Abstract
Background: HIV-induced systemic immune activation and inflammation have been associated with morbidity and mortality in virologically suppressed patients.
Objective: To evaluate the impact of treatment switch from a dual regimen with lamivudine (3TC) plus ritonavir-boosted protease inhibitors (PI/r) to 3TC plus dolutegravir (DTG) on the monocyte activation marker soluble CD14 (sCD14) and other inflammatory biomarkers, interleukin-6 (IL-6), C-reactive protein (CRP), intestinal fatty acid–binding protein (I-FABP) and D-dimer.
Methods: We performed a retrospective case-crossover study on integrase inhibitors-naïve virologically suppressed patients while on 3TC + PI/r dual maintenance therapy for ≥48 weeks who switched to 3TC + DTG and maintained this regimen for ≥48 weeks. Biomarkers plasma levels were tested by ELISA assays on stored samples at three time points: at switch (BL), 48 weeks before (−48 W) and 48 weeks after switch (+48 W).
Results: A total of 67 patients were included. Median sCD14 levels were stable from −48 W to BL (from 6.07 to 6.04 log10 pg/mL, p = 0.235) but showed a statistically significant decrease after switch: from 6.04 (IQR 5.92-6.12) at BL to 5.95 (IQR 5.84–6.07) log10 pg/mL at + W48 (p < 0.001). Concurrently, an improvement in lipid profile was observed, even thought it was not correlated to the change in sCD14. The levels of IL-6, CRP, I-FABP and D-dimer remained stable before and after the switch to 3TC + DTG.
Conclusions: In virologically suppressed HIV-infected patients on a 3TC + PI/r dual therapy, switching to 3TC + DTG was associated with a significant decline in sCD14. These data suggest reduced monocyte activation following substitution of boosted PI with DTG, which could have important clinical implications.
Introduction
Understanding the effects of different antiretroviral regimens on HIV-induced residual systemic inflammation is a topic of interest because it has been demonstrated that higher levels of circulating markers of immune activation (specifically monocyte activation) and inflammation can predict morbidity and mortality in treated HIV infection.Citation1–3 Persistent systemic inflammation, despite effective antiretroviral therapy (ART), may be due to several mechanisms such as low-level viral replication, microbial translocation, or direct effects of antiretrovirals on inflammatory pathways.Citation4
To date the impact of different antiretroviral classes or specific antiretrovirals on residual inflammation is largely unclear.
It might be hypothesized that integrase inhibitors (InSTI) favorably impact inflammation because these drugs are more lipid friendlyCitation5,Citation6 and might concentrate at higher levels in enterocytesCitation7 compared to other antiretroviral classes. Previous studies have shown several beneficial effects on inflammatory biomarker profiles following switch to raltegravir (RAL) in virologically suppressed patients.Citation8–10 However, data regarding dolutegravir (DTG), which is currently the most widely prescribed InSTI, are still lacking.
DTG is an InSTI with a high antiviral potency and resistance barrier; in combination with lamivudine (3TC), it has shown encouraging efficacy and safety as a two-drug maintenance regimen,Citation11 thus representing a potential good new option compared to the dual therapies with 3TC plus ritonavir-boosted protease inhibitors (PI/r), which are currently recommended by international guidelines.Citation12
The aim of our study was to evaluate in virologically suppressed patients the impact of switching ART from a dual regimen with 3TC + PI/r to 3TC + DTG on host inflammatory status, by measuring the monocyte activation marker soluble CD14 (sCD14) and other biomarkers of inflammation, interleukin 6 (IL-6), C-reactive protein (CRP), D-dimer, and enterocyte damage/microbial translocation, intestinal fatty acid-binding protein (I-FABP).
Our hypothesis was that the substitution of PI/r with DTG could ameliorate systemic inflammation in this population.
