Abstract
Neuromyelitis optica spectrum disorder (NMOSD) is a rare autoimmune inflammatory disease that can affect multiple generations and cause complications with long-term prednisolone treatment. This study aimed to evaluate the efficacy and safety of mycophenolate mofetil (MMF) in preventing NMOSD relapse while reducing prednisolone dosage. The trial involved nine patients with NMOSD who received MMF along with prednisolone dose reduction. MMF was effective in achieving prednisolone dose reduction without relapse in 77.8% of patients, with a significant decrease in mean annualized relapse rate. All adverse events were mild. The findings suggest that MMF could be a viable treatment option for middle-aged and older patients who require steroid reduction.
Clinical trial registration number: jRCT, jRCTs051180080. Registered February 27th, 2019-retrospectively registered, https://jrct.niph.go.jp/en-latest-detail/jRCTs051180080.
1. Introduction
Neuromyelitis optica spectrum disorder (NMOSD), which is characterized by severe optic neuritis and transverse myelitis, is a rare autoimmune inflammatory disease of the central nervous system. The estimated number of patients in Japan is approximately 4,000 [Citation1]. Most patients with NMOSD are women, with an older age of onset than that of multiple sclerosis (MS) [Citation2]. Since even a single attack can cause blindness or severe paraplegia, continuous treatment is needed to prevent relapse, even in older age. The core pathology is seropositivity for disease-specific autoantibody targeting aquaporin 4 (AQP4) in astrocytic foot processes, which is a distinguishing feature from MS [Citation3–5].
B-cell depletion, interleukin (IL)-6 receptor blocking, and complement-targeted therapies have all been successfully developed to treat NMOSD [Citation6–8]. However, no consensus has been reached on the treatment strategy for NMOSD, especially for middle-aged and older patients whose adverse events, including infection, may be more severe than those in young adults. In addition, cost-effectiveness, and different routes of administration of those novel treatments can also be concerning. Thus, conventional immunosuppressive therapy, including steroids, remains an important treatment option for NMOSD, but it is well known that long-term use of high-dose steroids can also cause a variety of risks in older patients, including osteoporosis and diabetes. Mycophenolate mofetil (MMF), as a non-steroidal immunosuppressive agent, has been reported to be effective for NMOSD, which may alleviate the adverse events associated with long-term administration of high-dose steroids. However, most studies have been retrospective [Citation9–17]. Only a few studies have prospectively implemented steroid reduction protocols, and the participants were relatively young (in their 30s and 40s) [Citation18,Citation19]. To date, no studies have been conducted in Japanese patients, and MMF is yet to be approved for NMOSD in Japan.
Therefore, in this study, we focused on MMF as a balanced drug, evaluated the efficacy and safety of MMF, and examined its use in steroid reduction. MMF is an antimetabolite that inhibits de novo synthesis of guanosine, a constituent of nucleic acids in T and B lymphocytes, thereby suppressing proliferation [Citation20]. The most common adverse events of MMF in NMOSD treatment were infections (33/594 patients) [Citation21], and elevated liver enzymes, diarrhea, anemia, leukopenia, and mild hair loss were also reported [Citation22].
2. Materials and methods
2.1. Study design and participants
This was a single-center, prospective, uncontrolled, open-label study. The diagnosis of NMOSD was based on the 2015 criteria [Citation23]. Patients with AQP4 antibody-positive NMOSD receiving oral prednisolone, aged 20–80 years, not receiving acute treatment for the active phase of NMOSD, with an Expanded Disability Status Scale score of ≤7.0, who provided written informed consent were enrolled. Patients with infectious diseases affecting their overall health, who were pregnant or lactating, with severe hepatic or renal dysfunction, and with a history of cancer within the past 5 years were excluded. The study was approved by the institutional ethics committee (No. C180054). The clinical trial registration number is jRCTs051180080.
