Lymphoprep™ (STEMCELL Technologies, Oslo, Norway) cushion, and cells were isolated according to the manufacturers’ instructions. Whole blood was collected by phlebotomy into a 7-mL sodium heparin vacutainer and inverted to ensure the mixing of blood with the anticoagulant. Then, 7 mL of blood was transferred into a 50 mL conical tube prefilled with 7 mL of phosphate-buffered saline (PBS) for a 1:1 dilution. This diluted blood was decanted into the upper chamber of a Lymphoprep tube. The filled Lymphoprep tube was centrifuged at 800 × g for 30 min at 21 °C without braking. Centrifugation resulted in an upper layer of plasma with a cloudy band of PBMCs and a lower layer of erythrocytes and polymorphonuclear cells, separated by the polyethylene insert. After centrifugation, the cloudy PBMC band was collected. Then, 10 mL of Dulbecco’s modified Eagle medium (DMEM) was added to enriched PBMCs and centrifuged at 300 × g for 10 min. The supernatant was carefully aspirated, and the cell pellet was resuspended in 10 mL (1 × 106/mL) of DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS).
2.2. Negative selection by magnetic separation
PBMC (1 × 107/mL) was suspended at 300 × g for 10 min, and the supernatant was aspirated completely. Cluster of differentiation (CD)14 MicroBeads (Miltenyi Biotec B.V. & Co. KG, Bergisch Gladbach, Germany) was added according to the instruction of the manual. CD14+ monocyte-depleted PBMCs were purified from PBMCs by negative selection using magnetic cell separation columns. Similarly, lymphocyte-depleted PBMCs were prepared from PBMCs by negative selection. Human CD3 Magnetic Beads Kit (Proteintech, Chicago, IL) was used for the depletion of human CD3+ T lymphocytes from PBMCs. Following incubation with biotinylated human CD3 antibody and streptavidin magnetic beads, the cell sample was placed on a magnet. After the depletion of CD3 T lymphocytes, CD19 MicroBeads (Miltenyi Biotec B.V. & Co. KG, Bergisch Gladbach, Germany) were added according to the manufacturers’ instructions. After the cell sample was placed on a magnet, CD19+ B lymphocyte-depleted samples were collected.
2.3. Enzyme-linked immunosorbent assay (ELISA) analysis
PBMCs (2 × 107/mL) were seeded in 12-well plates containing DMEM supplemented with 10% heat-inactivated FBS and stimulated with C5a (R&D Systems, Minneapolis, MN) for 18 h. Cell-free supernatants were assayed for IL-1β using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturers’ instructions.
2.4. Cell lysis and immunoblot analysis
Freshly isolated PBMCs were stimulated with C5a for 18 h, and cell-free supernatants were collected by centrifugation at 400 × g for 5 min. The supernatant was used for immunoblot analysis using anti-cleaved-IL-1β antibody (p17, D3A3Z) (CST, Danvers, MA). Cell pellets were washed with PBS and added with RIPA Lysis Buffer (Sigma-Aldrich, St. Louis, MO) supplemented with a proteinase inhibitor cocktail (Nacalai Tesque Inc., Kyoto, Japan) on ice. The cell lysates were centrifuged at 10,000 × g for 10 min at 4 °C, and the supernatant was collected. An equal amount (30 μg) was subjected to 12% sodium dodecyl-sulfate polyacrylamide gel electrophoresis and electrotransferred onto polyvinylidene fluoride membranes. The membrane was blocked for 1 h at room temperature with 5% bovine serum albumin. The membrane was incubated with primary antibodies against human pro-IL-1β (Proteintech, Chicago, IL), and cleaved-IL-1β (Cell Signaling Technology, Danvers, MA or Invitrogen, Carlsbad, CA), then incubated with secondary antibodies at room temperature, followed by visualization using ECL Reagent (Amersham, Little Chalfont, UK). Immunoblot detection was achieved by LAS-3000 Imaging System (Fuji Film, Tokyo, Japan).
