Abstract
Background: Antiretroviral therapy (ART) initiation is associated with decreases in bone mineral density (BMD).
Objectives: To plan for a larger trial, we sought to obtain preliminary estimates for the difference in the change in BMD at 48 weeks achieved with 24 weeks of prophylactic alendronate/vitamin D during ART initiation compared to no intervention, the within-group standard deviation of this change, and intra-patient correlation coefficient for repeated BMDs. Secondary objectives included assessing enrollment feasibility, treatment acceptability, adherence and safety.
Methods: We randomized treatment-naïve HIV-positive adults initiating tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat or abacavir/lamivudine/dolutegravir 1:1:1 to immediate alendronate/vitamin D3 70 mg/5600 IU for 24 weeks (concomitant treatment arm, CTA), the same intervention starting 24 weeks after study entry (delayed treatment arm, DTA), or no bone anti-resorptive therapy (standard of care, SOC). We assessed BMD, acceptability, adverse events and drug adherence at baseline, week 24 and week 48.
Results: Of 29 included participants, 72% initiated TDF/FTC/ELV/c and 28% initiated ABC/3TC/DTG. Median (IQR) CD4 count was 388 (303,525) cells/mm3 and median plasma HIV RNA was 4.45 (2.26, 4.84) log10 copies/mL. The mean (SD) percentage change in BMD for the CTA and DTA combined was 1.95% (2.53%), 0.38% (3.34%), and −0.57% (3.50%) at the lumbar spine, femoral neck and total hip respectively at 48 weeks. The ICC among repeated measurements of BMD was 0.978, 0.964, and 0.967 at these sites, respectively. Enrollment feasibility, drug acceptability, adherence, and tolerability were good.
Conclusions: Our findings inform the sample size for a larger trial of bone anti-resorptive therapy during ART initiation and support feasibility.
Introduction
Individuals living with HIV experience an increased rate of bone loss and higher risk of fracture compared to HIV-negative controls. Changes in bone strength can be quantified through the measurement of bone mineral density (BMD) at the lumbar spine and proximal femur, a validated surrogate marker for fracture risk based on studies in the general population.Citation1 For instance, numerous studies have shown prevalences of low BMD as high as 40–83% in people living with HIV.Citation2–8 A meta-analysis of 20 studies suggested that HIV-positive adults were 6.4 times more likely to have low BMD, and 3.7 times more likely to have osteoporosis, than HIV-negative controls.Citation9 Fracture prevalence has been estimated at 2.87 per 100 HIV-positive individuals compared with only 1.77 per 100 HIV-negative individuals (p < 0.0001), with an overrepresentation of fractures at fragility sites such as the vertebrae, hip and wrist.Citation10 Other studies have similarly shown increased rates and incidence of fractures in the setting of HIV.Citation11,Citation12
The etiology of these problems is likely multifactorial, and includes an over-representation of traditional risk factors such as smoking, hypogonadism, anorexia, low body mass index, and alcohol consumption;Citation13,Citation14 HIV proteins have been shown to upregulate receptor activator of nuclear factor kappa B ligand (RANKL), which in turn increases osteoclast activity.Citation15,Citation16 Importantly, antiretroviral therapy (ART) also plays a major role, and clinical trials of first-line ART among treatment-naïve HIV patients have demonstrated dramatic net BMD decreases of 2–6% during the first year after initiating therapy.Citation4,Citation17–19 However, the standard approach to bone health in HIV has typically ignored this critical time window. Instead, experts and clinical guidelines recommend monitoring for osteoporosis by assessing BMD in patients who have accumulated enough clinical risk for fracture over time.Citation20–22 For those with established osteoporosis, bone anti-resorptive therapies, such as the widely used bisphosphonate alendronate, have been shown to improve BMD in people living with HIV,Citation23–25 and clear evidence of decreased fracture risk with these drugs exists for the general population.Citation26
Given the striking and predictable decreases in BMD seen during ART initiation, however, an alternative strategy of giving short-course prophylactic anti-resorptive therapy concomitant with ART initiation is intuitively more attractive than waiting for osteoporosis to develop. Since BMD typically stabilizes after the first year on ART, a short course of therapy may be adequate, and could decrease or avoid the costs and toxicities of long-term osteoporosis treatment. An extensive literature has already shown that alendronate prevents BMD loss in postmenopausal women,Citation27–30 patients taking glucocorticoids,Citation31–33 and men receiving androgen deprivation therapy for prostate cancer.Citation34,Citation35 Indeed, high dose vitamin D with calcium supplementation has already been shown to attenuate BMD loss in HIV-positive patients initiating ART,Citation36 and a single phase 2 b clinical trial found that zoledronic acid reduced BMD loss by 65% at 24 weeks in adults initiating ART with tenofovir disoproxil fumarate (TDF), emtricitabine (FTC) and atazanavir/ritonavir, compared to placebo.Citation37 However, prophylactic bone anti-resorptive therapy has not been studied in those initiating ART with integrase strand transfer inhibitors (INSTIs), and the optimal timing of bisphosphonate initiation also remains uncertain. We conducted a pilot randomized controlled trial to inform the design of a larger clinical trial of prophylactic alendronate with INSTI-based ART initiation.