Materials and methods
Study participants
This was a retrospective case-crossover studyCitation13 conducted at “Fondazione A. Gemelli Hospital,” in Rome, Italy An observational cohort study is ongoing at our center; its aim is to collect data on novel prognostic and predictive biomarkers for patients with HIV. For this purpose, we prospectively record clinical and laboratory data in an electronic database and systematically recover residual samples obtained from routine analyses of HIV-infected patients during routine outpatient visits. This cohort study was approved by the local Ethic Committee and patients gave a written informed consent to participate. Within this cohort, we retrospectively selected treatment-experienced InSTI-naïve patients with plasma HIV-RNA <50 copies/mL who were on a dual regimen with 3TC + PI/r [ritonavir-boosted darunavir (DRV), atazanavir (ATV), or lopinavir (LPV)] from ≥48 weeks and who switched during routine clinical practice to a dual therapy with 3TC + DTG and maintained this regimen for at least 48 weeks. We evaluated the temporal trends of inflammatory biomarkers before and after the switch by measuring levels of sCD14, IL-6, CRP, D-dimer, and I-FABP on residual stored plasma samples at three different time points: 48 weeks before switching to the dual therapy (−48 W), at the time of the treatment switch (baseline, BL) and 48 weeks after the switch (+48 W). Trends in vitro-immunological and other laboratory parameters obtained for each patient from our database at the same time points were also evaluated.
Biomarkers assessment
Selected samples spanning a 4-year period (i.e. January 2014–December 2017) were assayed within the same time frame; patient samples at different time points were also tested within the same run to avoid inter-assay variability. Plasma samples were analyzed using specific standardized ELISA assays according to the manufacturers’ instructions: Quantikine ELISA (D6050, DC140 and DFBP20; R&D Systems, Minneapolis, MN, USA) for measuring IL-6 (limit of detection 700 pg/mL; inter-assay variability 3.8–6.4%), sCD14 (limit of detection 125 pg/mL; inter-assay variability 4.8–7.4%) and I-FABP (limit of detection 3.63 pg/mL; inter-assay variability 6.0–11.1%) and ELH-CRP and ELH-DDIMER (Raybiotech, Inc., Norcross, GA, USA) for CRP (limit of detection 34 pg/mL; inter-assay variability < 12%) and D-dimer (limit of detection 0.08 pg/mL; inter-assay variability <12%). Samples were run without any dilution for IL-6 quantification, at 1:200 dilution for sCD14, 1:5 for I-FABP, 1:20,000 for CRP, and 1:250,000 for D-dimer. Markers were measured in duplicate and values were averaged for analysis.
Statistical analysis
To assess the temporal trends of the log-transformed inflammatory biomarker levels as well as other continuous variables, we performed a general linear model (GLM) for repeated measures. Bonferroni adjustment was made when conducting multiple comparisons. Categorical variables were evaluated using a logistic regression with clustered standard errors to take into account the repeated evaluations at different times. The relationship between changes in biomarkers and other biological parameters was assessed using Spearman’s correlation. All statistical tests were two-sided and a p-value <0.05 was considered statistically significant. All analyses were performed using the IBM-SPSS Statistics version 22.0 (IBM Corp, Armonk, NY, USA).
Results
A total of 67 patients was included. Characteristic of patients at time of treatment switch (BL) are described in .
Table 1 Patients characteristics at baseline (n = 67).
For 64% (n = 43), 27% (n = 18), and 9% (n = 6) of the patients, the previous PI/r regimen was based on darunavir, atazanavir, and lopinavir, respectively. The main reasons for treatment switch were dyslipidemia (55.2%, n = 37) and gastrointestinal tract toxicity (13.4%, n = 9); drug interactions were reported in only a minority of patients (4.5%, n = 3), and in 18 subjects (26.9%) clinicians recommended a proactive switching strategy to avoid the risk of boosted-PI toxicity.
The evolution of biological parameters over time is shown in . All patients maintained virological suppression during the entire follow-up. Neither variation in co-morbidities arose nor changes in body mass index (BMI), concomitant medications, alcohol and smoking habits occurred over time.
Table 2 Evolution of biological parameters over time.