2.5. Flow cytometry and FLICA staining
PBMCs (1 × 106/mL) were seeded in 24-well plates containing DMEM supplemented with 10% heat-inactivated FBS and stimulated with C5a for 6 h. PBMCs stimulated with C5a were used for the flow cytometric analysis of the surface expressions of CD3, CD14 and CD19 using anti-human CD3 (PerCP-CY5.5; mouse no. 344806, 2:100 dilution; Biolegend, San Diego, CA), anti-human CD14 (APC; mouse no. 309808, 2:100; Biolegend, San Diego, CA) and anti-CD19 (APC; mouse no. 302211, 2:100; Biolegend, San Diego, CA) antibodies. The LIVE/DEAD Fixable Blue Dead Cell Stain Kit (Invitrogen, Carlsbad, CA) was used to evaluate cell viability by FACS. Before surface staining, PBMCs were stained with FAM-YVAD-FMK (fluorescent-labeled inhibitor of caspase-1 (FLICA); Immunochemistry Technologies, Bloomington, MN) for 1 h at 37 °C to determine caspase-1 activation. The procedures were performed according to the manufacturers’ instructions. Flow cytometry was performed on FACS-Canto II (BD Biosciences, Franklin Lakes, NJ), and data were analyzed using FlowJo (BD Biosciences, Franklin Lakes, NJ).
2.6. Statistical analysis
Between-group differences were examined for statistical significance using Student’s t-test. p Values of <.05 were considered significant. Bonferroni’s correction was performed for multiple comparisons.
Ethical approval for this study (no. 21003) was provided by the Ethics Committee of Fukushima Medical University and written informed consent was obtained from each individual.
3. Results
3.1. C5a induces IL-1β secretion from human PBMCs
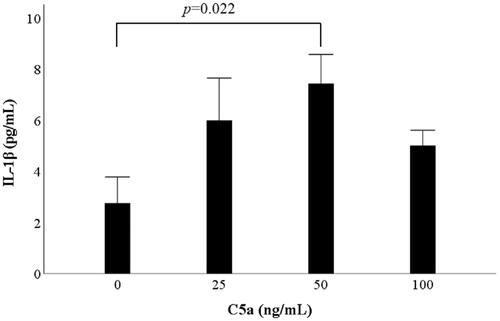
PBMCs were exposed to various concentrations of C5a for 18 h. The supernatants were analyzed for IL-1β using ELISA. Preliminary study revealed C5a stimulation dose-dependent induced IL-1β secretion from human PBMCs and reached a plateau at 50 ng/mL. To determine the optimal concentration of C5a, PBMCs were cultured in three concentrations of C5a (0, 25, 50 and 100 ng/mL). IL-1β secretion was significantly induced by C5a stimulation (). We examined whether a caspase-1 inhibitor, Ac-YVAD-CHO (Sigma-Aldrich, St. Louis, MO), inhibit IL-1β secretion. PBMCs were pretreated with Ac-YVAD-CHO for 1 h, and PBMCs were exposed to various concentrations of C5a for 18 h. The supernatants were analyzed for IL-1β using ELISA. Ac-YVAD-CHO inhibited IL-1β secretion from C5a-stimulated PBMCs (Supplemental Figure 1).
Figure 1. C5a significantly induces IL-1β production from human peripheral blood mononuclear cells. PBMCs were stimulated with three concentrations of C5a (25, 50 and 100 ng/mL) for 18 h. The supernatants were analyzed for IL-1β using ELISA. The data are expressed as the mean ± SE of six independent experiments. Student’s t-test and Bonferroni’s correction were performed. PBMCs: peripheral blood mononuclear cells; ELISA: enzyme-linked immunosorbent assay; SE: standard error.
3.2. C5a induces pro-IL-1β expression in human PBMCs
To determine whether C5a induces pro-IL-1β expression, PBMCs were stimulated with C5a, and cellular lysates were analyzed for pro-IL-1β protein by immunoblot analysis.