2.2. Treatment protocol
The 52-week study period included a 4-week observation period and a 48-week MMF treatment period. Patients visited the hospital every 4–8 weeks. During the treatment period, patients received oral MMF (CellCept® Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) in the range of 500–1500 mg/day twice daily. MMF was initiated at a 1000 mg/day for all patients and allowed to increase or decrease based on the dose reduction/suspension criteria (Supplementary Information 1, Supplementary Table 1). If patients received other immunosuppressive drugs before the initiation of MMF, they were switched without a washout period. After enrollment and observation, the treatment protocol was initiated and repeated as one course every 4 weeks for 24 weeks, then one course every 8 weeks thereafter, until the dose reduction/suspension criteria were met. Patients received prednisolone at their current dose for the first two courses after the initiation of MMF, and subsequently received 90% of the previous dose of prednisolone per course. Steroid tapering may be suspended based on clinical judgment.
2.3. Outcomes
The primary endpoint was the achievement of a prednisolone dose reduction without clinical relapse according to the treatment protocol for 48 weeks after MMF initiation. We defined the achievement of a prednisolone dose reduction to less than half of the initial dose at MMF initiation. In addition, secondary endpoints included prednisolone dose reduction rate, annualized relapse rate (ARR), magnetic resonance imaging (MRI), EDSS scores, and neuro-ophthalmologic examination (e.g. retinal nerve fiber layer [RNFL] thickness obtained by Cirrus high-definition optical coherence tomography [OCT, Carl Zeiss Meditec, Dublin, CA, USA]). Furthermore, as exploratory endpoints, we assayed cognitive tests (Paced Auditory Serial Addition Test [PASAT] and Symbol Digit Modalities Test [SDMT]), patient-reported scales (Guy’s Neurological Disability Scale [GNDS] and vitality scale), serum anti-AQP4 antibody titer, and serum inflammatory cytokine (Interferon-gamma [IFNγ], IL-6, IL-10, and IL-17A) levels measured using LEGENDplex™ (Biolegend, San Diego, CA, USA). Complete blood counts, blood chemistry, and urinalysis were assayed as safety endpoints. Blood tests were performed on every visit. Urinalysis, MRI, cognitive tests, and neuro-ophthalmologic examinations were performed at three time points: before the initiation of MMF, and at weeks 24 and 48. Any unfavorable or unintended symptoms that occurred after the initiation of MMF were recorded as adverse events, regardless of their causal relationship to MMF. Adverse events were evaluated according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE version 4.0).
2.4. Statistical analysis
Relapse occurs approximately once in two years with conventional prednisolone monotherapy [Citation24]. Hence, the success rate with MMF co-administration was estimated to be 50%, assuming that 50% of the patients would relapse within a year. On the other hand, 1.5 relapses per year (100% relapse) were expected in the absence of all treatment [Citation24]. We set a 10% threshold because it would be significantly sufficient to demonstrate success in the disease that relapses if left untreated. Under the success rate of 50% and a threshold of 10%, the required number of cases was seven when analyzed using a one-sample test of proportion with 80% power and a 5% significance level. Considering dropouts, the target sample size was set at 10 participants.
A one-sample proportion test (two-tailed) of the null hypothesis that ‘the proportion of success in the primary endpoint is 10%’ was performed at the 5% significance level as the analysis of the primary endpoint. Proportion and 95% confidence intervals (CI) were also estimated. In addition, summary statistics (number of cases, mean, standard deviation [SD], median, minimum, and maximum values) for the secondary and exploratory endpoints, including prednisolone dose reduction rate, ARR, EDSS, neuro-ophthalmologic examination, cognitive tests, patient-reported scales, serum anti-AQP4 antibody titer, and serum inflammatory cytokine levels, were also calculated. The paired t-test and repeated measures (RM) one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test were used to evaluate changes during the observation period. All statistical analyses were conducted using R (version 2.7-1) and GraphPad Prism (version 9.3.1).