Concerning immunological profile, no difference in median CD4 T cell count was observed at the three time points. The CD4/CD8 ratio showed a trend toward improvement, but did not reach a statistically significant difference when levels before and after the switch to 3TC + DTG were compared.
With regard to the inflammation biomarker pattern, the levels of IL-6, CRP, I-FABP and D-dimer remained stable at three time points; however, CRP showed a slight decrease between BL and +48 W, although it was not statistically significant.
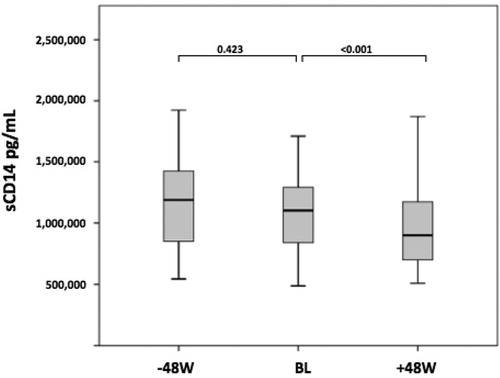
Notably, sCD14 showed a biphasic trend: levels were stable between −48 W and BL (p = 0.235) but significantly decreased between BL and +48 W (p < 0.001) (). Changes in sCD14 crude values are illustrated in ; between BL and +48 W a −15% median decrease was observed. Concurrently, total cholesterol, triglycerides, and LDL-cholesterol showed the same fluctuation with an improvement in the lipid profile after the switch to dual therapy with DTG ().
Figure 1 Crude median of sCD14 levels at the switch to DTG + 3TC (BL), 48 weeks before (-48 W) and 48 weeks after switch (+48 W). In the box plots, boundaries indicate the 25th and the 75th percentile, black lines within the box mark the median and whiskers above and below the box indicate the 10th and 90th percentiles. A GLM for repeated measures with Bonferroni adjustment was used for multiple comparisons; p-values for these comparisons are shown.
No correlation was observed between the decrease in sCD14 and the change in either triglycerides (p = 0.938), total cholesterol (p = 0.074), or LDL (p = 0.583).
We also performed a sub-group analysis to explore the effect of removing participants receiving statins. Overall, we found no major differences as compared to the main results.
The change in the sCD14 level between BL and +48 W consistently showed a significant reduction (p < 0.001); no difference in change was observed in the other markers over time. By contrast, the decrease in LDL levels (determined in 61% of subjects) showed a lack of statistical significance between BL and +48 W (p = 0.120). Triglycerides and total cholesterol levels confirmed a significant decrease after switch, without correlation with sCD14 change.
When patients were stratified according to the baseline regimen containing darunavir, atazanavir, or lopinavir, we observed no differences among the three groups for levels of the biomarkers at baseline or differences in changes in any markers over time as compared to the main results.
Discussion
Current guidelines recommend a 2-drug antiretroviral regimen as an alternative to triple ART in selected patients to reduce long-term toxicity and costs.Citation12
In the setting of virologically suppressed patients, the association of 3TC + boosted PI has the strongest evidence but 3TC + DTG is gaining popularity due to evidence from observational studies and recent clinical trials.Citation14
This is the first study describes that the effects of a treatment switch from a maintenance dual therapy with 3TC + PI/r to 3TC + DTG on markers of monocyte activation and inflammation. Our results show a significant decline in levels of sCD14 after substitution of PI/r with DTG. This finding may have important clinical implications because sCD14 has been associated with morbidity and all-cause mortality in HIV-treated infection.Citation2,Citation3
Although the percent decline in sCD14 in our study does not seem to be dramatic, it was actually markedly greater than the variation of the assay; moreover, even if the limitations of extrapolating data to different patient populations can be significant, data from previous studies suggest that the magnitude of the sCD14 reduction, we observed 48 weeks after switching to 3TC + DTG might have important clinical significance.Citation2,Citation3 Some previous observations suggest a potential beneficial role of InSTI on monocyte activation. A large cross-sectional study showed that lower sCD14 levels correlated with the use of InSTI, but no data were reported on specific drugs.Citation15 Moreover, a decrease in sCD14 was previously observed in virologically suppressed women who switched triple ART to RAL in a small randomized trial.Citation10 However, data for DTG are very scanty. To date, the only existing data are the preliminary results from the STRIIVING trial, which