Abstract
Background: Long-acting (LA) injectable antiretroviral therapy (ART) is a novel modality currently under development as an alternative to daily oral ART.
Objective: The LATTE-2 study (ClinicalTrials.gov identifier NCT02120352) showed that cabotegravir LA + rilpivirine LA maintained virologic suppression through 96 weeks and included further exploration of patient-reported treatment outcomes with an LA injectable form of treatment.
Methods: Two-hundred and eighty-six virologically suppressed participants on oral cabotegravir + abacavir/lamivudine once-daily tablets (induction period) were randomized to cabotegravir LA + rilpivirine LA once every 4 weeks (n = 115), once every 8 weeks (n = 115), or the continuation of the oral tablet regimen (n = 56) during the maintenance period. Patient-reported outcome measures included the HIV Medications Questionnaire (HIVMQ) and the HIV Treatment Satisfaction Questionnaire status (HIVTSQ[s]) and change (HIVTSQ[c]) versions at prespecified study visits through Week 96 of the randomized maintenance period.
Results: Most participants in the LA injectable groups reported injection-site–related adverse events; however, participants in the 4-week (median HIVTSQ[s] total score, 63.5; post hoc P = 0.02) and 8-week (65.0; post hoc P < 0.001) LA injectable groups were significantly more satisfied with treatment than participants in the oral maintenance group (60.0) at Week 96. This was consistent with results from the HIVTSQ[c] at Week 32, which revealed that participants in both LA groups were significantly more satisfied with therapy compared with patients receiving oral ART (both post hoc P < 0.001).
Conclusion: Participants who received LA injectable therapy had high levels of treatment satisfaction and favorably viewed convenience and lifestyle-related aspects of the therapy.
Introduction
Approaches to treatment of HIV-1 infection involve lifelong therapy with multiple antiretroviral medications from multiple drug classes.Citation1 Advances in these agents have resulted in improved antiviral effectiveness, transforming HIV-1 infection into a chronic, manageable condition.Citation2 Interest is growing in treatment options that take patient convenience, preferences, and medication accessibility into account.Citation3 Long-acting (LA) injectable antiretroviral therapy (ART) offers the potential for a convenient approach to managing HIV infection while avoiding daily oral dosing and the need to store and handle medications as patients undertake daily activities.Citation4
The LATTE-2 study investigated an LA injectable regimen composed of the integrase inhibitor cabotegravir and the non-nucleoside reverse transcriptase inhibitor (NNRTI) rilpivirine.Citation5 Once-daily oral cabotegravir is well tolerated and has an acceptable safety profile, a long half-life (40 h), and minimal drug–drug interactions.Citation6–8 Rilpivirine (TMC278) is an NNRTI approved as a 25-mg, once-daily oral medication for HIV-1 treatment.Citation9 Cabotegravir and rilpivirine have each been formulated as LA injectable preparations that maintain therapeutically relevant plasma concentrations for treatment of HIV-1 infection.Citation10 When administered intramuscularly (IM) every 4 or 8 weeks in the LATTE-2 study, 87% (4-week regimen) and 94% (8-week regimen) of participants on cabotegravir LA + rilpivirine LA maintained HIV-1 RNA <50 copies/mL at Week 96 (FDA snapshot algorithm) compared with 84% on oral cabotegravir + abacavir/lamivudine.Citation5
Because cabotegravir LA + rilpivirine LA is a novel antiretroviral regimen with a unique route of administration and dosing interval, detailed analysis of patient-reported acceptability, tolerability, and satisfaction with treatment is warranted. We report the patient-reported outcomes (PROs) associated with the LA injectable regimen at Week 96.
Methods
Study design
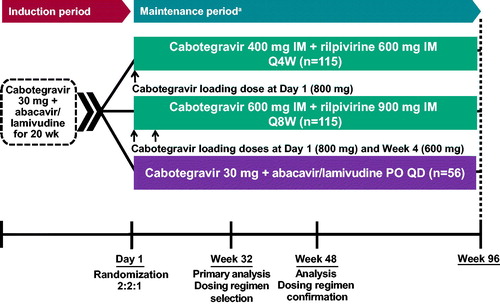
PROs were assessed in LATTE-2, an ongoing phase IIb, randomized, multicenter, open-label, parallel-group study conducted at 50 sites in the United States, Canada, Spain, France, and Germany.Citation5 An open-label design was used to minimize trial-design complexities and pill burden while eliminating the requirement for sham injections, as would have been required by a double-blind, double-dummy design. The study included a 20-week induction period and a 96-week maintenance period (). The first patient was screened in April 2014, and the last patient visit occurred in November 2016 (Week 96). The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants, and the protocol was approved by the institutional review board of each study site. The protocol summary was posted to www.clinicaltrials.gov (ClinicalTrials.gov identifier, NCT02120352).