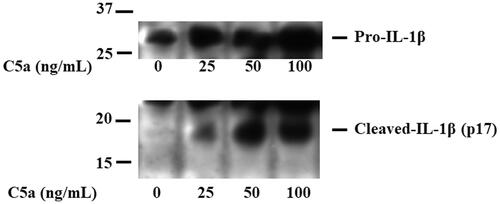
shows a significant increase in the protein levels of pro-IL-1β in the cellular lysates of C5a-stimulated PBMCs. Furthermore, the cleaved-IL-1β (p17) was detected in the same lysates of C5a-stimulated PBMCs. These findings suggest that pro-IL-1β processing was induced in C5a-stimulated PBMCs.
Figure 2. C5a-stimulation induces pro-IL-1β expressions in human peripheral blood mononuclear cells. PBMCs were stimulated with C5a (0 ng/mL, 25 ng/mL, 50 ng/mL and 100 ng/mL) for 18 h. Cellular lysates were analyzed by immunoblotting with antibody that recognize cleaved-IL-1β (p17) and pro-IL-1β. Data are representative of three independent experiments. PBMCs: peripheral blood mononuclear cells.
3.3. Expression of active caspase-1 on PBMCs
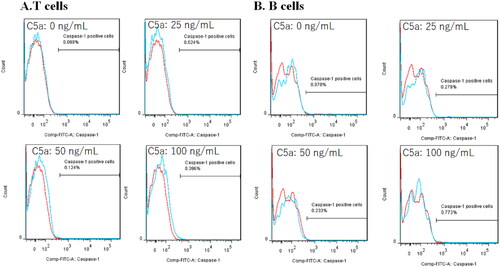
To identify IL-1β-releasing cells, flow cytometry was performed using FLICA, which demonstrates inflammasome activation by detecting active caspase-1 [Citation13]. PBMCs were stimulated with four concentrations of C5a (0, 25, 50 and 100 ng/mL) for 6 h. Flow cytometry gating strategies are shown in Supplemental Figure 2. Dead cells were excluded by LIVE/DEAD Fixable Blue Dead Cell staining. As shown in , active caspase-1 was detected in C5a-stimulated CD14-positive monocytes. The percentage of FLICA-positive cells in CD14-positive monocytes was increased in a C5a concentration-dependent manner (). Similarly, the gating strategy of CD3-positive T-cells and CD19-positive B-cells are shown in Supplemental Figure 3. On the contrary, CD3-positive T lymphocytes and CD19-positive B lymphocytes did not express active caspase-1 ().
Figure 3. C5a-stimulation induces pro-IL-1β expressions in human peripheral blood mononuclear cells. Flow cytometric analysis of CD14+ monocytes stimulated by C5a. PBMCs stimulated by C5a for 6 h. The expression of active caspase-1 was analyzed by flow cytometric analysis. Data are based on five independent experiments. (A) Flow cytometry overlay histograms for active caspase-1 demonstrate typical cell profiles comparing between C5a stimulated CD14+ monocytes and negative controls. (B) The histogram indicates the percentage of active caspase-1 positive cells in CD14+ monocytes stimulated by various concentrations of C5a. Active caspase-1 expression was detected using FAM-FLICA™ (blue histogram) or isotype control antibody (red histograms). The data are expressed as the mean ± SE. PBMCs: peripheral blood mononuclear cells.
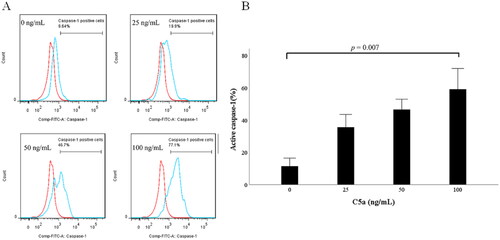
Figure 4. Flow cytometric analysis of CD3+ lymphocytes and CD19+ lymphocytes stimulated by C5a. PBMCs stimulated by C5a for 6 h. The expression of active caspase-1 was analyzed by flow cytometric analysis. (A) Flow cytometry overlay histograms for active caspase-1 demonstrate typical cell profiles comparing between C5a stimulated CD3+ T-cells and negative controls. Active caspase-1 expression was detected using FAM-FLICA™ (blue histogram) or isotype control antibody (red histograms). (B) Flow cytometry overlay histograms for active caspase-1 demonstrate typical cell profiles comparing between C5a stimulated CD19+ T-cells and negative controls. Active caspase-1 expression was detected using FAM-FLICA™ (blue histogram) or isotype control antibody (red histograms). PBMCs: peripheral blood mononuclear cells.