3. Results
3.1. Participant flow
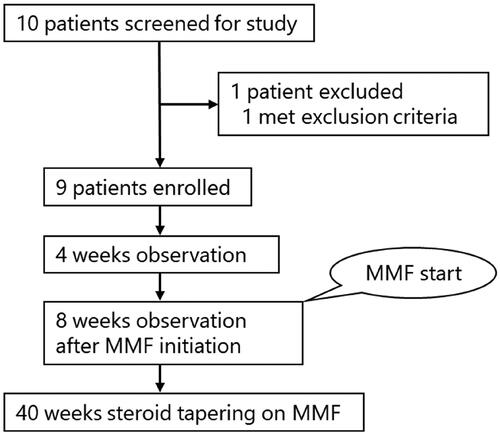
The participant flowchart for our clinical trial is shown in . Ten potentially eligible patients who visited Kobe University hospital between December 2018 and August 2020 were screened. One patient was excluded due to age exceeding the predetermined range, and the remaining nine patients were enrolled. MMF was initiated after a 4-week observation period, and prednisolone was tapered according to the treatment protocol.
3.2. Baseline characteristics
The demographic and clinical characteristics of the nine enrolled patients are shown in . The mean age was 56.4 (SD 8.92) years, with the mean age of onset of 51.5 (SD 9.39) years. All the patients were women. The mean disease duration was 59.3 (SD 51.6) months, annualized number of attacks in the last 2 years was 1.44 (SD 1.25), and the ARR (including first attacks) was 0.72 (SD 0.62). Furthermore, the mean number of attacks over the entire disease course was 3.88 (SD 3.11). At enrollment, three patients received 5–10 mg/day of prednisolone, whereas the remaining six received 11–20 mg/day. The mean duration of previous prednisolone treatment was 34.9 (SD 39.7) months. Three patients had received azathioprine (AZA), which was switched to MMF directly according to the treatment protocol described above. The mean EDSS score was 2.94 (SD 2.07).
Table 1. Baseline demographic and clinical characteristics of patients.
3.3. Primary outcome
Regarding the primary endpoint, seven of the nine patients were achieved of a prednisolone dose reduction without clinical relapse (77.8%, 95% CI 40–97%). The p-value of the one proportion test for exceeding the established 10% threshold was less than 0.0001. The dose of prednisolone administered to each patient is shown in Supplementary Information 2 (Supplementary Figure 1). Patient 5 (Pt.5) relapsed with nystagmus and taste disorder, with new lesions in the medulla oblongata on MRI under 14 mg/day prednisolone, 15 weeks after MMF initiation. Patient 9 (Pt.9) relapsed with bilateral weakness of the lower extremities under 4 mg/day of prednisolone, 40 weeks after MMF initiation, with transverse myelitis at Th1–4 evident on MRI.
3.4. Secondary and exploratory outcome
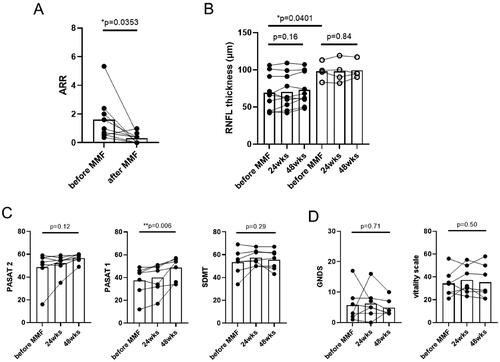
The frequency of the attacks for each patient is shown in . During the observation period, the mean ARR for all enrolled patients decreased from 0.72 to 0.22 (p < 0.05, paired t-test) ( and Citation3(A)). Conversely, the mean dose of prednisolone was reduced from 13.3 mg/day to 5.00 mg/day (Supplementary Information 2, Supplementary Figure 1). In the seven patients who achieved the primary endpoint, no new lesions were found on follow-up MRI. In addition, the mean EDSS score, which ranged from 2.43 to 2.35, did not worsen (p = 0.36, paired t-test, data not shown). Regarding neuro-ophthalmological examination, OCT revealed that the average RNFL thickness was lower in eyes with a history of optic neuritis than in those without a history of optic neuritis before the initiation of MMF; however, no change was observed during the treatment period (). Regarding cognitive tests, PASAT1 scores showed greater improvement (p < 0.01, RM one-way ANOVA with Tukey’s multiple comparison test, ), whereas PASAT2 and SDMT scores showed less changes. Patient-reported scales, including GNDS (range, 0–60; where higher scores indicate more severe neurological disabilities) [Citation25], and the vitality scale (range, 0–99; where higher scores indicate greater depression), remained unchanged (). Furthermore, the serum anti-AQP4 antibody titers showed less change (data not shown). Serum cytokine levels measured by cytometric bead array showed that IFNγ and IL-17A tended to decrease after MMF initiation. (Supplementary Information 3, Supplementary Figure 2).
