Abstract
Anti-nuclear matrix protein 2 (NXP2) antibody-positive dermatomyositis (DM) is characterized by extensive and severe myositis. In this study, we evaluated which cytokines/chemokines involved with the activity of the myositis. We performed quantitative immunoassays using the MILLIPLEX® Multiplex Assays Using Luminex to evaluate serum levels of interferon-γ, interleukin (IL)-1β, IL-6, IL-8, IL-12p40, and tumor necrosis factor-α in samples collected over time from a 9-year-old female with anti-NXP2 antibody-positive DM. In our case, the serum level of IL-8 was elevated when the myositis worsened, and decreased in accordance with the improvement of myositis, suggesting that the serum IL-8 levels were correlated with the myositis activity. Serum levels of IL-8 in samples from five patients with anti-NXP2 antibody-positive DM and five patients with anti-transcriptional intermediary factor 1γ (TIF1γ) antibody-positive DM without both interstitial lung disease (ILD) and malignancy before starting treatments, along with five healthy controls, were also evaluate by an enzyme-linked immunosorbent assay. Serum IL-8 levels were significantly elevated in anti-NXP2 or anti-TIF1γ antibody-positive DM patients with myositis but not ILD, than healthy controls. It was suggested that serum levels of IL-8 correlate with the activity of myositis in DM including anti-NXP2 antibody-positive DM.
1. Introduction
Dermatomyositis (DM), an idiopathic inflammatory myopathy (IIM), is characterized by myositis with cutaneous manifestations and/or interstitial lung disease (ILD) [Citation1], in which myositis-specific autoantibodies (MSAs) have been identified [Citation2]. Anti-nuclear matrix protein 2 (NXP2) antibody is an MSA detected in juvenile and adult IIMs, which we have reported is characterized by extensive muscular involvement with or without typical rash [Citation3].
It has been reported that serum levels of interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α correlated positively with changes in global, muscle, and extramuscular visual analog scale scores in juvenile and adult DM [Citation4]. A multivariate analysis revealed that IL-8 was the cytokine most significantly associated with anti-melanoma differentiation-associated gene 5 (MDA5) antibody-associated ILD, moreover, serum IL-6, IL-8, TNF-α, and CXCL10 correlate with disease activity in IIMs with anti- transcriptional intermediary factor 1γ (TIF1γ), anti-MDA5, anti-Mi-2β, and anti-signal recognition particle antibodies [Citation5]. Actually, another recent study indicated that serum IL-8 level was related with the disease activity of clinically amyopathic DM-associated ILD, which might be also associated to anti-MDA5 antibody [Citation6]. A previous case report of anti-NXP2-positive juvenile DM revealed the serum cytokine profile at the disease onset showing a CXCL10-dominant pattern [Citation7]. In this study, we evaluate which cytokines/chemokines are associated with the activity of myositis in patients with anti-NXP2 antibody-positive DM without ILD.
2. Case presentation
We prospectively observed a 9-year-old female, who was diagnosed with juvenile DM resulting in muscle weakness predominantly in the proximal muscles (manual muscle testing [MMT] of the deltoid, biceps, and iliopsoas, 6, 6, and 6, respectively), which was confirmed by muscle biopsy of the rectus femoris. The patient also presented with heliotrope rash, Gottron’s papules, and prominent pitting oedema of the face and limbs. Computed tomography showed calcification in the spinal erector muscle, but not ILD. Laboratory examinations revealed elevated serum levels of creatine kinase (CK, 19443 IU/L; normal, 46-230 IU/L) and aldolase (92.7 IU/L; normal, ≤ 6.0 IU/L). Anti-TIF1γ, anti-MDA5, anti-Mi-2β, and anti-aminoacyl tRNA synthetase antibodies were found to be negative by enzyme-linked immunosorbent assay (MESACUP™, Medial & Biological Laboratories, Tokyo, Japan), and positive for anti-NXP2 antibody by immunoprecipitation and western blotting [Citation3], and a line-blot assay (EUROLINE™ Autoimmune Inflammatory Myopathies 16 Ag (IgG), Euroimmun, Lübeck, Germany).