evaluated the effect of switching to single tablet DTG/abacavir/lamivudine on markers of immune activation/inflammation and showed a significant decline in sCD14 after 24 weeks in the switch arm when compared to the ongoing triple ART.Citation16 However, it should be noted that in that preliminary analysis, the effects on monocyte activation were likely to be not only related to the switch to DTG, because the backbone was modified in most patients as well; moreover, the pre-switch third agent was heterogeneous because it consisted of NNRTI, PI and included also InSTI. In the setting of dual therapy with 3TC, our analysis was not affected by the backbone; moreover, it specifically examined the effects of switching from PI/r- to DTG in InSTI-naïve subjects and, as aconsequence, was less influenced by confounders. On the basis of previous findings, it is tempting to hypothesize that in our study DTG resulted in more favorable changes in sCD14 levels than PI/r owing to a hypothetical intrinsic anti-inflammatory activity of the InSTI class, and more specifically to its positive effect on monocyte activation. A mechanistic hypothesis could be that InSTI may achieve higher levels in enterocytes.Citation7 Thus, DTG might result in a better control of local viral replication in gut-associated lymphoid tissues and resulting bacterial translocation than PI/r. This could be important because microbial translocation is a driver of immune activation in HIV infection and sCD14 is traditionally used as a marker of microbial translocation, even if it is not a direct and specific marker. To better explore how a potentially decreased microbial translocation could contribute to the observed decline in the sCD14 level, in this study, we also assessed changes in plasma levels of I-FABP, which is a marker of enterocyte damage that has also been correlated with intestinal impairment and microbial translocation.Citation17 However, we did not find any significant variation following the switch to DTG; as a consequence, this hypothesis cannot be confirmed by our findings. Alternatively, a decrease in sCD14 might be attributable to a beneficial effect on the lipid profile of InSTI-based regimens when compared to PI/r-based ones, as observed in our study and previously reported in others.Citation5,Citation6 Although we did not find any correlation between changes in monocyte activation marker and lipid levels, accordingly with other studies,Citation8,Citation18 we cannot exclude that DTG might have a favorable effect on proinflammatory oxidized lipid levels when compared to PI/r; thus, this hypothesis should be further investigated because some studies reported that oxidized lipids might be associated with monocyte activation in HIV infection.Citation19–20
We can also hypothesize that the dual regimen with DTG plus 3TC might be more able than the boosted PI-regimen to control residual viral replication in reservoirs such as monocyte subsets, although specific studies are certainly needed to address this complex issue. Clearly, this study has several limitations. First, the lack of matched control samples did not allow for comparison with a control group; moreover, the relatively small sample size. Nevertheless, we considered to have limited analysis bias with the crossover design; also, the magnitude in sCD14 improvement and the fact that our results were in keeping with previous observations with other InSTI strengthen our finding. Taken together, these factors lead us to be confident that the favorable variation in sCD14 represents a true finding. Another limitation is that we currently have no conclusive mechanistic explanation of how switching from 3TC + PI/r to 3TC + DTG decreases monocyte activation. Nevertheless, we can conclude that the decline in plasma levels of sCD14 observed after 48 weeks from switch to 3TC + DTG in virologically suppressed patients adds further important data in support of this maintenance strategy and suggests a possible association with a lower mortality and/or the development of comorbidities compared to 3TC + PI/r which warrants further investigations.
Acknowledgments
We would like to thank Dr Arianna Emiliozzi, Dr Chiara Picarelli, Dr Davide Moschese and Dr Alex Dusina from Catholic University of Sacred Heart, Institute of Clinical Infectious Diseases, Rome, for their contribution in data collection.