Figure 2. Long-term attacks with NMOSD before and after MMF. Frequency of attacks over the entire evaluable period in nine patients with NMOSD. The mean ARR decreased from 0.72 to 0.22 (p < 0.05, paired t-test). ARR: annualized relapse rate; MMF: mycophenolate mofetil; NMOSD: neuromyelitis optica spectrum disorder.
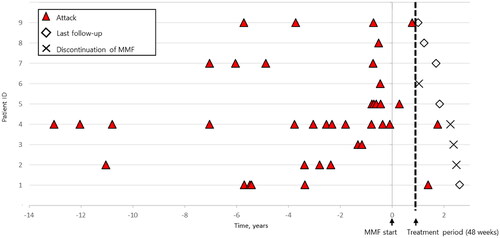
Figure 3. Secondary and exploratory outcomes. (A) ARR. Over the entire period, a reduction in ARR was observed in nine patients after MMF initiation (p < 0.05, paired t-test). (B) Optic coherence tomography findings. Patients (n = 7) who achieved the primary endpoint were analyzed separately for eyes with a history of optic neuritis (filled symbols, n = 10) and eyes without a history of optic neuritis (open symbols, n = 4). There was marked RNFL thinning in eyes with a history of optic neuritis compared to eyes without (p < 0.05, student’s t-test). However, no change was observed in either eye during the MMF treatment (RM one-way ANOVA with Tukey’s multiple comparisons test). (C) Cognitive test scores. PASAT1 scores showed greater improvement after MMF initiation, whereas PASAT2 and SDMT scores did not. (D) Patient-reported scales. GNDS and vitality scale scores remained unchanged. (C) and (D) were analyzed using RM one-way ANOVA with Tukey’s multiple comparison test. MMF: mycophenolate mofetil; ARR: annualized relapse rate; RNFL: retinal nerve fiber layer; PASAT: Paced Auditory Serial Addition Test; SDMT: Symbol Digit Modalities Test; GNDS: Guy’s Neurological Disability Scale.
3.5. Safety outcome
Complete blood counts, blood chemistry, and urinalysis revealed no abnormal findings. shows the adverse events reported during the treatment protocol period. Six of the nine patients enrolled in this study experienced one or more adverse events. Overall, ten events, including upper respiratory tract infections (n = 2), were reported. All adverse events were mild and equivalent to Grade 1 according to the CTCAE. None of the patients discontinued MMF due to adverse events, suggesting that MMF is well-tolerated.
Table 2. Adverse events after MMF initiation.
4. Discussion
This single-center, open-label, prospective cohort study demonstrated that MMF effectively prevents NMOSD relapse and may safely enable steroid reduction. It is notable that 77.8% of enrolled patients achieved the primary endpoint for treatment protocol including MMF administration and prednisolone dose reduction. Although the 95% CI (40–97%) was wide due to the limited sample size, it clearly exceeded the 10% threshold. In addition, the patients with NMOSD in our study were older than those reported in a recent study [Citation21], and their risk of osteoporosis and subsequent bone fractures would increase due to long-term steroid administration.
The efficacy of MMF in the treatment of NMOSD has been reported in several studies, including one by Jacob et al. in 2009 [Citation10]. However, most studies were retrospective (Supplementary Information 4, Supplementary Table 2) [Citation21,Citation26], and no study has aimed to evaluate the possibility of steroid reduction under MMF. Oral prednisolone is frequently used along with other immunosuppressive drugs, including MMF, AZA, tacrolimus, and rituximab (RTX), not only for NMOSD but also for other autoimmune diseases. A retrospective study conducted by Mealy et al. demonstrated that RTX and MMF were superior to AZA in reducing ARR [Citation13]. Although monoclonal antibodies are reported to be more effective than MMF [Citation27], MMF is well tolerated due to its less frequent adverse events, including elevated liver enzymes/hepatotoxicity, leukopenia, and hair loss, compared to AZA [Citation28], indicating its applicability for older patients. In addition, according to a report using a network meta-analysis, MMF has a lower incidence of leukopenia than tacrolimus and showed the lowest incidence of total adverse events followed by RTX among drugs including recently approved monoclonal antibodies [Citation29]. However, it is important to note that, similar to AZA, MMF for immunosuppression increases the risk of developing cancer [Citation22,Citation30]. In a study in Thailand, MMF and RTX showed better cost-effectiveness for patients with NMOSD [Citation31], suggesting their benefits compared to novel monoclonal antibodies. Taken together, MMF might be an alternate option for patients who have difficulty using AZA (e.g. genetic polymorphisms) [Citation32]. Additionally, after some periods of using potent monoclonal antibodies to suppress disease activity, switching to MMF may also be a useful strategy for long-term treatment.
