Abstract
Immune reconstitution inflammatory syndrome (IRIS) experienced in rheumatology practice is diverse and includes opportunistic infections such as herpes zoster (HZ). This study aimed to explore the risk of HZ in patients with rheumatic diseases in the perspective of IRIS. The study retrospectively reviewed the clinical courses of 20 patients with HZ and investigated the IRIS triggers such as the reduction or discontinuation of immunosuppressive drugs within 3 months and coronavirus disease 2019 (COVID-19) vaccination within 4 weeks prior to HZ development. Disease activity of the underlying rheumatic disease at HZ onset was evaluated using the physician’s global assessment. Thirteen patients developed HZ after reducing or discontinuing immunosuppressive drugs, with mild and stable disease activity. In four of these cases, disease activity increased after dose reduction or discontinuation, and HZ subsequently developed. Two of the seven patients who did not reduce or discontinue immunosuppressive drugs received the COVID-19 vaccination. Fifteen patients (75%) had at least one of the two IRIS triggers. Four of the five patients who developed HZ without any IRIS triggers were at HZ risk. To conclude, IRIS, caused by the reduction or discontinuation of immunosuppressive drugs, may be involved in the development of HZ in rheumatology practice.
1. Introduction
The appearance or exacerbation of inflammatory conditions, such as infectious and autoimmune diseases, due to the restoration of impaired immune function by drugs or other causes is called immune reconstitution inflammatory syndrome (IRIS). IRIS occurs in human immunodeficiency virus (HIV)-infected patients (HIV-IRIS) following antiretroviral therapy (ART); however, IRIS can also occur in non-HIV-infected individuals (non-HIV IRIS) [Citation1–3]. Non-HIV IRIS experienced in rheumatology practice is diverse and includes opportunistic infections that appear or worsen with dose reduction or discontinuation of immunosuppressive drugs, including glucocorticoids (GCs) and tumor necrosis factor α (TNF-α) inhibitors. Herpes zoster (HZ) is the most common clinical illness seen in patients with HIV-IRIS [Citation4]. HZ is also a common manifestation of non-HIV IRIS [Citation3]; however, its occurrence as an IRIS is less known in rheumatology practice. Therefore, previous studies exploring the risk of HZ development in patients with rheumatic diseases did not incorporate the perspective of IRIS. The coronavirus disease 2019 (COVID-19) vaccine has recently been suggested to be involved in the development of HZ [Citation5,Citation6], and there is a concept that IRIS is involved in its pathogenesis [Citation7]. Therefore, this study aimed to explore the risk of HZ in patients with rheumatic diseases in the perspective of IRIS triggers such as the reduction or discontinuation of immunosuppressive drugs and administration of COVID-19 vaccine.
2. Materials and methods
This study retrospectively reviewed the clinical course of 20 patients with rheumatic disease receiving immunosuppressive therapy at my department who developed HZ between October 2020 and May 2022. In this study, the following were defined as IRIS triggers: (1) reduction or discontinuation of immunosuppressive drugs, including GCs, within 3 months prior to HZ development and (2) COVID-19 vaccination within 4 weeks prior to HZ development. The COVID-19 vaccine administered was the mRNA-based vaccine, BNT162b2 (BioNTech/Pfizer) or mRNA-1273 (Moderna). Disease activity of the underlying rheumatic disease at HZ onset was evaluated by physician’s global assessment (PhGA), measured using a 0–100 mm visual analog scale. In this study, PhGA scores of 0–30, 31–60, and 61–100 were defined as mild, moderate, and severe disease activities, respectively. Arthritis status was represented by swollen joint count (SJC) and tender joint count (TJC). Disease activity before dose reduction or discontinuation was also observed in patients whose immunosuppressive drugs were reduced or discontinued within 3 months before HZ development. Erythrocyte sedimentation rate, serum C-reactive protein level, and patients’ global assessment score at the onset of HZ were excluded from this study because they were affected by HZ. The study was conducted in accordance with the principles stated in the Declaration of Helsinki. The Research Ethics Committee of Nishida Hospital approved the study protocol (Reference number: 202312-01). Written informed consent for participation in this study was obtained from all patients.
