Abstract
This study investigated the clinical features and prognostic relevance of decreased serum complement levels in patients with idiopathic inflammatory myositis (IIM). The clinical information of IIM patients with less than normal serum complement levels (L-Com) and that of those with normal serum complement levels (N-Com) was compared. In patients with interstitial lung disease (ILD), regression analyses were used to investigate the implication of L-Com in their PaO2/FiO2 (P/F) ratio. Prognostic outcomes of ILD were evaluated using the log-rank test. Of 94 IIM patients, 26 with L-Com (median age, 56.0 years) and 68 with N-Com (56.5 years) were included. The prevalence of women was significantly higher in patients with L-Com (92.3%) than in those with N-Com (67.6%). ILD was observed in 17 (65.4%) patients with L-Com and in 46 (67.6%) with N-Com. Among patients with ILD, the P/F ratio was significantly lower in those with L-Com than in those with N-Com. Serum C3 levels were correlated with decreased P/F ratio. Inferior prognosis of ILD was significantly demonstrated in patients with L-Com, especially in those positive for anti-melanoma differentiation-associated protein 5 antibody. L-Com may be implicated in reduced arterial oxygen levels and a poorer prognosis in patients with IIM-related ILD.
1. Introduction
Idiopathic inflammatory myopathies (IIM) are heterogeneous autoimmune diseases. These disease present with inflamed skeletal muscles with or without typical cutaneous eruptions and ulcers, leading to the classifications of dermatomyositis (DM), polymyositis (PM), clinically amyopathic DM (CADM), and inclusion body myositis [Citation1]. Meanwhile, IIM includes aspects of various systemic disorders, such as articular, gastrointestinal, and cardiac involvements. Notably, interstitial lung disease (ILD) frequently develops in patients with IIM; its comorbidity robustly impacts disease prognosis [Citation2–4]. Clinical phenotypes of IIM have been recently classified based on myositis-specific antibodies (MSA). These phenotypes, alongside some clinical evaluations such as respiratory function, pathological findings of skeletal muscle, and laboratory biomarkers, may provide practical knowledge about the clinical features and prognosis of IIM-related diseases [Citation4]. The presence of anti-melanoma differentiation-associated protein 5 (MDA-5) antibody has been linked to rapidly progressive ILD (RP-ILD), which may be life-threatening in patients with CADM [Citation5, Citation6]. In contrast, the long-term clinical course of anti-aminoacyl-tRNA synthetase (ARS) antibody-associated ILD is slow but progressive [Citation6, Citation7]. Inclusion body myositis and immune-mediated necrotizing myopathy, which is relatively novel and distinctly categorized [Citation8], are definitively determined using a pathological approach to the affected muscle [Citation1, Citation9]. In the pathological aspects of DM, the arteriolar deposition of complement proteins including C3, C4, and C5b–9 membranolytic attack complex in the endomysial and perimysial areas, along with perifascicular atrophy and inflammatory cell infiltration in the perifascicular area, have been demonstrated as the typical pathological features [Citation10, Citation11]. These findings suggest that the complement pathway may also be involved in the pathogenesis of DM. However, previous studies on IIM revealed inconsistent results, showing contrasted decreasing and increasing serum complement levels [Citation12, Citation13]. To our knowledge, serum complement levels of IIM patients have not been specifically analyzed when evaluating disease activity or pathogenesis. Therefore, the effect of serum complement levels on the clinical features and prognosis of patients diagnosed with IIM remains uncertain.
In this study, we investigated the clinical features in IIM patients presenting with low serum complement levels. Further, we analyzed the implications of serum complement levels in the prognosis.