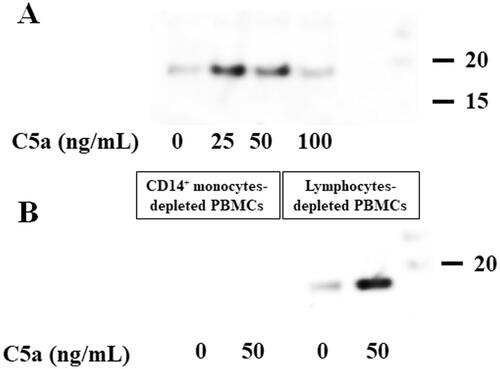
3.4. C5a induces cleaved-IL-1β secretion from C5a-stimulated PBMCs
To examine whether C5a stimulation induces the release of mature IL-1β from C5a-stimulated PBMCs, the culture supernatants were subjected to immunoblot analysis using an anti-cleaved-IL-1β (p17) antibody. The stimulation of PBMCs with C5a induced the production of cleaved-IL-1β (p17) (). To identify cleaved-IL-1β (p17)-releasing cells, CD14+ monocyte-depleted PBMCs and lymphocyte-depleted PBMCs were purified from PBMCs by negative selection using magnetic cell separation columns. Each purified PBMC was stimulated by C5a (50 ng/mL), and culture supernatants were analyzed for immunoblot analysis using an anti-cleaved-IL-1β (p17) antibody. C5a stimulation of lymphocyte-depleted PBMCs induced the production of cleaved-IL-1β (p17) (). However, the production of cleaved-IL-1β (p17) was not observed in the supernatant of CD14+ monocyte-depleted PBMCs.
Figure 5. C5a-stimulation induces cleaved-IL-1β (p17) production of human peripheral blood mononuclear cells stimulated with C5a. C5a-stimulation induces cleaved-IL-1β (p17) production of human peripheral blood mononuclear cells stimulated with C5a. (A) Human PBMCs were stimulated with C5a (0 ng/mL, 25 ng/mL, 50 ng/mL and 100 ng/mL) for 18 h. Culture supernatants were analyzed for immunoblot analysis using anti-cleaved-IL-1β (p17) antibody. Data are representative of three independent experiments. PBMCs: peripheral blood mononuclear cells. (B) Magnetic beads-isolated CD14+ monocytes-depleted PBMCs and lymphocytes-depleted PBMCs were stimulated with C5a (0 ng/mL and 100 ng/mL) for 18 h. Culture supernatants were analyzed for immunoblot analysis using anti-cleaved-IL-1β (p17) antibody. C5a-stimulation induces cleaved-IL-1β (p17) production of lymphocytes-depleted PBMCs stimulated with C5a. Data are representative of three independent experiments. PBMC: human peripheral blood mononuclear cells.
4. Discussion
The complement system is part of the innate arm of immunity and considered an archetypical danger sensing system [Citation14]. Pathogen recognition by complement results in the activation of this system in a cascade-like fashion and the generation of its most potent effector molecules, the opsonin C3b and the anaphylatoxins C3a and C5a [Citation14]. The previous reports suggest that the opsonin C3b and the anaphylatoxins C3a are associated with IL-1β secretion from human monocyte [Citation15,Citation16].