Study participants
Eligible patients were HIV-1 positive, aged ≥18 years, and had ≤10 days of previous ART, with HIV-1 RNA ≥1000 copies/mL and CD4+ T-cell counts ≥200 cells/mm3 at screening. Key exclusion criteria included the presence of any major antiretroviral resistance–associated mutation; pregnancy; moderate or severe hepatic impairment; clinically relevant hepatitis; hepatitis B virus infection; laboratory values of clinical concern; creatinine clearance <50 mL/minute; or need for long-term anticoagulants.
Study treatments
During the induction period, all eligible participants received oral cabotegravir 30 mg plus abacavir/lamivudine 600 mg/300 mg once daily for 20 weeks. Once-daily oral rilpivirine 25 mg was added at Week −4 (following 16 weeks of the induction regimen) and continued until the first injection visit (Day 1). Participants were eligible to enter the maintenance period at Day 1 if they tolerated the induction period regimen and achieved plasma HIV-1 RNA <50 copies/mL at Week −4. On Day 1, these participants were randomized to receive cabotegravir LA + rilpivirine LA as IM injections every 4 or 8 weeks or to continue taking oral cabotegravir + abacavir/lamivudine daily (or alternative nucleoside reverse transcriptase inhibitors if HLA-B*5701 positive). The LA injectable formulations contained cabotegravir 200 mg/mL and rilpivirine 300 mg/mL as separate injections into the gluteus medius. All participants had study visits every 4 weeks through Week 48. After Week 48, participants in the 8-week injection and oral treatment groups had study visits every 8 weeks, whereas the 4-week injection treatment group continued study visits every 4 weeks.
Participants in the 4-week LA injectable treatment group received an initial loading dose of cabotegravir LA 800 mg (two 2-mL injections) and rilpivirine LA 600 mg (one 2-mL injection) on Day 1 and then they received cabotegravir LA 400 mg (one 2-mL injection) and rilpivirine LA 600 mg (one 2-mL injection) every 4 weeks from Weeks 4 to 92. Participants in the 8-week LA injectable treatment group received an initial loading dose of cabotegravir LA 800 mg (two 2-mL injections) and rilpivirine LA 900 mg (one 3-mL injection) on Day 1; then they received a second loading dose, cabotegravir LA 600 mg (one 3-mL injection) at Week 4, and afterward received cabotegravir LA 600 mg (one 3-mL injection) and rilpivirine LA 900 mg (one 3-mL injection) every 8 weeks from Weeks 8 to 88.
Outcomes
The primary endpoints were percentage of participants who received at least one dose of study drug during the maintenance period and had HIV-1 RNA <50 copies/mL at Week 32 (according to the US Food and Drug Administration [FDA] snapshot algorithm), percentage of participants with protocol-defined virological failures, and safety results as assessed by adverse events (AEs) and laboratory abnormalities. Key secondary endpoints were tolerability, acceptability, and satisfaction of IM dosing of cabotegravir and rilpivirine. A key safety and tolerability domain monitored in LATTE-2 was the rate of injection-site reactions (ISRs), as represented by any of the following: pain, swelling, nodules, induration, pruritus, warmth, bruising, and erythema at the injection site.
The HIV Medication Questionnaire (HIVMQ; Health Psychology Research, Egham, UK), which was adapted from the Renal Medication Questionnaire (Health Psychology Research), was used to evaluate adherence to treatment and assess motivators and barriers to adherence associated with the LA injectable regimens compared with the oral regimen. The HIVMQ included six separate questions about each LA injectable and oral tablet medication. The responses were scored on a seven-point scale, with zero indicating “none of the time” or “none at all” and six representing “all of the time” or “a very great deal.” Item scores were reported and individually interpreted. The HIVMQ was administered 4 weeks before randomization; at Weeks 8, 32, 48, and 96; and at the withdrawal visit during the maintenance period.
Treatment satisfaction was measured using two versions of the revised HIV Treatment Satisfaction Questionnaire (HIVTSQ): the HIVTSQ[s] (status version) and the HIVTSQ[c] (change version).Citation11,Citation12 The contents of both versions are shown in . The HIVTSQ assessed change in within-participant treatment satisfaction over time (HIVTSQ[s]) and compared participants’ views of their current therapy with those of oral ART used during the induction phase (HIVTSQ[c]). The HIVTSQ[c] version helps account for the ceiling effects associated with the measure (ie, the inability to show substantial improvement at follow-up when participants have scored maximum or near maximum at baseline).Citation13 The HIVTSQ[s] was administered during the induction period (Weeks −16 and −4); on Day 1 (pre-dose); and at Weeks 8, 32, 48, and 96 (post-dose when possible) of the maintenance period. The HIVTSQ[c] was administered at Week 32 or at withdrawal for participants who withdrew. The original versions of the HIVTSQ included 10 items divided into general satisfaction/clinical and lifestyle/ease subscales.Citation11
Table 1 Item content in the HIVTSQ[s] and HIVTSQ[c] versions.Citation11,a
The revised HIVTSQ versions have the original 10 questionnaire items and 2 additional items assessing pain/discomfort and ease/difficulty of treatment. These two items were included to account for potential issues associated with injections. Eleven of these 12 items contribute to the total treatment satisfaction score, and the pain/discomfort item generates a stand-alone score.Citation12 A confirmatory factor analysis of HIVTSQ items showed high correlation between the pain/discomfort and side effect items, suggesting that pain/discomfort is regarded by patients as a side effect. However, inclusion of a pain/discomfort item ensures that this aspect of treatment, which is important for injectable modalities, is explicitly addressed.
