Abstract
Elevated liver enzymes are commonly observed among adult-onset Still’s disease (AOSD), but severe acute liver failure is extremely rare. Although severe acute liver failure associated with AOSD poses a life-threatening condition, the appropriate treatment is unclear. Some case reports have demonstrated the efficacy of high-dose prednisolone (PSL) and cyclosporin A (CyA), although the adverse effects of CyA led certain patients to cease its use. Therefore, an alternative treatment option is crucial, and thus far, there have been no reports of tocilizumab (TCZ) being used for this severe phenotype. Here, we report the first case of successful treatment using TCZ as maintenance therapy for severe ALF associated with AOSD. Following initial treatment with high-dose PSL and CyA, our case was switched to TCZ due to CyA-related side effects including alopecia and tremors. Our case highlights TCZ as a potential option for maintenance therapy of this severe condition.
1. Introduction
Adult-onset Still’s disease (AOSD) is a systemic inflammatory disorder characterized by spiking fever, sore throat, skin rash, arthralgia, and neutrophilic leukocytosis [Citation1]. Elevated liver enzymes are commonly observed among AOSD patients, accounting for 74% of cases [Citation2], but the development of severe acute liver failure (ALF) is extremely rare and has only been reported in isolated case reports [Citation3].
Although severe ALF in AOSD poses a life-threatening condition, the appropriate treatment is still unclear. Some case reports have shown the efficacy of high-dose prednisolone (PSL) and cyclosporin A (CyA) for inducing and maintaining remission in ALF associated with AOSD [Citation4,Citation5]. However, CyA has significant side effects, including alopecia, tremor, renal toxicity, and gastrointestinal symptoms, often necessitating discontinuation [Citation6]. On the other hand, considering the proved efficacy of tocilizumab (TCZ) in AOSD [Citation7], TCZ may be considered as one of the potential treatment choices for severe ALF. However, to date, there have been no reports on whether TCZ can be used in this severe phenotype. Furthermore, given that TCZ itself may induce liver damage and potentially trigger macrophage activation syndrome [Citation8], it is entirely unclear whether TCZ can be safely used in this severe condition.
Here, we present the first case of successful treatment using TCZ as maintenance therapy for severe ALF associated with AOSD. Following initial treatment with high-dose PSL and CyA, our case was switched to TCZ due to CyA-related side effects including alopecia and tremors. Thus, TCZ may be a safe and beneficial choice for maintenance therapy in severe AOSD-associated ALF.
2. Case report
A 49-year-old male was admitted to the hospital with a high fever that had lasted for two weeks, sore throat, and acute hepatitis. At that time, his body temperature was 40.0 °C, blood pressure was 135/70 mmHg. Physical examination revealed jaundice, but hepatic encephalopathy was not found. Blood tests revealed elevated levels of white blood cells (WBC; 10100/μL, 92% neutrophils, normal range ≤ 8600/μL), total bilirubin (T-Bil; 3.4 mg/dL, normal range ≤ 1.5 mg/dL), aspartate aminotransferase (AST; 1720 U/L, normal range ≤ 30 U/L), alanine aminotransferase (ALT; 1601 U/L, normal range ≤ 42 U/L), C-reactive protein (CRP; 1.014 mg/dL, normal range ≤ 0.14 mg/dL), and ferritin (7156 ng/mL, normal range ≤ 303.7 ng/mL). Levels of IgG (1419 mg/dL, normal range ≤ 1747 mg/dL), and prothrombin time-international normalized ratio (PT-INR; 1.16, normal range ≤ 1.2) were within normal range (). Results for blood cultures, hepatitis B surface antigen, anti-hepatitis B surface antibody, anti-hepatitis C virus antibody, anti-hepatitis A virus