2. Methods
2.1. Patients and study design
This study was performed using the clinical information of patients who were diagnosed with DM, CADM, or PM between April 2011 and March 2021 in our hospital. The disease classification was determined in accordance with the 2017 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria for IIM [Citation1]. We retrospectively extracted the clinical information at the diagnosis from electronic clinical records and performed the analyses. This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and the ethical guidelines for epidemiological research of the Ministry of Health, Labour and Welfare of Japan. This study was approved by the local ethics committee of Shinshu University (approval number: 604/5786), and all participants provided written informed consent. A manual muscle test in eight bilateral muscles (MMT-8) [Citation14] was used for evaluating muscular weakness. The diagnosis of ILD was determined by the imaging results of chest computed tomography (CT) performed at the diagnosis of IIM in all enrolled patients. For evaluating the extent of ILD spread as per imaging scans, CT scores were calculated according to previous studied methods [Citation15, Citation16]. Patients were classified into two groups: (a) those with normal serum levels of C3, C4, and CH50 (N-Com) and (b) those with less than normal serum levels of C3, C4, and/or CH50 (L-Com). The serum levels of C3 and C4 were measured using immunonephelometry, and those of CH50 were measured using liposome immunoassay. L-Com was defined as follows: C3 < 86 mg/dL, C4 < 17 mg/dL, and/or CH50 < 30 U/mL. The serum levels of C5a were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (abcam, Cambridge, UK). The titers of anti-ARS antibody and anti-MDA5 antibody were measured using ELISA with the MESACUP tests (Medical & Biological Laboratories, Tokyo, Japan). Patients who met the 2017 EULAR/ACR criteria for IIM and had adequate serum complement data were included in this study. Meanwhile, patients who had infections and/or malignancies upon diagnosis of IIM were excluded from this study. Further, those who fulfilled the classification criteria of systemic lupus erythematosus (SLE) [Citation17–19] were also excluded. Lastly, those who had insufficient clinical information for the analyses were excluded from this study.
2.2. Objective outcome setting
The primary objective outcome was to investigate the clinical differences between patients with L-Com and those with N-Com. All enrolled patients as well as those with ILD were investigated; functional respiratory tests, including the PaO2/FiO2 (P/F) ratio, were conducted in patients with ILD. The secondary objective outcome was to determine the prognosis of patients with ILD. The endpoint was estimated when exacerbation and/or death ascribable to ILD progression were observed up to 24 months after initial treatment. Disease exacerbation was based as follows: (a) chest CT findings of ILD-associated deterioration, (b) progression of respiratory symptoms, for example, cough, dyspnea, and/or hypoxia, and (c) additional immunosuppressant therapy and/or increase in the dose of corticosteroids.
2.3. Statistical analyses
For estimating the distribution of the data, the Kolmogorov–Smirnov test was preliminarily performed. All data were presented as medians with interquartile ranges (IQR). p-values of <0.05 were defined as statistically significant. The Mann–Whitney U and Fisher’s exact probability test were used to compare two independent groups. Single and multiple regression analyses were used to investigate the implications of the confounders, including age, sex, L-Com, and anti-ARS and anti-MDA5 antibodies, in the P/F ratio. Spearman’s rank correlation coefficient test was performed to evaluate the association between serum complement levels and the P/F ratio. These antibodies are robustly associated with the respiratory pathogenesis of IIM-related ILD [Citation6, Citation20]. The Kaplan-Meier method was employed for establishing survival curves. The log-rank test was used for quantitative evaluation of the outcomes. Statistical analyses were performed using BellCurve for Excel (SSRI, Tokyo, Japan) and JMP software version 14.3.0 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Flow of patients
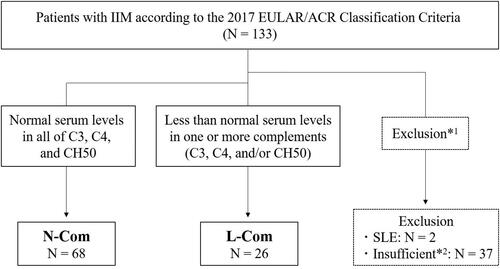
This study initially included 133 IIM patients with neither infections nor malignancies (). Two of these patients fulfilled the criteria of SLE. After excluding those with SLE and those who had insufficient clinical information, 94 patients were enrolled into the study and are as follows: 26 with L-Com (median age, 56.0 years) and 68 with N-Com (median age, 56.5 years).