For the HIVTSQ[s], higher scores indicate greater improvement in treatment satisfaction, with item scores ranging between 0 (very dissatisfied) and 6 (very satisfied) and the total treatment satisfaction score ranging from 0 to 66. For the HIVTSQ[c], item scores were between −3 (much less satisfied now) and 3 (much more satisfied now), and the total treatment satisfaction change score ranged from −33 to 33. Item responses were recoded and grouped from the questionnaire’s seven-point scale as a post hoc analysis: less favorable versus induction treatment, −3 to −1; neutral versus induction treatment, zero; more favorable versus induction treatment, 1 to 3.
Statistical analyses
The number and proportion of participants reporting AEs were tabulated for each group. The number and proportion of participants with each response to the items in the HIVTSQ[s] and HIVTSQ[c] were summarized by visit and group. Total satisfaction scores were summarized by visit and group using descriptive statistics (ie, mean, standard deviation [SD], median, interquartile range [IQR]). The statistical significance of treatment-group differences (each LA injectable regimen versus oral tablet regimen) for selected PRO endpoints was evaluated in post hoc analyses using the Wilcoxon rank sum test (two-sided α level of 0.05). Reported scores only included participants who remained on therapy at that time point. Results were evaluated using as-observed data, with no imputation for missing data. Data from the HIVMQ were presented separately for the cabotegravir LA and rilpivirine LA injections for pain/discomfort domains and were summarized across injections for the inconvenience/difficulty analysis.
Results
LATTE-2 enrolled 309 participants; 286 qualified for and entered the maintenance period (intention-to-treat maintenance exposed [ITT-ME] population).Citation5 Baseline characteristics were well balanced across the treatment groups. Among the participants in the ITT-ME population, 92% were male (n = 262) and 21% were nonwhite (n = 60). Through Week 96 of the maintenance period, 28 participants (10%) withdrew from the study—5 were in the 8-week LA injectable treatment group, 14 were in the 4-week LA injectable treatment group, and 9 were in the oral tablet treatment group. Of the 10 participants (3%) who reported an AE as the primary reason for withdrawal, one was in the 8-week LA injectable treatment group, eight were in the 4-week LA injectable treatment group, and one was in the oral tablet treatment group.
Most participants in the cabotegravir LA + rilpivirine LA 4-week and 8-week treatment groups reported an AE at the time of the Week 96 analysis. Injection-site pain was the most frequently reported AE in both LA injectable treatment groups through Week 96. The appearance of one or more episodes of injection-site swelling and nodules over time was also commonly reported (). Across the LA injectable treatment groups, most ISRs were considered mild (grade 1; 84% of ISRs) or moderate (grade 2; 15% of ISRs) and lasted a median of 3 days. The highest frequency of ISRs occurred on Day 1, with subsequent study visits through Week 96 reporting approximately one-half of the ISRs observed after the initial injections (). In addition, the percentage of participants receiving IM treatment who had moderate to severe ISR severity decreased from Day 1 (grade 2, 28%; grade 3, 3%) to subsequent study visits (Week 4: grade 2, 11%; grade 3, <1%). A difference in frequency was observed between the 4- and 8-week treatment groups in the rate of ISRs, with a mean of 1.19 ISRs per injection visit for the 8-week group compared with 0.89 ISRs per injection visit for the 4-week treatment group.
Figure 2 Participants reporting ISRs by visit through Week 96 of the maintenance period. AE, adverse event; IM, intramuscular; ISR, injection-site reaction; Q4W, every 4 weeks; Q8W, every 8 weeks. In the Q8W group, one participant completed the maintenance phase but did not receive injections at Week 96.
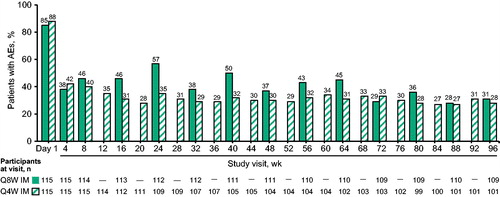
Table 2 Injection-site reactions in the cabotegravir + rilpivirine long-acting injectable treatment groups during the maintenance period through week 96.
The most commonly reported AEs other than ISRs through the combined induction and maintenance periods were nasopharyngitis (n = 39 [34%] in the 4-week group; n = 35 [30%] in the 8-week group; n = 22 [39%] in the oral treatment group), diarrhea (n = 32 [28%] in the 4-week group; n = 27 [23%] in the 8-week group; n = 11 [20%] in the oral treatment group), and headache (n = 27 [23%] in the 4-week group; n = 29 [25%] in the 8-week group; n = 14 [25%] in the oral treatment group).