antibody, anti-hepatitis E virus antibody, human immunodeficiency virus tests, anti-cytomegalovirus IgM, and anti-Epstein Barr virus IgM were all negative. Immunological tests revealed an anti-nuclear antibody titer of 1:40 (speckled pattern), rheumatoid factor of 20 U/mL (normal range ≤ 15 U/mL), and anti-liver/kidney microsome-1 antibody was negative. During 2 weeks after admission, liver enzymes continuously elevated, PT-INR increased to 1.5, and abdominal CT revealed the development of ascites (), therefore progression to ALF without hepatic coma was suspected based on the 2011 diagnostic criteria for ALF in Japan [Citation9]. At that time, blood tests revealed markedly elevated T-Bil (7.1 mg/dL, normal range ≤ 1.5 mg/dL), CRP (12.74 mg/dL, normal range ≤ 0.14 mg/dL), PT-INR (1.5, normal range ≤ 1.2), and ferritin (55968 ng/mL, normal range ≤ 303.7 ng/mL), without cytopenia (). Furthermore, the typical salmon-pink rash appeared during episodes of fever and resolved upon fever resolution (). We conducted a liver biopsy three weeks after admission, which revealed mild infiltration of inflammatory cells into lobules, lobular necrosis, and fibrous portal expansion (). Immunostaining of CD4, CD8 and CD68 revealed that CD8+ cells predominated over CD4+ cells, alongside an observed infiltration of CD68+ macrophages (). However, no specific findings indicative of lymphoma or autoimmune hepatitis, such as interface hepatitis, emperipolesis, or rosettes, were observed. We also conducted a bone marrow examination, which revealed no specific findings for hemophagocytosis or lymphoma.
Figure 1. Abdominal CT, skin, and pathological findings of liver in our present case. At the time of acute liver failure, abdominal CT revealed the development of ascites (A), and skin erythema appeared around the same time (B). Hematoxylin-eosin (H&E) staining showed mild infiltration of inflammatory cells into lobules (arrow) (original magnification: x100) (C), and silver staining showed fibrous portal expansion (arrow) (original magnification: x100) (D). Immunostaining of CD4 (E), CD8 (F), and CD68 (G) revealed the dominance of CD8 or CD68-positive cell infiltration (original magnification: x200).
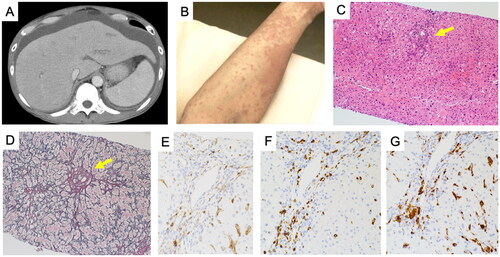
Table 1. Laboratory data of the present case on admission (day 1), day 14, and day 28.
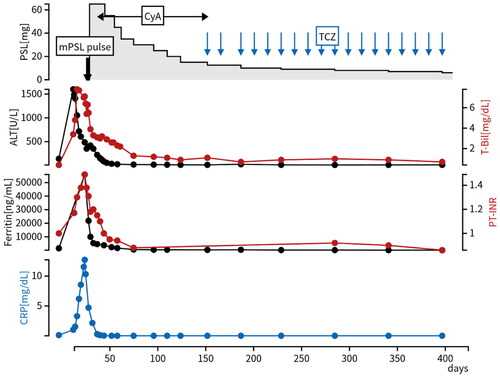
Considering persistent spiking fever, typical skin rash, leukocytosis, sore throat, markedly elevated ferritin levels and elevated levels of liver enzyme, we diagnosed him with AOSD complicated with severe ALF based on the Yamaguchi criteria [Citation1]. We started a pulse of methylprednisolone (1000 mg daily for three consecutive days), followed by high-dose prednisolone (1 mg/kg; 65 mg/day), CyA (3 mg/kg; 200 mg/day) and vitamin K supplementation for ALF. We started CyA simultaneously due to high disease activity and life-threatening condition, and adjusted the CyA dose to target trough levels in the range of 100-200 ng/mL. This approach aimed to balance therapeutic efficacy with minimizing potential toxicity, ensuring optimal management of the patient’s condition. After the treatment initiation, his fever, liver functions, ascites, and skin erythema all improved (, ). However, two months after initiation of CyA, alopecia and upper limb tremors appeared despite a trough level of CyA with 150 ng/mL, leading to the discontinuation of CyA and prompting a switch to intravenous TCZ (400 mg every two weeks). The discontinuation of CyA resulted in the disappearance of alopecia and tremor in one month. Furthermore, after the initiation of TCZ, his disease status remained stable without relapse of the disease or side effects for half a year. PSL was successfully tapered to 6 mg/day at the last visit ().