Figure 1. Flow diagram of the enrolled patients. Of the patients who were classified in idiopathic inflammatory myositis (IIM), including dermatomyositis, clinically amyopathic dermatomyositis, or polymyositis, in accordance with the 2017 European League Against rheumatism/American College of Rheumatology (EULAR/ACR) Classification criteria upon initial diagnosis, patients with normal serum levels of C3, C4, and CH50 (N-com) and those with lower than normal serum levels of one or more complements in C3, C4, and/or CH50 (L-com) were classified. *1To exclude patients with co-existing infection and/or malignancy, those who fulfilled the prevalent criteria of systemic lupus erythematosus (SLE), and *2those who had insufficient clinical information for performing the analyses and for determining N-Com and L-Com.
3.2. Epidemiological and clinical differences based on serum complement levels among IIM patients
The frequency of women was significantly higher in patients with L-Com than in those with N-Com (p = 0.017), while other factors were not significantly different between the two groups (). All patients positive for anti-ARS and anti-MDA5 antibodies also presented with ILD. Serum levels of complements, including C3, C4, and CH50, were significantly lower in patients with L-Com than in those with N-Com (p < 0.0001). Decreased serum levels of C3 (< 86 mg/dL) and C4 (< 17 mg/dL) were observed in 19 and 12 patients, respectively, whereas patients with decreased serum levels of CH50 (< 30 U/mL) were not observed. Serum C5a levels were not significantly different between patients with L-Com and those with N-Com (median [IQR] 1.96 [1.42–1.98] vs. 1.97 [1.96–1.98] ng/mL, respectively; p = 0.102) (Supplementary Figure 1).
Table 1. Epidemiologic and clinical findings between L-Com and N-Com among all IIM patients.
3.3. Clinical features of IIM patients with ILD in accordance with their serum complement levels
ILD was observed in 63 (67.0%) patients, including 17 with L-Com and 46 with N-Com. Among them, the frequency of women was higher in patients with L-Com than in those with N-Com, despite being not significantly different (). The P/F ratio was significantly lower in patients with L-Com than in those with N-Com (p = 0.033). A significantly higher utilization of ventilator was also observed in patients with L-Com than in those with N-Com (p = 0.041). Furthermore, in patients positive for anti-MDA5 antibody, a significantly higher utilization of ventilator was observed in those with L-Com than in those with N-Com (p = 0.037) despite being not significantly different in patients positive for anti-ARS antibody (p = 0.236) (Supplementary Table 1 and 2). There were no significant differences in the initial immunosuppressive therapies between the two groups.
Table 2. Epidemiologic and clinical findings between L-Com and N-Com among IIM patients with ILD.
The relevant factors associated with the P/F ratio were analyzed using regression analyses; we analyzed the implication of L-Com along with the adjustment for potential confounding variables, including age, sex, anti-ARS and anti-MDA5 antibodies. In the single regression analyses, significant inverse association with P/F ratio was observed in the elderly age (β coefficient −0.30 [95% confidence interval, CI −2.89 to −0.26], p = 0.019) and L-Com (β coefficient −0.28 [95% CI −49.37 to −2.31], p = 0.032), whereas significant associations were not observed in the multiple regression analyses (). When the titers of anti-ARS and anti-MDA5 antibodies were used as the adjustment for potential confounding variables, the multiple regression analyses showed a significant inverse association with P/F ratio in the elderly age (β coefficient −0.30 [95% CI −2.88 to −0.27], p = 0.019) (Supplementary Table 3). Serum C3 levels were also significantly correlated with the P/F ratio (p = 0.015) ().
Table 3. Confounders affecting the P/F ratio in IIM patients with ILD.
Table 4. Correlations between serum complement levels and P/F ratio in IIM patients with ILD.