The HIVMQ revealed no apparent differences between the cabotegravir and rilpivirine injections in patient-reported pain/discomfort between the LA injectable treatment groups (). The LA injectable regimen was associated with lower levels of inconvenience/difficulty in taking/receiving the medication compared with the oral tablet regimen in both the 4- and 8-week treatment groups (). The percentage of participants rating their pain/discomfort as zero (“none at all”) increased from Week 8—when the first PROs were collected—to Week 96 in both the 4- and 8-week treatment groups. It should be noted that the design of the LATTE-2 study may have resulted in the selection of participants who expected or hoped to receive LA therapy, potentially influencing treatment satisfaction.
Figure 3 Amount of pain/discomfort with cabotegravir LA + rilpivirine LA versus oral cabotegravir plus abacavir/lamivudine at Week 8 and Week 96 (HIVMQ, item F). Unlabeled bar values corresponding to item scores 3–6 represent ≤8% of respondents per bar. HIVMQ, HIV Medication Questionnaire; LA, long acting.
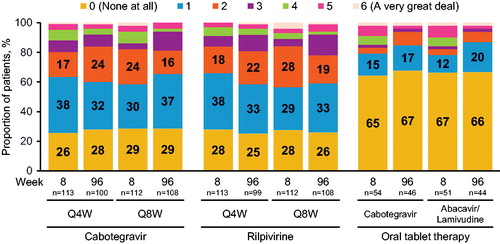
Table 3 Change over time in patient-reported inconvenience associated with cabotegravir and rilpivirine injections and oral tablet therapy (HIVMQ).
On the first HIVTSQ[s] administration at Week 8, 49% and 62% of participants in the 4- and 8-week LA injectable treatment groups, respectively, reported being “very satisfied” with the pain/discomfort associated with treatment (item score = 6). At Week 96, these proportions were 44% and 56%, respectively, indicating that participants in the less-frequent injection regimen were more satisfied. A higher proportion of participants in the injectable treatment groups reported being “very satisfied” with their current treatment compared with those in the oral tablet group at Week 96 (81% vs 76%). Higher proportions of participants in the injectable therapy groups reported that they would be “very satisfied” to continue with their current therapy (4-week, 88%; 8-week, 89%), compared with 43% in the oral tablet group. In addition, although treatment satisfaction was high in all groups, total satisfaction scores on the HIVTSQ[s] were significantly greater in the 4-week (median, 63.5; IQR [Q1–Q3], 59.0–66.0; n = 100; post hoc P = 0.02) and 8-week (median, 65.0; IQR [Q1–Q3], 60.5–66.0; n = 108; post hoc P < 0.001) LA injectable treatment groups compared with the oral tablet treatment group (median, 60; IQR [Q1–Q3], 56–64; n = 46) at Week 96, despite the occurrence of ISRs ().
Figure 4 (A) HIVTSQ[s] total scores at Day 1, Weeks 8, and 96. Boxplot values represent the median (center) and first and third quartiles (bottom and top); error bars represent the minimum and maximum scores. Comparisons made using the Wilcoxon rank sum test. Q4W, n = 100; Q8W, n = 108; PO, n = 46. (B) Response scores by item label on the general satisfaction/clinical subscale of the HIVTSQ[c] for participants on LA injectable therapy at Week 32 compared with oral treatment prior to randomization. Item responses were recoded and grouped from the questionnaire’s seven-point scale as a post hoc analysis: less favorable versus induction treatment, −3 to −1; neutral versus induction treatment, 0; more favorable versus induction treatment, 1–3. Unlabeled bar values correspond to ≤4% of respondents per bar. Q4W, n = 100; Q8W, n = 107; PO, n = 49. (C) Response scores by item label on the lifestyle/ease subscale of the HIVTSQ[c] for participants on LA injectable therapy at Week 32 compared with oral treatment prior to randomization. Item responses were recoded from questionnaire’s seven-point scale: less favorable versus induction treatment, −3 to −1; neutral versus induction treatment, 0; more favorable versus induction treatment, 1–3. Unlabeled bar values correspond to ≤4% of respondents per bar. Q4W, n = 100; Q8W, n = 107; PO, n = 49. HIVTSQ[c], HIV Treatment Satisfaction Questionnaire, change version; HIVTSQ[s], HIV Treatment Satisfaction Questionnaire, status version; LA: long acting; PO: once-daily oral tablets; SD: standard deviation; Q4W: cabotegravir LA + rilpivirine LA once every 4 weeks; Q8W: cabotegravir LA + rilpivirine LA once every 8 weeks. *P = 0.02 (post hoc) compared with oral dosing at Week 96. **P < 0.001 (post hoc) compared with oral dosing at Week 96. aItems added to the standard HIVTSQ specifically for the LATTE-2 study.