3. Results
3.1. Patient profile and clinical presentation of HZ
Twenty patients developed HZ during the observation period. The mean age was 72.0 (range, 46–91) years, and 16 patients (80%) were females. Fifteen patients (75%) had rheumatoid arthritis (RA) (including three with malignant RA). Immunosuppressive drugs used at HZ onset are shown in . Conventional synthetic disease-modifying anti-rheumatic drugs (DMARDs) such as bucillamine, salazosulfapyridine, and iguratimod, which are known to carry very low risk of infection, were excluded. In all patients, HZ skin lesions were localized to one dermatome. Two patients were rashless (zoster sine herpete). All patients received antiviral treatment, and the rash (or pain if rashless) resolved within 8 weeks. The time to resolution of the rash was within 3 weeks in 16 and 4–8 weeks in 4 (cases 6, 12, 14, and 16) patients. In case 11, GCs were administered because the rash appeared on the face. In two patients, immunosuppressive drugs were temporarily withdrawn during HZ treatment. In two patients, the pain lasted more than 3 months and was diagnosed as postherpetic neuralgia (PHN). Six patients had a history of HZ.
Table 1. Patient characteristics and information related to HZ.
3.2. Reduction or discontinuation of immunosuppressive drugs and COVID-19 vaccination prior to HZ onset
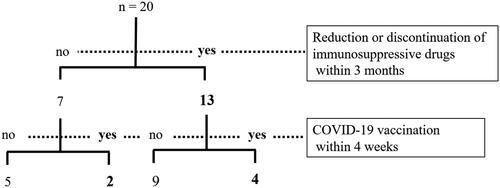
Any of the immunosuppressive drugs were reduced or discontinued to any degree prior to HZ onset in 13 of 20 patients (65%), with reduction or discontinuation 1–3 months prior in nine patients, within 1 month in three patients, and in both ways in one patient (, ). Two patients (cases 6 and 8) abruptly discontinued the drug due to self-determination, not based on the guidance of the attending physician. Fourteen patients had a history of COVID-19 vaccination prior to HZ onset, six of whom had been vaccinated within 4 weeks.
Table 2. The detailed information on patient’s clinical characteristics.
3.3. Disease activity of rheumatic diseases at HZ onset
Disease activity of rheumatic diseases at HZ onset was mild (PhGA 0-30) in 17 (85%) and moderate (PhGA 31-60) in three (15%) patients (). Nine mild cases had PhGA score 0 with no swollen or tender joint. Four patients (cases 6, 8, 13, and 18), including three with moderate disease activity, were originally stable with mild disease activity but showed increased activity after reducing or discontinuing immunosuppressive drugs within 3 months prior to HZ onset (see Supplementary Appendix). Cases 18 and 19 originally had 0 PhGA score, SJC, and TJC but showed increased disease activity within 1 week after COVID-19 vaccination and developed HZ a few weeks later (see Supplementary Appendix).
3.4. Details of patients with no IRIS triggers prior to HZ onset
Five of the 20 patients did not had IRIS triggers, as defined in this study, before HZ onset (). Two of these patients used Janus kinase (JAK) inhibitors (tofacitinib, upadacitinib, and filgotinib) for RA, which is a risk factor for HZ [Citation8], and also had a history of HZ, which has been reported as a risk factor for HZ in patients with RA treated with biologic DMARDs or targeted synthetic DMARDs [Citation9]. Case 9 involved a patient with long-term RA who also had impaired physical function [Citation10] and joint erosions/destructive changes [Citation11], which are risk factors for HZ. Case 20 had been on prednisolone (PSL), which is an HZ risk [Citation11], for more than 20 years. Case 10 was of an older patient who had received a quadrivalent inactivated influenza vaccine the day before HZ onset. The influenza vaccine has been reported to be a possible risk for HZ [Citation12], and being older is a known HZ risk [Citation10,Citation13]. Only case 12 did not had any HZ risk, as mentioned above.
Table 3. Clinical information on risk factors for HZ in patients with no IRIS triggers.