3.4. Prognostic outcomes of L-Com and N-Com in IIM patients with ILD
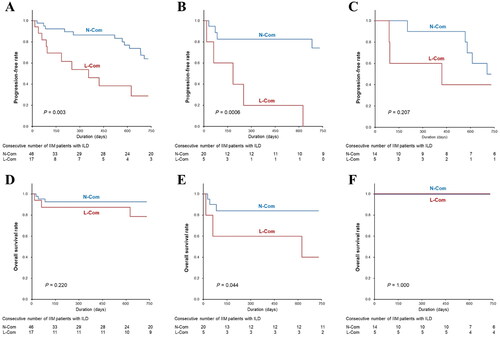
The prognosis ascribable to ILD exacerbation was evaluated in all patients with ILD. Among them, the progression-free rate was significantly lower in patients with L-Com than in those with N-Com (log-rank test, p = 0.003) (). Upon analyzing patients positive for anti-MDA5 antibody, significantly poorer prognosis was observed in those with L-Com than in those with N-Com (p = 0.0006) (). Upon analyzing patients positive for anti-ARS antibody, the progression-free rate was not significantly different between the two groups (p = 0.207) (). Of all patients with ILD, three (17.6%) with L-Com and three (6.5%) with N-Com died due to ILD exacerbation during the course of the study despite their frequencies being not significantly different, and their overall survival rate was not significantly different between the two groups (p = 0.220) (). All of the dead patients were positive for anti-MDA5 antibody, and overall survival rate in patients positive for anti-MDA5 antibody was significantly lower in those with L-Com than those with N-Com (p = 0.044) ().
Figure 2. Kaplan-Meier Survival analysis for evaluating outcome in patients with interstitial lung disease (ILD). The analyses were compared between patients with normal serum complement levels (N-com) and those with less than normal serum complement levels (L-com). The comparisons of progression-free rate were performed as follows: (A) in all patients with ILD, (B) in those positive for anti-MDA5 antibody, and (C) in those positive for anti-ARS antibody. The comparisons of overall survival rate were also performed as follows: (D) in all patients with ILD, (E) in those positive for anti-MDA5 antibody, and (F) in those positive for anti-ARS antibody.
Two patients with N-Com developed pneumocystis and cytomegalovirus pneumonia after IIM-related ILD exacerbation. One patient with N-Com presented with convulsion only upon initiating cyclosporin, whilst having no IIM-related ILD exacerbation throughout the observation period.
4. Discussion
To the best of our knowledge, this study is the first to focus on clinical features and prognostic outcomes of IIM patients and their association with reduced serum complement levels, although reduced serum complement levels have been described in some previous reports of IIM [Citation13, Citation21]. While IIM has been classified in 6.3% of patients with SLE [Citation22], we excluded patients with SLE from this study. Our study significantly demonstrated that female predominance, lower P/F ratios, and prognostic inferiority of ILD were associated with patients with L-Com when the clinical findings were compared to those with N-Com. In the physiologic immunities, sex-based differences in both females and males have been found to affect both innate and adaptive responses, which may be attributed to the impact of sex hormones and variations against self- or foreign-antigens [Citation23]. With regards to complement system, lower serum complement levels have been physiologically demonstrated in women than in men [Citation24, Citation25]. Moreover, the proportion of women was > 90% in IIM patients with L-Com; this may have been influenced by the aforementioned physiological feature of the complement system. On the other hand, in a Japanese epidemiologic study, the frequency of women among IIM patients was approximately 70% [Citation26]. Therefore, it is necessary to consider the influence of reduced serum complement levels on the clinical manifestations and immunopathological signals in IIM; in general, a stronger immune response has been observed in women than in men [Citation27].