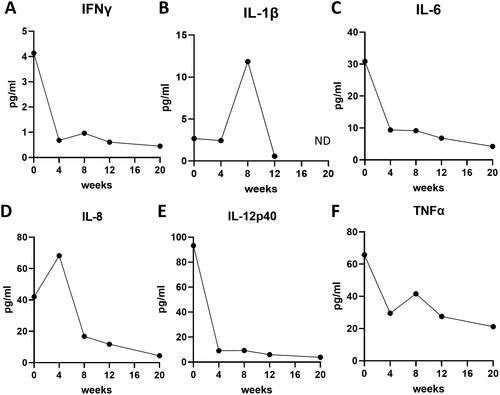
The patient was treated with oral prednisolone (30 mg/day) and methotrexate (6 mg/week, ), resulting in improvement of her rash (), but not her muscle symptoms, as indicated by high serum CK level (3616 U/L; ) and the development of dysphagia 4 weeks after initiation of the treatments (). Magnetic resonance imaging (MRI) revealed that myositis was still active in her proximal muscles of the upper limb, thigh, and gluteus muscles, and MMT for the deltoid, biceps, and iliopsoas worsened to 4, 7, and 4, respectively (). Subsequent examination of her serum cytokine levels, assessed by quantitative immunoassays, revealed that her initial serum levels of interferon (IFN)-γ, IL-1β, IL-6, IL-8, IL-12p40, and TNF-α were high (4.13 pg/mL, 2.67 pg/mL, 30.9 pg/mL, 42.0 pg/mL, 93.2 pg/mL, and 65.7 pg/mL, respectively; ). IFN-γ, IL-6, IL-12p40, and TNF-α serum levels quickly decreased in the 4 weeks after the treatment initiation (), while serum IL-8 level remained elevated (68.2 pg/mL; ). Methylprednisolone pulse therapy (900 mg/day for three days), azathioprine, and intravenous immunoglobulin were then administered and the doses of oral prednisolone and methotrexate were increased to 50 mg/day and 12 mg/week (). Her dysphagia and her muscle weakness gradually diminished (MMT for the deltoid, biceps, and iliopsoas were 10, 6, and 5, respectively; ), and her serum CK level decreased (39 U/L) 8 weeks after the treatment initiation (). Among the serum cytokines we measured, serum IL-8 also decreased in accordance with the improvement of myositis (). Oral prednisolone and methotrexate were tapered with no recurrence (), and MMT became almost full 20 weeks after the treatment initiation (). MRI revealed improvement of myositis in proximal muscles of the upper limb, thigh, and gluteus muscles. Low serum IL-8 levels were maintained through the inactive phase (), while serum levels of IFN-γ, IL-1β, and TNF-α were temporally elevated and did not correlate with the disease course (). She did not have any complications other than myositis and cutaneous manifestation, including arthritis, ILD, and heart and gut disorders, throughout the study period.
Figure 1. Clinical course of treatment and disease activity in the juvenile patient with anti-nuclear matrix protein 2 (NXP2) antibody-positive dermatomyositis (DM). (A–D) treatment course and dysphagia (a), and transitions in cutaneous dermatomyositis disease area and severity index (CDASI, B), the serum creatine kinase (CK) level (C), and manual muscle testing (MMT) of deltoid, biceps, and iliopsoas (D). IVMP: intravenous methylprednisolone pulse therapy; MTX: methotrexate; AZP: azathioprine; IVIG: intravenous immunoglobulin.
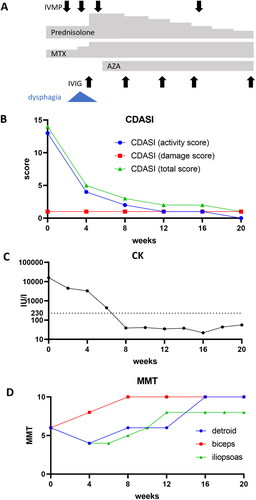
Figure 2. The serum levels of interleukin (IL)-8 levels correlate with the activity of myositis in the juvenile patient with anti-NXP2 antibody-positive DM. (A–F) Serum levels of interferon (IFN)-γ (a), IL-1β (B), IL-6 (C), IL-8 (D), IL-12p40 (E), tumor necrosis factor (TNF)-α (F). Time points are before treatment (0), and 4, 8, 12, 20 weeks after the initiation of treatment. ND: not detected.
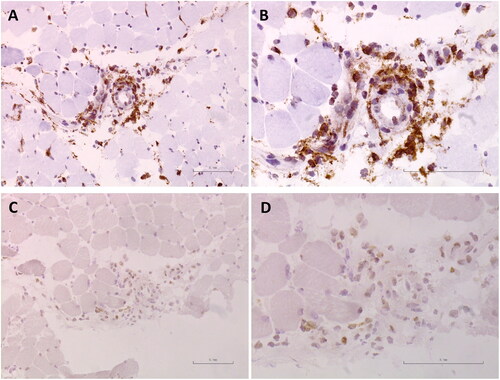
Immunohistochemical (IHC) staining were performed to determine the location of IL-8 expression in muscle tissue. Frozen section (6 µm) from her muscle biopsy of the rectus femoris was stained with rabbit anti-human IL-8 antibodies (Product number: ab106350, dilution 1:1000; Abcam) and anti-CD68 antibodies (Product number: # 76437, dilution 1:400; Cell Signaling Technology). Non-specific staining was blocked with 5% bovine serum albumin. Bound antibodies were visualized with peroxidase-labeled anti-rabbit/mouse IgG antibody (Envision™+ Dual Link System-HRP, Dako, Carpinteria, CA), and associated substrates (Liquid DAB + Substrate Chromogen System, Dako). Her muscle biopsy showed that inflammatory cells, almost of which expressed CD68 (), infiltrated around the blood vessels, and expressed IL-8 ().