4. Discussion
IRIS is a concept proposed in a series of reports of paradoxical infections and inflammatory reactions after ART in HIV-infected patients [Citation14], but it is now known that IRIS occurs in non-HIV-infected patients as well [Citation1–3]. In the field of rheumatology, opportunistic infections that appear or worsen with the reduction or discontinuation of immunosuppressive drugs, immune-related adverse events with immune checkpoint blockade, paradoxical reactions during treatment with TNF-α blockades, and the exacerbation of rheumatic disease activity after childbirth can be interpreted as IRIS [Citation2,Citation15–17]. However, although HZ is the most common HIV-IRIS, it is seldom discussed in the field of rheumatology from the perspective of IRIS. HZ is caused by the reactivation of the latent varicella-zoster virus in the dorsal root ganglia after primary infection. Clinically, HZ is characterized by a unilateral vesicular rash that can cause multiple neurological complications, the most frequent being PHN. Because HZ reactivation is a complication of immunosuppressive therapy and is more frequent in patients with rheumatic diseases than in the general population [Citation18], understanding its pathogenesis is essential to improve the quality of rheumatology care. However, no studies have been reported on whether reduction or discontinuation of immunosuppressive drugs, a routine practice in rheumatology, is a risk for HZ reactivation in patients with rheumatic diseases.
In this study, 13 of 20 patients developed HZ after reducing or discontinuing immunosuppressive drugs, whereas the disease activity in rheumatic diseases was mild and stable. In four of these patients, the disease activity increased after dose reduction or discontinuation, and HZ developed subsequently, which was particularly reminiscent of IRIS. The seven patients who did not reduce or discontinue immunosuppressive drugs were not scheduled for intensified therapy because their rheumatic disease activity was mild. Two of these seven patients received COVID-19 vaccination within 4 weeks prior to the onset of HZ. In other words, 15 patients (75%) had at least one of the two IRIS triggers defined in this study (). These results support the possibility that HZ is caused by IRIS. However, although proposed by Sueki et al. [Citation2], diagnostic criteria for non-HIV IRIS have not yet been established and no specific test exists; therefore, it is not possible to diagnose IRIS even if there is a likely trigger. In all patients, the skin lesions of HZ were limited to a single dermatome. HZ occurring as IRIS in immunosuppressed individuals has been reported to be associated with mild inflammation [Citation3], which is consistent with our study. In four of the six patients who developed HZ after COVID-19 vaccination, dose reduction or discontinuation was also performed, and the two patients who developed PHN were included. Whether the combination of drug reduction or discontinuation and COVID-19 vaccination further increases the risk of HZ remains unknown, and is a subject for future studies. Four of the five patients who developed HZ without IRIS triggers were at some risk of developing HZ, leading to the hypothesis that patients with risk factors for HZ reactivation are more prone to develop HZ even without obvious IRIS triggers.
This study had several major limitations. First, because this was a single-center, retrospective study of a small population with no control population, it was not possible to prove a causal relationship between dose reduction or discontinuation of immunosuppressive drugs or COVID-19 vaccination and the development of HZ. Second, the validity of setting the time between the reduction or discontinuation of immunosuppressive drugs and HZ onset is unclear. It is set to 3 months in this study because HZ is more likely to develop 1–3 months after the last course of chemotherapy in patients with cancer [Citation3]. However, HZ in non-HIV IRIS has been reported to have a more variable time to onset than in HIV-IRIS [Citation3]. The time from COVID-19 vaccination to HZ onset was set within 4 weeks, based on previous reports [Citation19,Citation20]; however, its validity remains unclear. Third, although it is generally believed that IRIS is triggered by a relatively large dose or rapid reduction or discontinuation of immunosuppressive drugs [Citation1–3], in this study, even a small dose or slow reduction or discontinuation was also considered a trigger. In clinical practice, a small dose, slow reduction, or discontinuation can be followed by a flare-up of disease activity in rheumatic diseases, which could also be a possible trigger for IRIS. Future studies are needed to determine whether there are differences in the incidence, severity, and time to onset of HZ depending on the dose and speed of immunosuppressive drugs to be reduced or discontinued, as well as the type of drug used, dose administered, and cumulative duration of administration.