![Figure 4 (A) HIVTSQ[s] total scores at Day 1, Weeks 8, and 96. Boxplot values represent the median (center) and first and third quartiles (bottom and top); error bars represent the minimum and maximum scores. Comparisons made using the Wilcoxon rank sum test. Q4W, n = 100; Q8W, n = 108; PO, n = 46. (B) Response scores by item label on the general satisfaction/clinical subscale of the HIVTSQ[c] for participants on LA injectable therapy at Week 32 compared with oral treatment prior to randomization. Item responses were recoded and grouped from the questionnaire’s seven-point scale as a post hoc analysis: less favorable versus induction treatment, −3 to −1; neutral versus induction treatment, 0; more favorable versus induction treatment, 1–3. Unlabeled bar values correspond to ≤4% of respondents per bar. Q4W, n = 100; Q8W, n = 107; PO, n = 49. (C) Response scores by item label on the lifestyle/ease subscale of the HIVTSQ[c] for participants on LA injectable therapy at Week 32 compared with oral treatment prior to randomization. Item responses were recoded from questionnaire’s seven-point scale: less favorable versus induction treatment, −3 to −1; neutral versus induction treatment, 0; more favorable versus induction treatment, 1–3. Unlabeled bar values correspond to ≤4% of respondents per bar. Q4W, n = 100; Q8W, n = 107; PO, n = 49. HIVTSQ[c], HIV Treatment Satisfaction Questionnaire, change version; HIVTSQ[s], HIV Treatment Satisfaction Questionnaire, status version; LA: long acting; PO: once-daily oral tablets; SD: standard deviation; Q4W: cabotegravir LA + rilpivirine LA once every 4 weeks; Q8W: cabotegravir LA + rilpivirine LA once every 8 weeks. *P = 0.02 (post hoc) compared with oral dosing at Week 96. **P < 0.001 (post hoc) compared with oral dosing at Week 96. aItems added to the standard HIVTSQ specifically for the LATTE-2 study.](/cms/asset/f48cac80-8fc2-411e-991c-eefa938dc0a8/yhct_a_1661696_f0004_c.jpg)
At Week 32, participants in the 4- and 8-week LA injectable treatment groups reported being more satisfied with their LA injectable therapy compared with the oral cabotegravir + abacavir/lamivudine tablet therapy received in the 20-week induction phase prior to randomization across all 12 items on the HIVTSQ[c] (). Total satisfaction scores on the HIVTSQ[c] were significantly greater in the 4-week (median, 29; IQR [Q1–Q3], 24–33; n = 100; post hoc P < 0.001) and 8-week (median, 32; IQR [Q1–Q3], 27–33; n = 107; post hoc P < 0.001) LA injectable treatment groups compared with the oral tablet treatment group (median, 24; IQR [Q1–Q3], 4–31; n = 49) at Week 32). The item related to “willingness to continue therapy” received the highest scores, followed by “would recommend to a friend,” whereas the item assessing satisfaction with side effects received the lowest scores. On all HIVTSQ[c] items, a higher percentage of participants who received LA injectable therapy every 8 weeks reported being “much more satisfied” compared with those who received LA injectable therapy every 4 weeks.
Discussion
Cabotegravir LA + rilpivirine LA, administered by IM injections every 4 or 8 weeks, was developed to provide viable antiretroviral options for patients living with HIV-1 infection who prefer alternatives to daily oral therapy. Whether this novel regimen will satisfy patients’ expectations required a detailed investigation of patient-reported health outcomes and treatment satisfaction. The LATTE-2 study established the robust antiviral efficacy and acceptable safety profile of the cabotegravir LA + rilpivirine LA regimen.Citation5 This study reports planned secondary endpoints of LATTE-2 that supplement the clinical data with insights on patients’ perceptions of treatment tolerability and satisfaction; analyses of the statistical significance of PRO endpoint results (eg, P value calculations) were conducted post hoc.
One of the major findings is that patient-reported tolerability, acceptability, and preference for an IM injection were maintained through 96 weeks. Compared with the results at Week 32, the responses were the same or improved at Week 96, demonstrating that the LA injectable regimen is sustainable over time.
The clinical safety profile reported in the LATTE-2 study supported further clinical development of cabotegravir LA + rilpivirine LA, with few withdrawals related to AEs and no serious AEs considered to be drug related.Citation5 However, it is important to further characterize the ISRs that were observed in the majority of participants. These ISRs were most commonly identified as injection-site pain, of mild or moderate severity, and lasting on average approximately 3 days. Consistent with the AE data, participant responses on the HIVMQ indicated that both LA therapies were associated with more pain/discomfort than the oral regimen. Although participants reported pain with LA injectable therapy, most remained satisfied and were willing to continue with the injections, as indicated on the HIVTSQ[c]. It was also noted that the rate of reported ISRs was substantially lower following subsequent treatment administrations compared with Day 1, suggesting that the first injection may be more universally described as painful, but subsequent injections are better tolerated. In addition to patients becoming accustomed to the injections, accumulating health care provider experience with administration may lead to improved injection technique, thereby contributing to increased tolerability of subsequent injections. The improvement in tolerability observed after Day 1 may also be due to the extra injection given for the loading dose of cabotegravir, which required two injections on Day 1. Therefore, including the injection of rilpivirine, participants received a total of three injections on Day 1, whereas only two injections were received at all other study visits. In this study, injections were given into the gluteus medius; however, it is possible that tolerability may vary if another injection site were to be used.