Previous studies reported a decrease in the ARR with the initiation of MMF, consistent with our findings. Our cohort had a lower baseline ARR (0.72) than those reported previously, suggesting that this study included patients with less disease activity. As NMOSD is a chronic disease, EDSS scores may reflect residual sequelae from previous attacks, and the patients’ scores did not change during MMF treatment in this study. Although there have been reports of EDSS scores improving after MMF initiation [Citation9,Citation18,Citation33], it should be noted that those studies may have included patients still affected by their most recent attacks, and that their EDSS scores before MMF initiation may have been overestimated. Recently, several studies on OCT findings in NMOSD have been reported, including comparisons with MS (e.g. changes in RNFL) [Citation34–36]. Our study did not show any remarkable changes on RNFL thickness over the treatment protocol period, and long-term follow-up is needed. In this study, PASAT1 scores improved after MMF initiation; however, to our knowledge, no report has shown that MMF improves cognitive function, which might be a secondary effect of prednisolone dose reduction. In addition, despite conducting the cognitive tests six months apart to minimize the effects of repeated application, we could not completely rule out the practice effects [Citation37]. In this study, serum cytokine levels were measured to assess the immune backgrounds of the treated patients. Although no significant change was observed, probably due to the small sample size, a trend of reduction in pro-inflammatory cytokines after MMF initiation was observed that corresponded to the reduction in the ARR.
In practice, each clinician makes individualized and integrated decisions regarding each patient’s long-term treatment plan. The practice involves complex decision-making (e.g. steroid reduction rate) considering various parameters, including disease activity (based on the frequency of relapses), the severity of neurological disabilities, imaging findings, comorbidities, and background lifestyle. Recently, a study showed that the risk of relapse is higher in patients with NMOSD who are in the cluster phase [Citation38]; the steroid dose may not be reduced in some cases due to several clinical issues. We believe that the strength of our study is that the primary endpoint was achieved, even with a protocol aimed at steroid reduction in all patients. A few prospective studies have implemented steroid reduction in their protocols [Citation18,Citation19]; however, their protocols for MMF initiation with high-dose oral prednisolone (1 mg/kg/day) were not intended to target steroid reduction.
This study has several limitations. First, it was a single-center, single-arm study with a small sample size. However, we believe that the prospective design and analysis of a one-sample proportion test demonstrated the efficacy of MMF for steroid reduction. It is controversial whether a randomized controlled trial is necessary for MMF, which has been on the market for decades and is widely used [Citation39]. Second, long-term evaluation of each parameter was challenging in our 48-week trial period but would be preferable in future studies.
5. Conclusions
We provide class IV evidence that MMF is effective for relapse prevention and enables steroid reduction in middle-aged and older patients with NMOSD without marked adverse events. Further studies are needed to determine how pathophysiological parameters of NMOSD change over long-term treatment with MMF.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Kobe University Hospital (No.C180054). Written informed consent was obtained from all patients prior to inclusion in the study.
Author contributions
NC and TO planed and designed the trial. RA, NC, SK, SM, TK and MN acquired the clinical data. HT, YO, TU, TO, KS and RM supervised the study. RA and NC analyzed the data, and drafted the manuscript. RA and NC contributed equally as co-first authors. All authors read and approved the final version of the manuscript.