If future studies reveal that HZ in patients with rheumatic disease can occur through the IRIS mechanism, the following strategies should be considered: (1) Reiterate patient guidance regarding HZ, for example, avoiding sleep deprivation [Citation21] and consulting a doctor immediately if a painful rash develops (since it is fundamental to initiate antiviral treatment as soon as possible after the appearance of the first vesicles to limit the disease severity) before reducing or discontinuing immunosuppressive drugs. (2) Refrain from reducing or discontinuing immunosuppressive drugs close to the date of COVID-19 vaccination. (3) No temporary cessation of non-GC immunosuppressive drugs when HZ occurs. In rheumatology practice, it is common to temporarily suspend immunosuppressive drugs other than GC when a patient develops HZ [Citation8]. However, whether HZ treatment outcomes differ with or without drug withdrawal has not been adequately investigated in patients with rheumatic diseases. For non-HIV IRIS, immunosuppressive therapy should be considered in addition to antimicrobial therapy for infections [Citation1–3]. Therefore, if IRIS is involved in HZ reactivation in patients with rheumatic diseases, withdrawal of immunosuppressive drugs may be undesirable. Most patients in this study had been treated by dermatologists or otolaryngologists at other hospitals for HZ, and as a result, only two patients were withdrawn from immunosuppressive drugs other than GC, but all responded well to antiviral therapy. Incidentally, one of the two cases of PHN involved the withdrawal of immunosuppressive drugs other than GC. (4) Prophylactic administration of antivirals, as our understanding of the pathogenesis of HZ reactivation improves the accuracy of its prediction, such as acyclovir during hematopoietic stem cell transplantation [Citation22], may become a promising option. (5) Recommend vaccination for HZ. Recombinant zoster vaccines have recently been recommended for patients with rheumatic diseases, aged >18 years, on immunosuppressive therapy [Citation23]. This would be generally beneficial in rheumatology practice regardless of whether IRIS is involved. The vaccine was not administered to any of the patients in this study.
In conclusion, IRIS, caused by the reduction or discontinuation of immunosuppressive drugs, may be involved in the development of HZ in rheumatology practice. Although this study could not prove a causal relationship, it offers a useful new perspective in the practice of rheumatology. The IRIS perspective is essential to investigate the risk of HZ associated with COVID-19 mRNA vaccination in patients with rheumatic diseases. Furthermore, the IRIS perspective should be considered in clinical studies on JAK inhibitors and anifrolumab [Citation24], which are known to increase the risk of HZ.
Geolocation information
Oita, Japan
Supplemental Material
Download MS Word (16.7 KB)Acknowledgments
I thank the patients for making this work possible and have obtained her written informed consent to publish the material. I also thank Editage (www.editage.jp) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sun HY, Singh N. Immune reconstitution inflammatory syndrome in non-HIV immunocompromised patients. Curr Opin Infect Dis. 2009;22(4):394–402. doi:10.1097/QCO.0b013e32832d7aff.
- Sueki H, Mizukawa Y, Aoyama Y. Immune reconstitution inflammatory syndrome in non-HIV immunosuppressed patients. J Dermatol. 2018;45(1):3–9. doi:10.1111/1346-8138.14074.
- Shiohara T, Kurata M, Mizukawa Y, et al. Recognition of immune reconstitution syndrome necessary for better management of patients with severe drug eruptions and those under immunosuppressive therapy. Allergol Int. 2010;59(4):333–343. doi:10.2332/allergolint.10-RAI-0260.
- Jevtović DJ, Salemović D, Ranin J, et al. The prevalence and risk of immune restoration disease in HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 2005;6(2):140–143. doi:10.1111/j.1468-1293.2005.00277.x.
- Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60(SI):SI90–SI95. doi:10.1093/rheumatology/keab345.
- Hertel M, Heiland M, Nahles S, et al. Real-world evidence from over one million COVID-19 vaccinations is consistent with reactivation of the varicella-zoster virus. J Eur Acad Dermatol Venereol. 2022;36(8):1342–1348. doi:10.1111/jdv.18184.
- Ota M. SARS-CoV-2 mRNA vaccination and subsequent herpes zoster: possible immune reconstitution by mRNA vaccination. JAAD Case Rep. 2022;23:166–167. doi:10.1016/j.jdcr.2022.02.035.