Our results revealed a significantly decreased P/F ratio in patients with L-Com; the consumption of serum complements in developing ILD might be implicated in aggravating respiratory conditions. The complement system acts as a host defense mechanism against invading pathogens. Meanwhile, it indirectly stimulates both the innate and adaptive immune systems as an inflammatory mediator, which may in turn result in self-organ damage during infections, trauma, and autoimmune diseases [Citation28, Citation29]. The activation of complement has been shown to precipitate acute lung injury by inducing inflammation and cytokine storm [Citation30]. Additionally, focal activation and deposition of complement proteins contribute to vascular injury, which is pivotally implicated in the pathogenesis of DM as the C3 component plays a crucial role in early immune response [Citation20, Citation31]. In our study, serum C3 levels were correlated with the changes in P/F ratio, suggesting that reduced serum C3 levels may be attributed to the consumption of C3 by focal immune reaction in the lung tissue. The proteomic analyses using plasma demonstrated that the implication of the complement system in the pathogenic pathway was particularly observed in anti-MDA5 antibody-positive DM patients with RP-ILD compared to those without RP-ILD and healthy individuals [Citation32]. Moreover, stronger expression of C3 in the lung tissues was significantly observed in patients with ILD related to DM, particularly in those positive for anti-MDA5-antibody, than in patients with idiopathic pulmonary fibrosis [Citation33]. In addition, patients with L-Com revealed decreased serum levels of C3 or C4 despite the absence of decreased serum levels of CH50, which is a comprehensive test for screening the total activity of the classical complement pathway [Citation34]. The C3 activity is principally implicated in the alternative pathway; therefore, no impact of slightly decreased serum C3 levels can be observed on serum CH50 levels. Meanwhile, the status of common terminal complements, including C5 to C9, can pivotally affect serum levels of CH50 [Citation35], suggesting that slightly decreased serum C4 levels in the early cascade of the classical pathway have less impact on serum CH50 levels. Moreover, serum C5a levels were not significantly different between patients with L-Com and N-Com. Accordingly, the complement pathway may be partially implicated in the pathogenesis of IIM-ILD.
Hypocomplementemia is a common laboratory finding in SLE, which is ascribable to the focal visceral deposition of immune complexes including complement proteins and specific autoantibodies [Citation36, Citation37]. Reduced serum complement levels have been demonstrated in antineutrophil cytoplastic antibody-associated vasculitis [Citation38–42], which has been typically referred to as “pauci-immune.” This disease demonstrated the deposition of complement proteins along the vessel wall [Citation43], suggesting that reduced serum complement levels may be associated with inflammatory vasculopathy as the result of focal immune response. Single regression analyses revealed significant associations of L-Com and aging with decreased P/F ratio. Older age has been suggested as a predictive factor for the prognosis of IIM-related ILD [Citation20, Citation44]. However, the implication of L-Com in decreased P/F ratio could not be demonstrated after adjusting for age, sex, anti-ARS and anti-MDA5 antibodies. Thus, L-Com was not independently associated with reduced P/F ratio. Accordingly, complement activation was suggested to be partially implicated in the respiratory manifestations associated with IIM-related ILD.
An inferior prognosis associated with ILD was also significantly demonstrated in patients with L-Com, suggesting the implication of a reduced P/F ratio. Low arterial oxygenation upon diagnosis may be predictive of an unfavorable outcome in patients with IIM-related ILD [Citation44]. ILD has been found in > 70% of patients with IIM; notably, RP-ILD is frequently observed in patients positive for anti-MDA5 antibody [Citation4]. Further, of the patients positive for anti-MDA5 antibody in our study, those with L-Com showed a significantly poor prognosis associated with ILD than those with N-Com. Considering the innate immune reactions against exogenous antigens, the MDA5 protein participates in the activation of the type I interferon (IFN-I) pathway, as well as the production of proinflammatory cytokines, as the pattern recognition receptor, especially against viral double-strand RNA [Citation45]. It was suggested that persistent activation of MDA5/IFN-I pathway has been suggested to ultimately promote MDA5 overexpression, leading anti-MDA5 antibody production and immune complexation in anti-MDA5-positive DM-related ILD [Citation46]. Although the complement system and its association with the MDA5/IFN-I pathway remain elusive in the development of DM-related ILD, its role as an activator of early-phase innate immunity has been fundamentally established [Citation28]. Given that the MDA5/IFN-I and complement pathways are both activated during innate immune responses, our study suggests that reduced serum complement levels may be partially implicated in developing IIM-related ILD.