Supplemental Material
Download PDF (602.9 KB)Acknowledgements
We would like to thank Clinical & Translational Research Center, Kobe University Hospital, Kobe, Japan for statistical advice and support for this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Miyamoto K. Epidemiology of multiple sclerosis and neuromyelitis optica. Nihon Rinsho. 2014;72(11):1903–1907.
- Papp V, Magyari M, Aktas O, et al. Worldwide incidence and prevalence of neuromyelitis optica: a systematic review. Neurology. 2021;96(2):59–77. doi: 10.1212/WNL.0000000000011153.
- Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74.
- Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X.
- Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477. doi: 10.1084/jem.20050304.
- Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352–1363. doi: 10.1016/S0140-6736(19)31817-3.
- Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-Positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614–625. doi: 10.1056/NEJMoa1900866.
- Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(22):2114–2124. doi: 10.1056/NEJMoa1901747.
- Huh SY, Kim SH, Hyun JW, et al. Mycophenolate mofetil in the treatment of neuromyelitis optica spectrum disorder. JAMA Neurol. 2014;71(11):1372–1378. doi: 10.1001/jamaneurol.2014.2057.
- Jacob A, Matiello M, Weinshenker BG, et al. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch Neurol. 2009;66(9):1128–1133. doi: 10.1001/archneurol.2009.175.
- Jeong IH, Park B, Kim SH, et al. Comparative analysis of treatment outcomes in patients with neuromyelitis optica spectrum disorder using multifaceted endpoints. Mult Scler. 2016;22(3):329–339. doi: 10.1177/1352458515587752.
- Jiao Y, Cui L, Zhang W, et al. Dose effects of mycophenolate mofetil in chinese patients with neuromyelitis optica spectrum disorders: a case series study. BMC Neurol. 2018;18(1):47. doi: 10.1186/s12883-018-1056-x.
- Mealy MA, Wingerchuk DM, Palace J, et al. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol. 2014;71(3):324–330. doi: 10.1001/jamaneurol.2013.5699.
- Montcuquet A, Collongues N, Papeix C, et al. Effectiveness of mycophenolate mofetil as first-line therapy in AQP4-IgG, MOG-IgG, and seronegative neuromyelitis optica spectrum disorders. Mult Scler. 2017;23(10):1377–1384. doi: 10.1177/1352458516678474.
- Torres J, Pruitt A, Balcer L, et al. Analysis of the treatment of neuromyelitis optica. J Neurol Sci. 2015;351(1-2):31–35. doi: 10.1016/j.jns.2015.02.012.
- Yang Y, Chen L, Wu L, et al. Effective rituximab treatment in patients with neuromyelitis optica spectrum disorders compared with azathioprine and mycophenolate. Neurol Ther. 2022;11(1):137–149. doi: 10.1007/s40120-021-00298-5.
- Zhang L, Tian J, Dong X, et al. Efficacy of azathioprine, mycophenolate mofetil, and rituximab in the treatment of neuromyelitis optica spectrum disorder and analysis of prognostic factors. Neurol Sci. 2022;43(4):2651–2658. doi: 10.1007/s10072-021-05609-0.
- Xu Y, Wang Q, Ren HT, et al. Comparison of efficacy and tolerability of azathioprine, mycophenolate mofetil, and cyclophosphamide among patients with neuromyelitis optica spectrum disorder: a prospective cohort study. J Neurol Sci. 2016;370:224–228. doi: 10.1016/j.jns.2016.09.035.
- Yang Y, Wang CJ, Wang BJ, et al. Comparison of efficacy and tolerability of azathioprine, mycophenolate mofetil, and lower dosages of rituximab among patients with neuromyelitis optica spectrum disorder. J Neurol Sci. 2018;385:192–197. doi: 10.1016/j.jns.2017.12.034.
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2-3):85–118. doi: 10.1016/s0162-3109(00)00188-0.
- Songwisit S, Kosiyakul P, Jitprapaikulsan J, et al. Efficacy and safety of mycophenolate mofetil therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. Sci Rep. 2020;10(1):16727. doi: 10.1038/s41598-020-73882-8.
- Huang Q, Wang J, Zhou Y, et al. Low-Dose mycophenolate mofetil for treatment of neuromyelitis optica spectrum disorders: a prospective multicenter study in South China. Front Immunol. 2018;9:2066. doi: 10.3389/fimmu.2018.02066.
- Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729.
- Watanabe S, Misu T, Miyazawa I, et al. Low-dose corticosteroids reduce relapses in neuromyelitis optica: a retrospective analysis. Mult Scler. 2007;13(8):968–974. doi: 10.1177/1352458507077189.
- Sharrack B, Hughes RA. The guy’s neurological disability scale (GNDS): a new disability measure for multiple sclerosis. Mult Scler. 1999;5(4):223–233. doi: 10.1177/135245859900500406.
- Wang Y, Ma J, Chang H, et al. Efficacy of mycophenolate mofetil in the treatment of neuromyelitis optica spectrum disorders: an update systematic review and meta -analysis. Mult Scler Relat Disord. 2021;55:103181. doi: 10.1016/j.msard.2021.103181.
- Luo J, Yu J, Sui Z, et al. Comparison on the effect of seven drugs to prevent relapses of neuromyelitis optica spectrum disorders: a modeling analysis of literature aggregate data. Int Immunopharmacol. 2022;110:109004. doi: 10.1016/j.intimp.2022.109004.
- Espiritu AI, Pasco PMD. Efficacy and tolerability of azathioprine for neuromyelitis optica spectrum disorder: a systematic review and meta-analysis. Mult Scler Relat Disord. 2019;33:22–32. doi: 10.1016/j.msard.2019.05.011.
- Yin Z, Qiu Y, Duan A, et al. Different monoclonal antibodies and immunosuppressants administration in patients with neuromyelitis optica spectrum disorder: a bayesian network meta-analysis. J Neurol. 2023;270(6):2950–2963. doi: 10.1007/s00415-023-11641-1.
- Poupart J, Giovannelli J, Deschamps R, et al. Evaluation of efficacy and tolerability of first-line therapies in NMOSD. Neurology. 2020;94(15):e1645–e56. doi: 10.1212/WNL.0000000000009245.
- Aungsumart S, Apiwattanakul M. Cost effectiveness of rituximab and mycophenolate mofetil for neuromyelitis optica spectrum disorder in Thailand: economic evaluation and budget impact analysis. PLOS One. 2020;15(2):e0229028. doi: 10.1371/journal.pone.0229028.
- Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46(9):1017–1020. doi: 10.1038/ng.3060.
- Chen H, Zhang Y, Shi Z, et al. The efficacy and tolerability of mycophenolate mofetil in treating neuromyelitis optica and neuromyelitis optica spectrum disorder in Western China. Clin Neuropharmacol. 2016;39(2):81–87. doi: 10.1097/WNF.0000000000000131.
- Lu A, Zimmermann HG, Specovius S, et al. Astrocytic outer retinal layer thinning is not a feature in AQP4-IgG seropositive neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2022;93(2):188–195. doi: 10.1136/jnnp-2021-327412.
- Oertel FC, Specovius S, Zimmermann HG, et al. Retinal optical coherence tomography in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1068. doi: 10.1212/NXI.0000000000001068.
- Pisa M, Ratti F, Vabanesi M, et al. Subclinical neurodegeneration in multiple sclerosis and neuromyelitis optica spectrum disorder revealed by optical coherence tomography. Mult Scler. 2020;26(10):1197–1206. doi: 10.1177/1352458519861603.
- Castrogiovanni N, Mostert J, Repovic P, et al. Longitudinal changes in cognitive test scores in patients with Relapsing-Remitting multiple sclerosis: an analysis of the DECIDE dataset. Neurology. 2023;101(1):e1–e11. doi: 10.1212/WNL.0000000000207301.
- Akaishi T, Nakashima I, Takahashi T, et al. Neuromyelitis optica spectrum disorders with unevenly clustered attack occurrence. Neurol Neuroimmunol Neuroinflamm. 2020;7(1):e640. doi: 10.1212/NXI.0000000000000640.
- Cree B. Mycophenolate mofetil to treat neuromyelitis optica: is it time for a randomized trial? JAMA Neurol. 2014;71(11):1354–1357. doi: 10.1001/jamaneurol.2014.2359.