The presence of self-limited ISRs did not appear to negatively impact the LATTE-2 study participants’ satisfaction with the overall treatment regimen. Participants reported a high degree of satisfaction with their study medication as indicated by responses on the HIVTSQ[s] and HIVTSQ[c]. A statistically significant difference in satisfaction was detected in the total HIVTSQ[s] score at Week 96, indicating higher satisfaction with injections compared with oral treatment. All participants in both LA injectable treatment groups expressed high willingness to continue treatment at Week 96, and HIVTSQ[c] items for convenience and lifestyle were associated with high satisfaction levels at Week 32. These results provide evidence that participants preferred the lifestyle and convenience factors associated with LA injectable therapy over those for daily oral therapy.
However, barriers to the acceptability and tolerability of LA injectable treatment approaches may remain. A recent focus-group study identified factors that could influence the acceptability of LA injectable treatments, including pain intensity, number of injections per dose, and side effects.Citation14 Patient characteristics may also influence the acceptability of LA injectable treatments; for example, patients experienced with ART expressed less enthusiasm for switching from established dosing routines, and patients taking multiple medications for other chronic conditions may see little benefit in reducing a small number of daily pills.Citation14 Other factors identified, such as the location of treatment facilities, body site for injections, and medication frequency and effectiveness, are likely to support the potential acceptability of cabotegravir LA + rilpivirine LA. In line with the treatment parameters used in the LATTE-2 study,Citation5 participants in the focus group study indicated that a treatment interval longer than one week was important for acceptability and that monthly or quarterly dosing was preferred.Citation14
In a separate substudy of participants (n = 27) in the LATTE-2 study, qualitative interviews were conducted at sites in both the United States (n = 11) and Spain (n = 16) to explore participants’ experiences with cabotegravir LA + rilpivirine LA.Citation15 Findings underscored the convenience of LA injectable therapy versus daily oral pills and the emotional benefits such as ameliorating the experience of internalized stigma and the related “daily reminder of HIV.” These logistical and psychosocial benefits helped participants live with more flexibility and with less anxiety over daily dosing and the risk of accidental HIV disclosure. Despite many participants describing some degree of pain related to injections, there was broad agreement that it was “worth it.” Participants generally reported feeling very comfortable visiting the clinics on a monthly or every two months’ basis to receive injections. However, several participants expressed concern about the number of clinic visits involved and the associated potential for unwanted HIV status disclosure.
There were limitations in the conduct of this study. The LATTE-2 study enrolled mostly white male individuals from middle and high income countries and excluded individuals with CD4+ T-cell counts <200 cells/mm3, as well as children and adolescents; these characteristics may limit the generalization of results to the broader population of those living with HIV.Citation16 Patient-reported treatment satisfaction could have been confounded by the patient population, which may have been self-selected to be more enthusiastic about an injectable regimen. Patient-reported outcomes collected at study withdrawal were not included in the observed case analyses at Weeks 8 and 32, thereby introducing a small degree of selection bias. All participants were required to attend visits at the same schedule until Week 48, and therefore the effect of the frequency of visits was not considered in the analysis of participant satisfaction. Lastly, the open-label design introduced limitations on using patient-reported preference data to compare injectable and oral ART.
Patient-reported tolerability, acceptability, and satisfaction likely influence the acceptability and uptake of a novel simplification approach such as cabotegravir LA + rilpivirine LA, which differs from approved therapies regarding its components, administration route, and dose timing. These factors will play a large role in determining the demographic and clinical characteristics of patients who may be best suited for this regimen. These secondary endpoints of LATTE-2 help contextualize the safety data from the trial by providing insight regarding how ISRs influence patients’ satisfaction with injectable therapies. Most LATTE-2 participants were willing to continue and/or recommend cabotegravir LA + rilpivirine LA to others, indicating that the lifestyle features of the regimen resonate with patients living with HIV infection despite the high incidence of ISRs.
Data availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Acknowledgments
All listed authors meet the criteria for authorship set forth by the International Committee of Medical Journal Editors. All authors had full access to the data and are responsible for the veracity and completeness of the reported data. The corresponding author had final responsibility for the decision to submit the manuscript for publication. Editorial assistance was provided under the direction of the authors by Leila Strickland, PhD, and Sherri Damlo, MedThink SciCom, and was funded by ViiV Healthcare.