This study has some limitations. First, this was performed as a retrospective single-center study. Hence, we included only a small number of patients and their clinical information for the analyses, with missing values still being incorporated. In the multiple regression analyses, it was deemed appropriate to use additional potential confounders for larger patient numbers to retrieve more precise results associated with decreased P/F ratio. Furthermore, finding other predictive factors was ideal in analyzing the prognosis of ILD. Second, consecutive information about serum complements could not be enrolled in this study, because the serum complement levels of patients have not been explored throughout their clinical course after initiating treatment. Therefore, the alteration of serum complement levels in association with the clinical state of the disease should be investigated, in order to elucidate the definite implication of the complement system in the pathogenesis of IIM-related ILD.
In conclusion, our study analyzed the clinical features of IIM patients presenting with L-Com. Female predominance was significantly demonstrated in these patients. Of the patients with IIM-related ILD, reduced arterial oxygenation as well as an inferior prognosis were significantly observed in those with L-Com compared with those with N-Com. Notably, patients positive for anti-MDA5 antibody significantly demonstrated a poorer prognosis for ILD, especially in those with L-Com than with N-Com. In contrast, positive anti-ARS antibody led to no significant difference in ILD prognosis observed between those with L-Com and with N-Com. Therefore, this study suggests that IIM-related L-Com contributes to the pathogenesis of ILD by reducing arterial oxygenation, and may be predictive for the prognosis of ILD. Meanwhile, it is necessary to perform further analyses using larger patient numbers to elucidate more precise mechanisms underlying the implication of the complement system in the development of IIM-related ILD.
Authors’ Contributions
S-N, Y-SHI, D-K, T-I, A-M, and Y-SE were involved in the study design, developed the structure and argument for this study. S-N, Y-SHI, D-K, and T-I recruited clinical data. S-N and Y-SHI analyzed the obtained data, and S-N, Y-SHI, D-K, T-I, A-M, and Y-SE interpreted the analyzed data. Y-SHI prepared the draft of this manuscript, and contributed to revising the manuscript. All authors revised and approved of the final manuscript.
Supplementary Tables and Figure.docx
Download MS Word (72.6 KB)Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
The authors declare that they have no financial or personal conflicts of interest.
Data availability statement
The data for the analyses in this study are available upon reasonable request.
Additional information
Funding
References
- Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 2017;69(12):2271–2282. doi: 10.1002/art.40320.
- Wang H, Lv J, He J, et al. The prevalence and effects of treatments of rapidly progressive interstitial lung disease of dermatomyositis/polymyositis adults: a systematic review and meta-analysis. Autoimmun Rev. 2023;22(8):103335. doi: 10.1016/j.autrev.2023.103335.
- Fairley JL, Wicks I, Peters S, et al. Defining cardiac involvement in idiopathic inflammatory myopathies: a systematic review. Rheumatology (Oxford). 2021;61(1):103–120. doi: 10.1093/rheumatology/keab573.
- Lundberg IE, Fujimoto M, Vencovsky J, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers. 2021;7(1):86. doi: 10.1038/s41572-021-00321-x.
- Koga T, Fujikawa K, Horai Y, et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology (Oxford). 2012;51(7):1278–1284. doi: 10.1093/rheumatology/ker518.
- Sato S, Masui K, Nishina N, et al. Initial predictors of poor survival in myositis-associated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology (Oxford). 2018;57(7):1212–1221. doi: 10.1093/rheumatology/key060.
- Tanizawa K, Handa T, Nakashima R, et al. The long-term outcome of interstitial lung disease with anti-aminoacyl-tRNA synthetase antibodies. Respir Med. 2017;127:57–64. doi: 10.1016/j.rmed.2017.04.007.
- Day JA, Limaye V. Immune-mediated necrotising myopathy: a critical review of current concepts. Semin Arthritis Rheum. 2019;49(3):420–429. doi: 10.1016/j.semarthrit.2019.04.002.