C5a is a highly potent inflammatory anaphylatoxin that activates neutrophils, mast cells, basophils and monocytes and drives the production of inflammatory mediators, including histamine, and various cytokines and chemokines [Citation17]. Critical illness is accompanied by the release of anaphylatoxin, C5a. C5a-induced immune cell activation was considered [Citation6]; however, it remains unclear whether C5a contributes to the inflammasome activation process. Inflammasome is a multimeric protein complex that mediates the processing of pro-IL-1β to mature IL-1β and the release of the cytokine and contributes to autoinflammatory disorders [Citation11,Citation18,Citation19]. IL-1β-mediated diseases are caused by the release of the active form of IL-1β driven by endogenous molecules acting on the monocyte/macrophage [Citation20]. IL-1β induces the release of other proinflammatory cytokines such as TNF-α and IL-6 [Citation9]. The processing of the inactive IL-1β precursor and secretion of active IL-1β are tightly controlled by caspase-1 via inflammasome [Citation16,Citation21]. Some previous studies show that co-stimulation with C5a and lipopolysaccharide (LPS) induces IL-1β production in PBMCs [Citation22,Citation23]. In addition, An et al. reported C5a is able to induce pro-caspase-1 and IL-1β in human primary monocytes [Citation24]. However, confirmation of cleaved IL-1β (p17) is important for the demonstration of activated IL-1β. In this regard, previous reports cannot confirm the release of active IL-1β by C5a alone. Therefore, the involvement of complement-activation products in the maturation process of pro-IL-1β has not been completely elucidated. In this study, we demonstrated that C5a stimulation alone induces human pro-IL-1β expression and cleaved IL-1β secretions from PBMCs. Furthermore, C5a induces the activation of caspase-1 and sub-sequent pro-IL-1β processing in human PBMCs, a mixed population of lymphocytes and monocytes. However, these effects of C5a are presumed to be a cell-type-specific phenomenon. Caspase-1 activation and cleaved-IL-1β secretion in response to C5a stimulation were detected in lymphocyte-depleted PBMCs, but not in CD14+ monocyte-depleted PBMCs. Our data suggest that C5a stimulation induced caspase-1 activation and cleaved-IL-1β production by affecting CD14+ monocyte populations. Although the mechanism by which C5a-mediated pro-IL-1β processing was induced exclusively in monocyte remains unknown, C5a-mediated intracellular pathways may differ between lymphocytes and monocytes [Citation25], reflecting differential contribution to the differential inflammasome activation status between monocytes and lymphocytes. However, it cannot be completely denied that cellular interactions between monocytes and lymphocytes contribute to C5a-mediated caspase-1 activation and subsequent pro-IL-1β processing in our mixture culture system using PBMCs.
In contrast to previous reports, where in vitro differentiated murine DC stimulated by anaphylatoxins (C3a or C5a) did not activate inflammasome [Citation26], we found that C5a stimulation induces mature IL-1β production by primary PBMCs. We demonstrated that C5a induces pro-IL-1β accumulation and pro-IL-1β processing in human PBMCs. However, inconsistent results have been reported regarding the production of immunoreactive IL-1β, in which C5a provides primarily a transcriptional but not a translational signal for IL-1β [Citation24]. Our data did not completely elucidate whether C5a activates inflammasome against which immune cell populations directly or indirectly. However, our data suggest that C5a acts on CD14+ monocytes to activate caspase-1 and pro-IL-1β processing and secretion. These findings may indicate that caspase-1 activation and subsequent pro-IL-1β processing may mainly occur in myeloid-lineage immune cells, such as monocytes.
In our data, stimulation with C5a 50 ng/mL produced the most cleaved-IL-1β, whereas stimulation with 100 ng/mL C5a decreased their production. In addition, the caspase-1 activity increased in a dose-dependent manner up to 100 ng/mL in response to C5a stimulation. Generally, the production of IL-1β is necessary to both priming step and activation step [Citation10]. Our result might suggest C5a activate caspase-1 in a dose-dependent manner, but the effect of C5a to priming step is the bell-shaped dose response. The bell-shaped dose response to C5a is consistent with previous studies [Citation27]. Decreased response to higher concentrations of C5a is a well-known phenomenon termed deactivation [Citation27].