Disclosure statement
A.B. has received nonfinancial support from Bristol-Myers Squibb and ViiV Healthcare, and personal fees from Gilead Sciences. G. B. has received a travel grant from Bristol-Myers Squibb. R. C. has been an advisor for Gilead, Janssen-Cilag and Basel Pharmaceutical, has received speakers’ honoraria from ViiV Healthcare, Bristol-Myers Squibb, Merck Sharp & Dohme, Abbott, Gilead and Janssen-Cilag, and has received research support from ‘Fondazione Roma’. M.F. received speakers’ honoraria and support for travel to meetings from Bristol-Myers Squibb, Merck Sharp & Dohme, Janssen-Cilag, ViiV Health Care and Gilead. S. D. G. was a paid consultant or member of advisory boards for Gilead, ViiV Healthcare, Janssen-Cilag, Merck Sharp & Dohme and Bristol-Myers Squibb. All other authors: none to declare.
Additional information
Funding
Notes on contributors
Francesca Lombardi
Dr. Francesca Lombardi received Degree in Biology, Sapienza University of Rome; specialist degree in “Clinical Biochemistry” at University of Rome “Tor Vergata”. She worked at Children’s Hospital Los Angeles from 2009 to 2011, her main interests of research was HIV. Since 2012 she has worked at the Institute of “Clinical Infectious Diseases” at Catholic University of Sacred Heart of Rome in the HIV research field, participating in randomized clinical trials and performing observational studies mainly focused on antiretroviral therapy.
Simone Belmonti
Dr. Simone Belmonti is a Clinical Laboratory Technologist, graduated from Sapienza University of Rome. He worked at the Italian National Institute of Health and San Raffaele Scientific Institute, Milan, and since 2011 he has been a member of the HIV research team at the Institute of “Clinical Infectious Diseases” at Catholic University of Sacred Heart of Rome.
Alberto Borghetti
Dr. Alberto Borghetti graduated in Medicine and Surgery at Catholic University of Sacred Heart of Rome in 2012 and became specialist in Infectious Diseases in 2019. Since 2012 he has worked in the field of HIV research and clinic, participating in randomized clinical trials and performing observational studies mainly focused on antiretroviral therapy and management of chronic HIV infection. Since 2019 he has worked as an Infectious Diseases consultant in medical and surgical wards at Fondazione Policlinico A. Gemelli in Rome, while continuing his research activity on HIV.
Arturo Ciccullo
Dr. Arturo Ciccullo is a resident in infectious diseases and tropical medicine at Catholic University of the Sacred Heart in Rome since 2016. He received his degree in Medicine and Surgery there in 2015. At the moment, his research activity mainly focuses on HIV infection management and antiretroviral therapy. He authored 20 publications in international peer-reviewed journals.
Gianmaria Baldin
Dr. Gian M. Baldin graduated at University of Padua, Italy, in 2014 and became specialist in Infectious and Tropical Diseases at Catholic University of Sacred Heart in 2018. His research interests are HIV antiretroviral therapy. He performed a trainee in Ikonda hospital in Tanzania from October to December 2017.
Roberto Cauda
Dr. Roberto Cauda MD, is the Chairman of Infectious Diseases and Director of Specialisation School in Infectious Diseases and Tropical Medicine at Catholic University of Sacred Heart of Rome, Fondazione Policlinico A. Gemelli. Adjunct Professor at the University of Alabama in Birmingham, and Texas Tech University. Visiting Professor in Slovak Universities; honorary fellow of ESCMID, Royal Society of Tropical Medicine and Hygiene, ESBIC. Research: antibiotic resistance and nosocomial infections, clinical and epidemiological aspects of HIV infection/disease, viral hepatitis, herpetic and tropical diseases.
Massimiliano Fabbiani
Dr. Massimiliano Fabbiani graduated in Medicine and Surgery at University of Siena in 2003 and became specialist in Infectious Diseases there in 2007. He obtained his PhD in biological and clinical research on infectious and tropical disease. He was Infectivology Physician at Catholic University of Sacred Heart of Rome and later San Gerardo Hospital, University of Milano-Bicocca, Monza. Currently he works at the Clinic of Infectious and Tropical Diseases of Foundation IRCCS Polyclinic San Matteo-University of Pavia.