- De Bleecker JL, De Paepe B, Aronica E, et al. 205th ENMC International Workshop: pathology diagnosis of idiopathic inflammatory myopathies part II 28-30 March 2014, Naarden, The Netherlands. Neuromuscul Disord. 2015;25(3):268–272. doi: 10.1016/j.nmd.2014.12.001.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971–982. doi: 10.1016/s0140-6736(03)14368-1.
- Dalakas MC. Review: an update on inflammatory and autoimmune myopathies. Neuropathol Appl Neurobiol. 2011;37(3):226–242. doi: 10.1111/j.1365-2990.2010.01153.x.
- Cavalli S, Lonati PA, Gerosa M, et al. Beyond systemic lupus erythematosus and anti-phospholipid syndrome: the relevance of complement from pathogenesis to pregnancy outcome in other systemic rheumatologic diseases. Front Pharmacol. 2022;13:841785. doi: 10.3389/fphar.2022.841785.
- Behan WM, Behan PO. Complement abnormalities in polymyositis. J Neurol Sci. 1977;34(2):241–246. doi: 10.1016/0022-510x(77)90072-7.
- Rider LG, Koziol D, Giannini EH, et al. Validation of manual muscle testing and a subset of eight muscles for adult and juvenile idiopathic inflammatory myopathies. Arthritis Care Res (Hoboken). 2010;62(4):465–472. doi: 10.1002/acr.20035.
- Shimojima Y, Ishii W, Matsuda M, et al. Coadministration of cyclosporin a with prednisolone in acute interstitial pneumonia complicating polymyositis/dermatomyositis. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:43–52. doi: 10.4137/cmamd.S9398.
- Shimojima Y, Ishii W, Matsuda M, et al. Effective use of calcineurin inhibitor in combination therapy for interstitial lung disease in patients with dermatomyositis and polymyositis. J Clin Rheumatol. 2017;23(2):87–93. doi: 10.1097/rhu.0000000000000487.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725–1725. doi: 10.1002/1529-0131(199709)40:9<1725::aid-art29>3.0.co;2-y.
- Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151–1159. doi: 10.1136/annrheumdis-2018-214819.
- Cao H, Huang J, Chang J, et al. Predictors of progression in idiopathic inflammatory myopathies with interstitial lung disease. J Transl Int Med. 2023;11(1):46–56. doi: 10.2478/jtim-2022-0029.
- Behan WMH, Barkas T, Behan PO. Detection of immune complexes in polymyositis. Acta Neurol Scand. 1982;65(4):320–334. doi: 10.1111/j.1600-0404.1982.tb03089.x.
- Bitencourt N, Solow EB, Wright T, et al. Inflammatory myositis in systemic lupus erythematosus. Lupus. 2020;29(7):776–781. doi: 10.1177/0961203320918021.
- Roved J, Westerdahl H, Hasselquist D. Sex differences in immune responses: hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav. 2017;88:95–105. doi: 10.1016/j.yhbeh.2016.11.017.
- Troldborg A, Hansen A, Hansen SW, et al. Lectin complement pathway proteins in healthy individuals. Clin Exp Immunol. 2017;188(1):138–147. doi: 10.1111/cei.12909.
- Gaya da Costa M, Poppelaars F, van Kooten C, et al. Age and sex-associated changes of complement activity and complement levels in a healthy Caucasian population. Front Immunol. 2018;9:2664. doi: 10.3389/fimmu.2018.02664.
- Tomimitsu H, Ohta A, Nagai M, et al. Epidemiologic analysis of the clinical features of Japanese patients with polymyositis and dermatomyositis. Mod Rheumatol. 2016;26(3):398–402. doi: 10.3109/14397595.2015.1091137.
- Giefing-Kröll C, Berger P, Lepperdinger G, et al. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–321. doi: 10.1111/acel.12326.
- Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. doi: 10.1038/cr.2009.139.
- Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol. 2014;32(1):433–459. doi: 10.1146/annurev-immunol-032713-120154.