Disclosure statement
M.M. was an employee of ViiV Healthcare at the time of the study. S.G., J.M., W.S., and D.M. are currently employed by ViiV Healthcare and are shareholders in GlaxoSmithKline. A.M. has received personal fees from Gilead Sciences, ViiV Healthcare, and Merck Sharp & Dohme. F.P. has received personal fees from Gilead Sciences, Janssen Pharmaceuticals, Merck Sharp & Dohme, and ViiV Healthcare. V.C. was an employee of Johnson & Johnson – Janssen Pharmaceuticals at the time of the study. D.D. is currently employed by and is a shareholder in GlaxoSmithKline. M.R. has participated in advisory boards for Merck, ViiV Healthcare, and Gilead Sciences; and has been a speaker for AbbVie, Allergan, and Gilead Sciences. R.L. and H.J. have nothing to disclose.
Additional information
Funding
Notes on contributors
Miranda Murray
Miranda Murray, PhD, was the Head of the Health Outcomes group within the medical organization at ViiV Healthcare at the time of the study. Prior to joining ViiV Healthcare, Dr Murray worked at the English Health Protection Agency on Surveillance and Epidemiology of Healthcare Associated Infections. Her current research interests include behavioral research; patient/physician communication; and patient-reported outcomes measures in clinical trials. Dr Murray holds a PhD from Johns Hopkins University, Bloomberg School of Public Health.
Federico Pulido
Federico Pulido, MD, is Associate Professor of Medicine in the Department of Health Sciences at the Complutense University of Madrid, Spain. He also serves as Associate Director of the HIV Unit at the 12 de Octubre University Hospital in Madrid. He acts as a primary care provider for HIV-infected persons and as a teacher for medical students, residents, and others on the fundamentals of HIV medicine and clinical research. Dr Pulido has been involved in the care of HIV-infected patients since the earliest stages of the epidemic, providing care for more than 2300 HIV-infected persons. He is a senior researcher in the Instituto de Investigación Sanitaria Hospital 12 de Octubre with a diverse research agenda that includes participation in the Spanish AIDS Study Group and the Spanish AIDS Research Networks. He is also a member of the expert panel of the AIDS Study Group of the Spanish Society of Infectious Diseases and Clinical Microbiology and the National AIDS Plan. Dr Pulido received his medical degree from Autonoma University School of Medicine, Madrid, and completed his residency in Internal Medicine at 12 de Octubre University Hospital.
Anthony Mills
Anthony Mills, MD, is Chief Medical Officer of the SoCal Men’s Medical Group, Assistant Professor of Clinical Medicine at the University of California, Los Angeles, and a member of the editorial advisory board of Muscle & Fitness Magazine. A leading clinician in the fields of men’s health and HIV, Dr. Mills serves as the primary care provider for more than 2000 patients, including approximately 1000 living with HIV Dr. Mills is a member of the Infectious Disease Society of America, HIV Medical Association, International AIDS Society, IAS-USA, and the American Academy of HIV Medicine. He earned his undergraduate and medical degrees from Duke University and then completed an internship in internal medicine, his residency in anesthesiology, and a fellowship in cardiovascular anesthesiology at the University of California, San Francisco.
Moti Ramgopal
Moti Ramgopal, MD, FACP, FIDSA, is Consultant Physician and Director of Associates in Infectious Diseases; Founder of Midway Immunology and Research Center, Midway Specialty Care Center, Midway Primary Care, and Asvins.org; and Associate Professor of Medicine at Florida State University Medical School. He has been in practice for more than 20 years. Dr. Ramgopal is certified by the American Board of Internal Medicine and board certified in infectious diseases. Dr. Ramgopal received his medical degree from the University of the West Indies Faculty of Medical Sciences, completed his residency in internal medicine at Bon Secours Hospital in Michigan, and earned a fellowship in infectious diseases at Jackson Memorial and the University of Miami School of Medicine.
Roger LeBlanc
Roger LeBlanc, MD, is Cofounder of the OPUS clinic in Montreal, Canada, and Assistant Professor at McGill University. Dr. LeBlanc has managed a clinical practice with a large urban community with people living with HIV, LGBTQ patients, and patients with hepatitis B and C. His main areas of research are the primary and tertiary care of people with HIV, sexually transmitted infections, disease prevention, and harm reduction through lifestyle modifications and the long-term safety of drug therapy. Much of his research interest lies in addiction medicine, medical care for transgendered persons, and the screening and treatment of hepatitis C. He earned a bachelor’s degree in biochemistry, his medical degree from McGill University, and completed residencies in internal medicine and infectious diseases at the Royal Victoria Hospital in Montreal.
Hans Jaeger
Hans Jaeger, MD, is Medical Director of MVZ Karlsplatz, HIV Research and Clinical Care Centre, Munich; Founder of MUC Research; and Chairman of the Board of Directors of KIS - Curatorium for Immunedeficiency. Between 1983-1989, he was Clinical Director of the Out-Patient Department for Immunedeficiency at the Academic Teaching Hospital Munich-Schwabing. His scientific activities include coordinating and/or principal investigation of several clinical studies, especially on HIV/AIDS. He has served as chairman of numerous scientific national conferences on HIV/AIDS and as an advisor for national and international boards. Dr. Jaeger received his medical degree from the Technical University Munich and completed fellowships in oncology at the University of Rochester, New York, and Memorial Sloan Kettering Cancer Center, New York.