Figure 2. Clinical course of a present case. T-Bil, ALT, ferritin, PT-INR, and CRP improved after initiation of high-dose PSL and CyA, and his disease status remained stable without relapse for half a year after we switched CyA to TCZ. Abbreviations: CyA: cyclosporine; mPSL: methylprednisolone; PSL: prednisolone; TCZ: tocilizumab.
3. Discussion
We presented our case of severe ALF associated with AOSD, which was effectively managed with high-dose PSL and CyA during induction therapy and maintained in remission after transitioning from CyA to TCZ. With no established consensus on treating AOSD complicated by severe ALF, our case emphasizes the potential effectiveness of TCZ as a maintenance therapy for this life-threatening complication.
Involvement of a pro-inflammatory cytokine cascade is believed to underlie the pathogenesis of AOSD. Excessive production of IL-1β and IL-18, triggered by macrophage activation due to pathogens or damage-associated molecular patterns, leads to elevated levels of IL-6, IFN-γ, and tumor necrosis factor α, establishing a cytokine loop that manifests systemically [Citation10]. Glucocorticoids effectively suppress these cytokines and are considered the first-line therapy in AOSD. However, its long-term use is problematic due to side effects, and many cases experience relapse during glucocorticoid tapering [Citation11], especially in cases with severe hepatitis [Citation12]. Therefore, advanced therapies that can prevent disease relapse and reduce glucocorticoid doses are essential. Several candidates for advanced therapy are utilized for AOSD, including IL-1 receptor inhibitors, IL-6 receptor inhibitors like TCZ, tumor necrosis factor (TNF) inhibitors, and Janus kinase (JAK) inhibitors. TCZ, a monoclonal anti-IL-6 receptor antibody, has consistently shown efficacy in ameliorating systemic symptoms, preventing disease relapse, and reducing glucocorticoid doses [Citation13,Citation14]. Importantly, TCZ is the only agent demonstrating efficacy in a randomized, double-blind, placebo-controlled phase III trial [Citation7]. In contrast, IL-1 receptor inhibitors have shown efficacy only in retrospective studies for improving systemic symptoms [Citation15]. Data on TNF inhibitors and JAK inhibitors are more limited, with TNF inhibitors primarily studied in arthritis cases without systemic symptoms [Citation13,Citation16]. Considering TCZ’s efficacy in managing systemic symptoms, preventing disease relapse, and its glucocorticoid-sparing effects, we deemed it the most suitable and effective option for maintenance therapy in our current case.
The etiology of liver involvement in AOSD remains poorly understood. A recent report indicated significantly elevated IL-18 levels in AOSD patients with acute hepatitis, with high expression observed in CD68+ macrophages within the liver tissue [Citation17]. Additionally, another study documented a dominant infiltration of CD8+ T cells over CD4+ T cells in the liver tissue of AOSD cases with severe hepatitis [Citation4], which is consistent with our findings. Therefore, it is plausible to hypothesize that liver macrophages and CD8+ T lymphocytes are involved in the pathogenesis of acute hepatitis associated with AOSD [Citation4].
Considering the involvement of T cells in the pathogenesis of acute hepatitis associated with AOSD, CyA has been selected and used as remission induction and maintenance therapy for AOSD with severe ALF in some cases [Citation3,Citation4]. A retrospective study reported that PSL monotherapy failed in 79% of cases with severe hepatitis, whereas 80% of cases were successfully treated with the combination of PSL and CyA [Citation3]. On the other hand, there are no reports on the use of TCZ, although it has been shown to be effective in AOSD [Citation7]. Our present case suggests that TCZ therapy is a potentially viable treatment option for maintenance therapy of severe ALF associated with AOSD.