- Bosmann M, Ward PA. Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv Exp Med Biol. 2012;946:147–159. doi: 10.1007/978-1-4614-0106-3_9.
- Dalakas MC. Mechanisms of disease: signaling pathways and immunobiology of inflammatory myopathies. Nat Clin Pract Rheumatol. 2006;2(4):219–227. doi: 10.1038/ncprheum0140.
- Qiu Y, Feng X, Liu C, et al. Proteomic profiling identifies SPP1 associated with rapidly progressive interstitial lung disease in anti-MDA5-positive dermatomyositis. Arthritis Res Ther. 2024;26(1):9. doi: 10.1186/s13075-023-03243-z.
- Zaizen Y, Okamoto M, Azuma K, et al. Enhanced immune complex formation in the lungs of patients with dermatomyositis. Respir Res. 2023;24(1):86. doi: 10.1186/s12931-023-02362-0.
- Prohászka Z, Kirschfink M, Frazer-Abel A. Complement analysis in the era of targeted therapeutics. Mol Immunol. 2018;102:84–88. doi: 10.1016/j.molimm.2018.06.001.
- Coss SL, Zhou D, Chua GT, et al. The complement system and human autoimmune diseases. J Autoimmun. 2023;137:102979. doi: 10.1016/j.jaut.2022.102979.
- Takamatsu R, Shimojima Y, Kishida D, et al. The impact of normal serum complement levels on the disease classification and clinical characteristics in systemic lupus erythematosus. Adv Rheumatol. 2022;62(1):49. doi: 10.1186/s42358-022-00283-y.
- Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. 2020;16(10):565–579. doi: 10.1038/s41584-020-0480-7.
- Deshayes S, Aouba A, Khoy K, et al. Hypocomplementemia is associated with worse renal survival in ANCA-positive granulomatosis with polyangiitis and microscopic polyangiitis. PLoS One. 2018;13(4):e0195680. doi: 10.1371/journal.pone.0195680.
- Crnogorac M, Horvatic I, Kacinari P, et al. Serum C3 complement levels in ANCA associated vasculitis at diagnosis is a predictor of patient and renal outcome. J Nephrol. 2018;31(2):257–262. doi: 10.1007/s40620-017-0445-3.
- García L, Pena CE, Maldonado R, et al. Increased renal damage in hypocomplementemic patients with ANCA-associated vasculitis: retrospective cohort study. Clin Rheumatol. 2019;38(10):2819–2824. doi: 10.1007/s10067-019-04636-9.
- Chalkia A, Thomas K, Giannou P, et al. Hypocomplementemia is associated with more severe renal disease and worse renal outcomes in patients with ANCA-associated vasculitis: a retrospective cohort study. Ren Fail. 2020;42(1):845–852. doi: 10.1080/0886022x.2020.1803086.
- Hakroush S, Tampe D, Korsten P, et al. Complement components C3 and C4 indicate vasculitis manifestations to distinct renal compartments in ANCA-associated glomerulonephritis. Int J Mol Sci. 2021;22(12):6588. doi: 10.3390/ijms22126588.
- Nomura S, Shimojima Y, Ichikawa T, et al. Immunopathological features of myopathy associated with small-to-medium-sized vessel vasculitis and differences from autoimmune myositis. Clin Exp Rheumatol. 2024;42(4):786–794. doi: 10.55563/clinexprheumatol/hpoapl.
- Motegi SI, Sekiguchi A, Toki S, et al. Clinical features and poor prognostic factors of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with rapid progressive interstitial lung disease. Eur J Dermatol. 2019;29(5):511–517. doi: 10.1684/ejd.2019.3634.
- Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20(9):537–551. doi: 10.1038/s41577-020-0288-3.
- He C, Li W, Xie Q, et al. Rituximab in the treatment of interstitial lung diseases related to anti-melanoma differentiation-associated gene 5 dermatomyositis: a systematic review. Front Immunol. 2021;12:820163. doi: 10.3389/fimmu.2021.820163.