Viviam Canon
Viviam Canon was a medical physician overseeing the clinical development of long-acting rilpivirine for Janssen Research & Development at the time of the study.
David Dorey
David Dorey, MMATH, is Statistics Manager at GlaxoSmithKline. His research interests include the design and analysis of clinical trials. He earned a master’s degree in Mathematics from the University of Waterloo.
Sandy Griffith
Sandy Griffith, PharmD, is Director of Clinical Development at ViiV Healthcare.
Joseph Mrus
Joseph Mrus, MD, MSc, is Executive Medical Director for the North America Medical Affairs organization at ViiV Healthcare. Prior to joining ViiV Healthcare, Dr Mrus worked for Johnson & Johnson, GlaxoSmithKline, and the University of Cincinnati. His career has been predominately focused on optimizing care for patients with HIV. He is a board-certified internist and pediatrician. Dr Mrus earned his medical degree from the University of Texas Health Science Center, Houston, and masters’ degrees in Biomedical Engineering from the University of Texas and in Epidemiology from the Harvard School of Public Health.
William Spreen
William Spreen, PharmD, is the Director of Research and Development at ViiV Healthcare and the Medicine Development Leader for the HIV integrase inhibitor cabotegravir, a long-acting injectable agent under investigation for both HIV prevention and treatment. In more than 20 years with GSK, he has led global development programs for a number of small-molecule antiretroviral agents, culminating in approval of dolutegravir, abacavir, and the combination products Trizivir® and Epzicom/Kivexa®. Dr Spreen and colleagues also led GSK’s efforts to validate the clinical utility of a genetic-based screening test for abacavir hypersensitivity reaction. He received his PharmD from The Ohio State University.
David Margolis
David Margolis, MD, is the Director of Clinical Development and R&D at ViiV Healthcare and serves as the Project Physician Lead for the long-acting integrase inhibitor cabotegravir. At GSK and ViiV Healthcare, his research focus has been on HIV drug development and HIV clinical trials. Dr Margolis earned his MPH at the University of North Carolina at Chapel Hill and earned his MD at Duke Medical University School of Medicine. He was a resident in internal medicine at the University of Colorado and completed a fellowship in infectious diseases at the University of California, San Diego.
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Rockville, MD: US Department of Health and Human Services; 2018. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed January 22, 2019.
- Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194(1):11–19.
- Vitoria M, Ford N, Doherty M, Flexner C. Simplification of antiretroviral therapy: a necessary step in the public health response to HIV/AIDS in resource-limited settings. Antivir Ther. 2014;19(suppl 3):31–37.
- Margolis DA, Boffito M. Long-acting antiviral agents for HIV treatment. Curr Opin HIV Aids. 2015;10(4):246–252.
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510.
- Spreen WR, Margolis DA, Pottage J. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013;8(6):565–571.
- Spreen W, Min S, Ford SL, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013;14(5):192–203.
- Ford SL, Gould E, Chen S, et al. Lack of pharmacokinetic interaction between rilpivirine and integrase inhibitors dolutegravir and GSK1265744. Antimicrob Agents Chemother. 2013;57(11):5472–5477.
- Edurant [package insert]. Titusville, NJ: Janssen Therapeutics;2011; revised 2017. https://www.janssencarepath.com/sites/www.janssencarepath.com/files/prescribing-information-edurant.pdf. Accessed January 22, 2019.
- Williams PE, Crauwels HM, Basstanie ED. Formulation and pharmacology of long-acting rilpivirine. Curr Opin HIV Aids. 2015;10(4):233–238.
- Woodcock A, Bradley C. Validation of the revised 10-item HIV Treatment Satisfaction Questionnaire status version and new change version. Value Health. 2006;9(5):320–333.
- Romaine J, Murray M, Bradley C. Psychometric evaluation of the revised HIV Treatment Satisfaction Questionnaire (HIVTSQ). Abstract presented at: Annual European Congress of the International Society For Pharmacoeconomics and Outcomes Research; October 29–November 2, 2016; Vienna, Austria.
- The Health Psychology Research Group. HIVTSQ Guidelines. London: Royal Holloway of the University of London; 2015.
- Simoni JM, Beima-Sofie K, Mohmed ZH, Collier AC, Ho R. Acceptability of long-acting injectable ART: provider and patient preferences. Poster presented at Adherence 2017; June 4–6, 2017; Miami, FL.
- Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: A qualitative study among PLHIV participating in a phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One. 2018;13(1):e0190487.
- World Health Organization (WHO) Support for Country Impact. Progress Report 2016: Prevent HIV, Test and Treat All. Geneva: WHO; 2016. http://www.who.int/hiv/pub/progressreports/2016-progress-report/en. Accessed January 22, 2019.