C5a has been recognized as a powerful proinflammatory mediator, interacting with its receptors C5aR1 and C5aR2 [Citation28]. Both C5aR1 and C5aR2 are expressed on monocytes. The C5aR1 receptor primarily drives pro-inflammatory effects, while numerous studies have suggested highly complex interactions of C5a with its receptors, including the conflicting pro- or anti-inflammatory roles of C5aR2 [Citation28,Citation29]. Cotreatment with C5a and LPS demonstrated NLR family pyrin domain containing 3 (NLRP3)-mediated IL-1β production in mouse peritoneal macrophages [Citation30]. IL-1β production co-stimulated with C5a and LPS was only marginally affected by C5aR1 deficiency, implying that the downregulation was primarily dependent on C5aR2 [Citation30]. On the other hand, the stimulation of C5a alone did not indicate activation of the inflammasome [Citation30]. However, C5aR-mediated downstream signals and the mechanisms of inflammasome activation are still unclear in our data. Thus, the role of C5aR in each immune cell type should be verified, which will give an accurate implication for complement-mediated disorders.
This study has some limitations. Human PBMCs may not be suitable for investigating immune-phenotype-specific functions. Therefore, isolation procedures that purify each immune-phenotype-specific cell population are needed, and the negative selection procedure is ideal to avoid receptor-mediated stimuli. However, we circumvented this procedure because we could not obtain a sufficient number of purified immune cells using limited amounts of peripheral blood. We performed the functional assays using the lymphocyte or CD14+ monocyte-deleted PBMCs; however, limitations are inherent to the use of these cell populations. Additionally, we did not investigate the C5a-receptor-mediated signaling because of the limited number and mixture of immune cells.
5. Conclusions
Our data indicated that C5a alone induces mature IL-1β production through caspase-1 activation in PBMCs. The results provide novel insight into the crosstalk between C5a and pro-IL-1β processing as an immunomodulatory function. This crosstalk is likely to play a prominent role in regulating IL-1β production in rheumatic disorders with inflammatory states. Further studies are needed to elucidate C5a-mediated inflammasome activation processes.
supplemental_figure_1.tif
Download TIFF Image (82.1 KB)Supplemental_figure_2.tif
Download TIFF Image (630.3 KB)Supplemental_figure_3.tif
Download TIFF Image (622.1 KB)Acknowledgements
The authors acknowledge Ms. Sachiyo Kanno for an excellent support about the experiments.
Disclosure statement
KM has received research grants from Chugai, Pfizer and AbbVie. Rest of the authors declares that they have no competing interests.
Additional information
Funding
References
- Merle NS, Noe R, Halbwachs-Mecarelli L, et al. Complement system part II: role in immunity. Front Immunol. 2015;6:257. doi: 10.3389/fimmu.2015.00257.
- Ballanti E, Perricone C, Greco E, et al. Complement and autoimmunity. Immunol Res. 2013;56(2–3):477–491. doi: 10.1007/s12026-013-8422-y.
- Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34(3):J276–J286. doi: 10.1016/j.jaut.2009.11.014.
- Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol. 2014;32(1):433–459. doi: 10.1146/annurev-immunol-032713-120154.
- Walport MJ. Complement. N Engl J Med. 2001;344(15):1140–1144. doi: 10.1056/NEJM200104123441506.
- Wood AJ, Vassallo A, Summers C, et al. C5a anaphylatoxin and its role in critical illness-induced organ dysfunction. Eur J Clin Invest. 2018;48(12):e13028.
- Horiuchi T, Tsukamoto H. Complement-targeted therapy: development of C5- and C5a-targeted inhibition. Inflamm Regen. 2016;36(1):11.
- Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008. doi: 10.3390/ijms20236008.
- Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007.