Simona Di Giambenedetto
Simona Di Giambenedetto MD, graduated in Medicine and Surgery at Catholic University of Sacred Heart of Rome in 1998. She obtained the title of specialist in Infectious Diseases in 2003. From 2004 to 2008 she worked as a MD with a contract at the Institute of “Clinical Infectious Diseases” at Policlinico Gemelli and since 2009 she has worked as a Clinical Researcher at the Institute of “Clinical Infectious Diseases” at Catholic University of Sacred Heart of Rome. Her research activity is predominantly focused on HIV infection, opportunistic infections in AIDS and antiretroviral therapy.
References
- Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;8:1248–1259.
- Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790.
- Wada NI, Bream JH, Martínez-Maza O, et al. Inflammatory biomarkers and mortality risk among HIV-suppressed men: a multisite prospective cohort study. Clin Infect Dis. 2016;63(7):984–990.
- Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep. 2017;14(3):93–100.
- Gatell JM, Assoumou L, Moyle G, et al. Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS 2017;31(18):2503–2514.
- Martinez E, Larrousse M, Llibre JM, et al. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS 2010;24(11):1697–1707.
- Patterson KB, Prince HA, Stevens T, et al. Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure. AIDS 2013;27(9):1413.
- Martínez E, D’Albuquerque PM, Llibre JM, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS 2012;26(18):2315–2326.
- Silva EF, Charreau I, Gourmel B, et al. Decreases in inflammatory and coagulation biomarkers levels in HIV-infected patients switching from enfuvirtide to raltegravir: ANRS 138 substudy. J Infect Dis. 2013;208(6):892–897.
- Lake J, Mccomsey GA, Hulgan T, et al. Switch to raltegravir decreases soluble CD14 in virologically suppressed overweight women: the women, integrase and fat accumulation trial. HIV Med. 2014;15(7):431–441.
- Taiwo BO, Marconi VC, Berzins B, et al. Dolutegravir plus lamivudine maintain HIV-1 suppression through week 48 in a pilot randomized trial. Clin Infect Dis. 2018;66(11):1794–1797.
- Panel on antiretroviral guidelines for adults and adolescents DHHS. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV Web site. https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed October, 2017.
- Delaney JA, Suissa S. The case-crossover study design in pharmacoepidemiology. Stat Methods Med Res. 2009;18(1):53–65.
- Rossetti B, Montagnani F, De Luca A. Current and emerging two-drug approaches for HIV-1 therapy in ART-naïve and ART-experienced, virologically suppressed patients. Expert Opin Pharmacother. 2018;19(7):713–738.
- Castley A, Williams L, James I, et al. Plasma CXCL10, sCD163 and sCD14 levels have distinct associations with antiretroviral treatment and cardiovascular disease risk factors. PLoS ONE. 2016;11(6):e0158169.
- Lake J, Currier J, Koteff J, et al. Cardiovascular biomarkers after switching to ABC/DTG/3TC : the STRIVING study. Paper presented at: 23rd Conference on Retroviruses and Opportunistic Infections, 2016; Boston, MA.
- Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26(1):2–18.
- Belmonti S, Lombardi F, Quiros-Roldan E, et al. Systemic inflammation markers after simplification to atazanavir/ritonavir plus lamivudine in virologically suppressed HIV-1-infected patients: ATLAS-M substudy. J Antimicrob Chemother. 2018;73(7):1949–1954.
- Kelesidis T, Jackson N, McComsey GA, et al. Oxidized lipoproteins are associated with markers of inflammation and immune activation in HIV-1 infection. AIDS 2016;30(17):2625–2633.
- Zidar DA, Juchnowski S, Ferrari B, et al. Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J Acquir Immune Defic Syndr. 2015;69(2):154–160.