IL-6 is a multifunctional cytokine that not only induces acute phase proteins such as CRP, but also accelerates the differentiation of cytotoxic CD8 + T cells, and inhibits the differentiation of regulatory T cells (Tregs) [Citation18]. A recent study demonstrated the significant decrease and functional impairment of Tregs in active AOSD cases, and improvement after remission induction therapy including TCZ [Citation19]. Inhibition of IL-6 by TCZ increases the proportion of regulatory Tregs in several autoimmune diseases [Citation20–22], and enhances the migration of Tregs into the inflammatory organs and improves disease activity [Citation21,Citation23]. Although there are no pathological findings on Tregs interaction in AOSD, it is plausible to hypothesize that Tregs activated by TCZ suppress cytotoxic CD8+ T cells and control T cell-mediated AOSD disease activity, including hepatitis.
There have been no reports on the effectiveness and safety of TCZ for inducing remission in severe ALF associated with AOSD. Although TCZ has demonstrated efficacy in treating AOSD, some cases of macrophage activation syndrome have been reported to be triggered by TCZ [Citation8]. Considering that extremely elevated ferritin levels, indicative of macrophage activation, have been identified as a risk factor for macrophage activation syndrome following TCZ therapy [Citation8], AOSD cases with severe ALF may face risks when initiating TCZ as remission induction therapy. Furthermore, our case was switched to TCZ due to CyA-related side effects during the maintenance phase, but the optimal timing of switching remains unknown, and earlier switching from CyA to TCZ soon after the improvement of ALF can be an option. Further accumulation of cases is needed to establish an appropriate treatment strategy in this life-threatening condition.
In conclusion, we presented a case of severe ALF associated with AOSD, effectively maintained in remission with TCZ. Our case highlights the potential of TCZ in serving as a cornerstone in stabilizing this critical condition as a maintenance therapy.
Patient consent
Written informed consent for publication of this report was obtained from the patient.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424–430.
- Asanuma YF, Mimura T, Tsuboi H, et al. Nationwide epidemiological survey of 169 patients with adult Still’s disease in Japan. Mod Rheumatol. 2015;25(3):393–400. doi:10.3109/14397595.2014.974881.
- Muller R, Briantais A, Faucher B, et al. Acute severe hepatitis in adult-onset Still’s disease: case report and comprehensive review of a life-threatening manifestation. Clin Rheumatol. 2021;40(6):2467–2476. doi:10.1007/s10067-020-05383-y.
- Kurokawa M, Hioki T, Aoyagi T, et al. Clinicopathologic features of adult-onset still’s disease complicated by severe liver injury. Intern Med. 2024;63(4):503–511. doi:10.2169/internalmedicine.2043-23.
- Nagashima T, Aoki Y, Onishi S, et al. Steroid-refractory severe hepatic failure in adult onset Still’s disease responding to cyclosporine. Clin Rheumatol. 2008;27(11):1451–1453. doi:10.1007/s10067-008-0950-9.
- Nakamura H, Fujieda Y, Tarumi M, et al. Calcineurin inhibitors for adult-onset Still’s disease: a multicentre retrospective cohort study. Clin Exp Rheumatol. 2020;38(Suppl 127):11–16.
- Kaneko Y, Kameda H, Ikeda K, et al. Tocilizumab in patients with adult-onset still’s disease refractory to glucocorticoid treatment: a randomised, double-blind, placebo-controlled phase III trial. Ann Rheum Dis. 2018;77(12):1720–1729. doi:10.1136/annrheumdis-2018-213920.
- Adachi S, Takase-Minegishi K, Maeda A, et al. Risk of macrophage activation syndrome in patients with adult-onset still’s disease treated with IL-1 and IL-6 inhibitors: a meta-analysis and single-center experience. Rheumatol Ther. 2023;10(6):1623–1636. doi:10.1007/s40744-023-00600-x.