- Netea MG, Simon A, van de Veerdonk F, et al. IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6(2):e1000661. doi: 10.1371/journal.ppat.1000661.
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6(4):232–241. doi: 10.1038/nrrheum.2010.4.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. doi: 10.3390/ijms20133328.
- Grabarek J, Darzynkiewicz Z. In situ activation of caspases and serine proteases during apoptosis detected by affinity labeling their enzyme active centers with fluorochrome-tagged inhibitors. Exp Hematol. 2002;30(9):982–989. doi: 10.1016/s0301-472x(02)00886-x.
- Ricklin D, Hajishengallis G, Yang K, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923.
- Bacle F, Haeffner-Cavaillon N, Laude M, et al. Induction of IL-1 release through stimulation of the C3b/C4b complement receptor type one (CR1, CD35) on human monocytes. J Immunol. 1990;144(1):147–152. doi: 10.4049/jimmunol.144.1.147.
- Haeffner-Cavaillon N, Cavaillon JM, Laude M, et al. C3a(C3adesArg) induces production and release of interleukin 1 by cultured human monocytes. J Immunol. 1987;139(3):794–799. doi: 10.4049/jimmunol.139.3.794.
- Colley CS, Popovic B, Sridharan S, et al. Structure and characterization of a high affinity C5a monoclonal antibody that blocks binding to C5aR1 and C5aR2 receptors. MAbs. 2018;10(1):104–117. doi: 10.1080/19420862.2017.1384892.
- Hoffman HM, Wanderer AA. Inflammasome and IL-1β-mediated disorders. Curr Allergy Asthma Rep. 2010;10(4):229–235. doi: 10.1007/s11882-010-0109-z.
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. doi: 10.1038/nri2691.
- Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203–1217. doi: 10.1002/eji.201141550.
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3.
- Cavaillon J-M, Fitting C, Haeffner-Cavaillon N. Recombinant C5a enhances interleukin 1 and tumor necrosis factor release by lipopolysaccharide-stimulated monocytes and macrophages. Eur J Immunol. 1990;20(2):253–257. doi: 10.1002/eji.1830200204.
- Morin M, Schindler R, Wakabayashi G, et al. Picogram concentrations of endotoxin stimulate synthesis of IL-1 beta and TNF alpha by human peripheral blood mononuclear cells exposed to recombinant human C5a. Eur Cytok Netw. 1991;2(1):27–30.
- An L-L, Mehta P, Xu L, et al. Complement C5a potentiates uric acid crystal-induced IL-1β production. Eur J Immunol. 2014;44(12):3669–3679. doi: 10.1002/eji.201444560.
- Soruri A, Riggert J, Schlott T, et al. Anaphylatoxin C5a induces monocyte recruitment and differentiation into dendritic cells by TNF-α and prostaglandin E2-dependent mechanisms. J Immunol. 2003;171(5):2631–2636. doi: 10.4049/jimmunol.171.5.2631.
- Laudisi F, Spreafico R, Evrard M, et al. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1β release. J Immunol. 2013;191(3):1006–1010. doi: 10.4049/jimmunol.1300489.
- Miller AM, Stella N. Microglial cell migration stimulated by ATP and C5a involve distinct molecular mechanisms: quantification of migration by a novel near-infrared method. Glia. 2009;57(8):875–883. doi: 10.1002/glia.20813.
- Li XX, Lee JD, Kemper C, et al. The complement receptor C5aR2: a powerful modulator of innate and adaptive immunity. J Immunol. 2019;202(12):3339–3348. doi: 10.4049/jimmunol.1900371.
- Zhang T, Garstka MA, Li K. The controversial C5a receptor C5aR2: its role in health and disease. J Immunol Res. 2017;2017:8193932. doi: 10.1155/2017/8193932.
- Haggadone MD, Grailer JJ, Fattahi F, et al. Bidirectional crosstalk between C5a receptors and the NLRP3 inflammasome in macrophages and monocytes. Mediators Inflamm. 2016;2016:1340156. doi: 10.1155/2016/1340156.