3. Serum IL-8 levels were elevated in anti-NXP2 and anti-TIF1γ antibody-positive DM
Based on the above analysis, serum levels of IL-8 in five patients with anti-NXP2 antibody-positive DM, five age-matched patients with anti-TIF1γ antibody-positive DM without both ILD and malignancy, before starting any treatments (), and five age-matched healthy controls, were also evaluated by ELISA (LEGEND MAX™ Human IL-8 ELISA Kit 8, BioLegend, San Diego, CA). As shown in , serum IL-8 levels were significantly elevated in both anti-NXP2 antibody-positive DM and anti-TIF1γ antibody-positive DM, in which patients suffered from myositis and rash but not ILD or malignancy, compared to healthy controls (p = 0.0215 and 0.0265, respectively, by Kruskal-Wallis test with Dunn’s multiple comparisons test). Serum IL-8 levels were not significantly higher in anti-NXP2 or anti-TIF1γ antibody-positive DM patients with dysphagia compared to those without dysphagia (Supplementary Figure 1 (A,B)).
Figure 4. The serum levels of IL-8 in patients with anti-NXP2 and anti-transcriptional intermediary factor 1γ (TIF1γ) antibody-positive DM. The serum levels of IL-8 from patients with anti-NXP2 and anti-TIF1γ antibody-positive DM prior to treatment, along with age-matched five healthy controls. Bars represent medians. *p = 0.0215, **p = 0.0265 by Kruskal-Wallis test with Dunn’s multiple comparisons test.
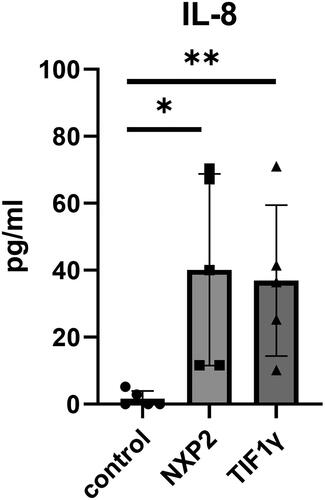
Table 1. Clinical features of cases of anti-NXP2 antibody-positive and anti-TIF1γ antibody-positive dermatomyositis.
4. Discussion
In our study, we identified serum levels of IL-8, but not IFN-γ, IL-1β, IL-6, IL-12p40, and TNF-α, were correlated to the activity of myositis in our juvenile female case of anti-NXP2 antibody-positive DM. While her serum levels of all cytokines were elevated before treatments, the serum levels of IFN-γ, IL-6, IL-12p40, and TNF-α were decreased after a 4-week immunosuppressive treatment period in spite of insufficient therapeutic effects for myositis. Her serum levels of IL-8 only reflected her high serum CK levels, muscle weakness and dysphagia, and low serum IL-8 levels were maintained through the inactive phase. Her cutaneous manifestations were improved quickly by 4 weeks after the treatment initiation while serum IL-8 level remained elevated, in other words, the serum IL-8 levels were not correlated to the activity of cutaneous manifestations. Moreover, elevated serum IL-8 levels were significantly elevated in both groups of anti-NXP2 antibody-positive DM and anti-TIF1γ antibody-positive DM, in which patients suffered from myositis and rash without other extramuscular manifestations.
It has been reported that serum levels of IL-8 were correlated positively with changes in global, muscle, and extramuscular visual analog scale scores in juvenile and adult DM4. Muscle strength loss was also reported to be associated with changes in serum IL-8 levels in inclusion body myositis patients [Citation8]. A previous report showed that CD40 expression on muscle cells was enhanced in polymyositis/DM, and that its ligand, CD40L, markedly increased the production of IFN-γ, IL-6, IL-8 and IL-15 from myoblasts [Citation9]. IL-6, IL-8 and IL-15 have been termed as ‘myokines’, which are directly produced from muscle tissue by exercise. However, IHC staining of the muscle biopsy from our case showed IL-8 expression on infiltrating inflammatory cells, not myocytes. Almost inflammatory cells infiltrating into her muscle tissue were CD68-positive macrophages producing IL-8. Because elevated serum levels of IL-8 were detected in DM cases with severe myositis without ILD/malignancy, IL-8 mainly from macrophages may enhance myositis. IL-8 is considered to act locally as a chemoattractive molecule of neutrophils and macrophages in an endocrine or paracrine fashion [Citation10]. Moreover, IL-8 is well-known to be a stimulator of angiogenesis via CXC receptor 1 and 2 expressed on microvascular endothelial cells [Citation11].