- Mochida S, Takikawa Y, Nakayama N, et al. Diagnostic criteria of acute liver failure: a report by the Intractable Hepato-Biliary Diseases Study Group of Japan. Hepatol Res. 2011;41(9):805–812. doi:10.1111/j.1872-034X.2011.00860.x.
- Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat Rev Rheumatol. 2018;14(10):603–618. doi:10.1038/s41584-018-0081-x.
- Sampalis JS, Esdaile JM, Medsger TA, et al. A controlled study of the long-term prognosis of adult Still’s disease. Am J Med. 1995;98(4):384–388. doi:10.1016/s0002-9343(99)80318-0.
- Chi H, Wang Z, Meng J, et al. A cohort study of liver involvement in patients with adult-onset still’s disease: prevalence, characteristics and impact on prognosis. Front Med (Lausanne). 2020;7:621005. doi:10.3389/fmed.2020.621005.
- Leavis HL, van Daele PLA, Mulders-Manders C, et al. Management of adult-onset Still’s disease: evidence- and consensus-based recommendations by experts. Rheumatology. 2023;63(6):1656–1663. doi:10.1093/rheumatology/kead461.
- Suematsu R, Ohta A, Matsuura E, et al. Therapeutic response of patients with adult Still’s disease to biologic agents: multicenter results in Japan. Mod Rheumatol. 2012;22(5):712–719. doi:10.3109/s10165-011-0569-6.
- Colafrancesco S, Manara M, Bortoluzzi A, et al. Management of adult-onset Still’s disease with interleukin-1 inhibitors: evidence- and consensus-based statements by a panel of Italian experts. Arthritis Res Ther. 2019;21(1):275. doi:10.1186/s13075-019-2021-9.
- Ruscitti P, Cantarini L, Nigrovic PA, et al. Recent advances and evolving concepts in Still’s disease. Nat Rev Rheumatol. 2024;20(2):116–132. doi:10.1038/s41584-023-01065-6.
- Priori R, Barone F, Alessandri C, et al. Markedly increased IL-18 liver expression in adult-onset Still’s disease-related hepatitis. Rheumatology. 2011;50(4):776–780. doi:10.1093/rheumatology/keq397.
- Tanaka T, Narazaki M, Masuda K, et al. Interleukin-6; pathogenesis and treatment of autoimmune inflammatory diseases. Inflamm Regen. 2013;33(1):054–065. doi:10.2492/inflammregen.33.054.
- Shimojima Y, Ichikawa T, Kishida D, et al. Circulating regulatory T cells in adult-onset Still’s disease: focusing on their plasticity and stability. Clin Exp Immunol. 2021;206(2):184–195. doi:10.1111/cei.13648.
- Yoshida H, Magi M, Tamai H, et al. Effects of interleukin-6 signal inhibition on Treg subpopulations and association of Tregs with clinical outcomes in rheumatoid arthritis. Rheumatology. 2024;1–10. doi:10.1093/rheumatology/keae196.
- Sakai R, Ito M, Yoshimoto K, et al. Tocilizumab monotherapy uncovered the role of the CCL22/17-CCR4+ Treg axis during remission of crescentic glomerulonephritis. Clin Transl Immunology. 2020;9(11):e1203. doi:10.1002/cti2.1203.
- Kikuchi J, Hashizume M, Kaneko Y, et al. Peripheral blood CD4(+)CD25(+)CD127(low) regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: increase in regulatory T cells correlates with clinical response. Arthritis Res Ther. 2015;17(1):10. doi:10.1186/s13075-015-0526-4.
- Scholz GA, Fux M, Christ L, et al. Divergent regulatory T cell responses to high-dose methylprednisolone and tocilizumab in giant cell arteritis. J Autoimmun. 2022;133:102909. doi:10.1016/j.jaut.2022.102909.