- Atzeni F, Gozza F, Riva A, et al. Conventional, biological disease-modifying anti-rheumatic drugs and Janus kinase inhibitors and varicella zoster virus. Expert Opin Pharmacother. 2023;24(6):679–689. doi:10.1080/14656566.2023.2195050.
- Jeong S, Choi S, Park SM, et al. Incident and recurrent herpes zoster for first-line bDMARD and tsDMARD users in seropositive rheumatoid arthritis patients: a nationwide cohort study. Arthritis Res Ther. 2022;24(1):180. doi:10.1186/s13075-022-02871-1.
- Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology (Oxford). 2006;45(11):1370–1375. doi:10.1093/rheumatology/kel328.
- Veetil BM, Myasoedova E, Matteson EL, et al. Incidence and time trends of herpes zoster in rheumatoid arthritis: a population-based cohort study. Arthritis Care Res (Hoboken). 2013;65(6):854–861. doi:10.1002/acr.21928.
- Walter R, Hartmann K, Fleisch F, et al. Reactivation of herpesvirus infections after vaccinations? Lancet. 1999;353(9155):810. doi:10.1016/S0140-6736(99)00623-6.
- Marra F, Parhar K, Huang B, et al. Risk factors for herpes zoster infection: a meta-analysis. Open Forum Infect Dis. 2020;7(1):ofaa005. doi:10.1093/ofid/ofaa005.
- French MA. Antiretroviral therapy. Immune restoration disease in HIV-infected patients on HAART. AIDS Read. 1999;9(8):548. 54–55, 59–62.
- Fujita J. Immune reconstitution inflammatory syndrome in the lung in non-human immunodeficiency virus patients. Respir Investig. 2020;58(1):36–44. doi:10.1016/j.resinv.2019.11.001.
- Harigai M, Mochida S, Mimura T, et al. A proposal for management of rheumatic disease patients with hepatitis B virus infection receiving immunosuppressive therapy. Mod Rheumatol. 2014;24(1):1–7. doi:10.3109/14397595.2013.852834.
- Gupta M, Jafri K, Sharim R, et al. Immune reconstitution inflammatory syndrome associated with biologic therapy. Curr Allergy Asthma Rep. 2015;15(2):499. doi:10.1007/s11882-014-0499-4.
- Furer V, Rondaan C, Heijstek M, et al. Incidence and prevalence of vaccine preventable infections in adult patients with autoimmune inflammatory rheumatic diseases (AIIRD): a systemic literature review informing the 2019 update of the EULAR recommendations for vaccination in adult patients with AIIRD. RMD Open. 2019;5(2):e001041. doi:10.1136/rmdopen-2019-001041.
- Iwanaga J, Fukuoka H, Fukuoka N, et al. A narrative review and clinical anatomy of herpes zoster infection following COVID-19 vaccination. Clin Anat. 2022;35(1):45–51. doi:10.1002/ca.23790.
- Lladó I, Fernández-Bernáldez A, Rodríguez-Jiménez P. Varicella zoster virus reactivation and mRNA vaccines as a trigger. JAAD Case Rep. 2021;15:62–63. doi:10.1016/j.jdcr.2021.07.011.
- Chung WS, Lin HH, Cheng NC. The incidence and risk of herpes zoster in patients with sleep disorders: a population-based cohort study. Medicine (Baltimore). 2016;95(11):e2195. doi:10.1097/MD.0000000000002195.
- Wada-Shimosato Y, Tanoshima R, Hiratoko K, et al. Effectiveness of acyclovir prophylaxis against varicella zoster virus disease after allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Transpl Infect Dis. 2019;21(3):e13061. doi:10.1111/tid.13061.
- Bass AR, Chakravarty E, Akl EA, et al. 2022 American College of Rheumatology Guideline for Vaccinations in Patients With Rheumatic and Musculoskeletal Diseases. Arthritis Care Res (Hoboken). 2023;75(3):449–464. doi:10.1002/acr.25045.
- Tummala R, Abreu G, Pineda L, et al. Safety profile of anifrolumab in patients with active SLE: an integrated analysis of phase II and III trials. Lupus Sci Med. 2021;8(1):e000464. doi:10.1136/lupus-2020-000464.