Both anti-TIF1γ-positive and anti-NXP2-positive DM patients more frequently develop dysphagia than DM patients with other MSAs [Citation12,Citation13]. In this study, there was no significant correlation between serum IL-8 levels and the presence of dysphagia in both groups of anti-NXP2 and anti-TIF1γ antibody-positive DM patients.
Our results suggest that serum levels of IL-8, which has been previously reported to be related to DM-associated ILD, correlate with the activity of myositis in DM including anti-NXP2 antibody-positive DM.
Ethical approval
This study is in compliance with the Helsinki Declaration. Informed consent was obtained from all participants. The Medical Ethics Committees of University of Tsukuba Hospital and Tokyo Medical and Dental University, Japan, approved this study (R02-323 and M2021-347, respectively).
NXP2_DM_IL8_IM_FigS1.tif
Download TIFF Image (355 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Callen JP. Dermatomyositis. Lancet. 2000;355(9197):53–57. doi:10.1016/S0140-6736(99)05157-0.
- Okiyama N. Clinical features and cutaneous manifestations of juvenile and adult patients of dermatomyositis associated with myositis-specific autoantibodies. J Clin Med. 2021;10(8), 1725.
- Ichimura Y, Konishi R, Shobo M, et al. Anti-nuclear matrix protein 2 antibody-positive inflammatory myopathies represent extensive myositis without dermatomyositis-specific rash. Rheumatology (Oxford); 2021;61(3):1222–1227.
- Reed AM, Peterson E, Bilgic H, et al. Changes in novel biomarkers of disease activity in juvenile and adult dermatomyositis are sensitive biomarkers of disease course. Arthritis Rheum. 2012;64(12):4078–4086. doi:10.1002/art.34659.
- Gono T, Kaneko H, Kawaguchi Y, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology (Oxford). 2014;53(12):2196–2203. doi:10.1093/rheumatology/keu258.
- Zou J, Chen J, Yan Q, et al. Serum IL8 and mRNA level of CD11b in circulating neutrophils are increased in clinically amyopathic dermatomyositis with active interstitial lung disease. Clin Rheumatol. 2016;35(1):117–125. doi:10.1007/s10067-015-3080-1.
- Nagamori T, Ishibazawa E, Yoshida Y, et al. A continuous increase in CXC-Motif chemokine ligand 10 in a case of anti-nuclear matrix protein-2-positive juvenile dermatomyositis. J Med Cases. 2022;13(6):290–296. doi:10.14740/jmc3940.
- Badrising UA, Tsonaka R, Hiller M, et al. Cytokine profiling of serum allows monitoring of disease progression in inclusion body myositis. J Neuromuscul Dis. 2017;4(4):327–335. doi:10.3233/JND-170234.
- Sugiura T, Kawaguchi Y, Harigai M, et al. Increased CD40 expression on muscle cells of polymyositis and dermatomyositis: role of CD40-CD40 ligand interaction in IL-6, IL-8, IL-15, and monocyte chemoattractant protein-1 production. J Immunol. 2000;164(12):6593–6600. doi:10.4049/jimmunol.164.12.6593.
- Nielsen S, Pedersen BK. Skeletal muscle as an immunogenic organ. Curr Opin Pharmacol. 2008;8(3):346–351. doi:10.1016/j.coph.2008.02.005.
- Norrby K. Interleukin-8 and de novo mammalian angiogenesis. Cell Prolif. 1996;29(6):315–323. doi:10.1111/j.1365-2184.1996.tb01583.x.
- Kotobuki Y, Tonomura K, Fujimoto M. Transcriptional intermediary factor 1 (TIF1) and anti-TIF1γ antibody-positive dermatomyositis. Immunol Med. 2021;44(1):23–29. doi:10.1080/25785826.2020.1791402.
- Ichimura Y, Konishi R, Shobo M, et al. Anti-nuclear matrix protein 2 antibody-positive inflammatory myopathies represent extensive myositis without dermatomyositis-specific rash. Rheumatology (Oxford). 2022;61(3):1222–1227. doi:10.1093/rheumatology/keab518.