Methods
Study overview and objectives
We conducted a two-site, three-arm, open-label, pilot randomized controlled trial of bone anti-resorptive therapy (co-formulated alendronate/vitamin D) in treatment-naïve HIV-positive adults initiating one of two eligible INSTI-based regimens. Our primary objective was to obtain preliminary estimates for the difference in the change in lumbar spine and proximal femur BMD at 48 weeks that can be achieved with 24 weeks of co-formulated alendronate plus vitamin D during antiretroviral initiation compared to no intervention, the standard deviation of the change at 48 weeks, and the intra-patient correlation coefficient of repeated BMD measurements. In keeping with our design as a pilot study,Citation38 our rationale for estimating these parameters was to inform the sample size calculations for a larger phase IV clinical trial of bone anti-resorptive therapy with ART initiation, rather than to generate definitive estimates for these differences. As a secondary objective, we further sought to determine whether the timing of alendronate plus vitamin D (immediate vs. delayed by 24 weeks) during ART initiation might impact on the change in BMD observed at 48 weeks. Additional secondary objectives were to provide preliminary information on enrollment feasibility, treatment acceptability, safety and drug adherence.
The study was approved by the Research Ethics Boards of the University Health Network and of St. Michael’s Hospital. All participants provided written informed consent prior to undertaking any study activities.
Eligibility criteria
Treatment naive HIV-positive adults aged 18 years or older were eligible to participate if they were within two weeks of initiating either tenofovir disoproxil fumarate/emtricitabine/elvitegravir/cobicistat (TDF/FTC/ELV/c; Stribild®) or abacavir/lamivudine/doltutegravir (ABC/3TC/DTG; Triumeq®). Participants also had to have a low (<10%) ten-year risk of fracture as assessed by the FRAX score validated for Canadian populations, with age assigned a value of 40 for those aged under 40 years (www.shef.ac.uk/FRAX/), since guidelines recommend consideration of active pharmacotherapy in those with higher risk. The choice of antiretroviral treatment regimen was a clinical decision made prior to patients being approached for possible participation in the study. Those with a known history of osteoporosis, requirement for concomitant medication with a known effect on bone density (eg. systemic corticosteroids, hormone therapy), pregnancy, hypocalcemia, creatinine clearance <35 mL/min, inability to communicate in English, or any contraindication to alendronate use (hypersensitivity, esophageal abnormalities etc) were excluded.
Study procedures
We randomized participants 1:1:1 to three arms in permuted blocks of three and six, and stratified by ART treatment regimen, using a secure web-based system that employed a computer-generated randomization scheme and provided complete allocation concealment. The concomitant treatment arm (CTA) received 24 weeks of weekly open-label co-formulated alendronate 70 mg with 5600 IU vitamin D3 (Fosavance®) immediately upon study entry. The delayed treatment arm (DTA) received the same intervention but starting 24 weeks after study entry. The standard of care arm (SOC) received no bone anti-resorptive therapy. Participants randomized to one of the bisphosphonate arms were advised to take their study drug with plenty of water and to remain upright for at least half an hour after ingestion to decrease the risk of esophagitis. They also underwent counseling on side effects such as atypical hip fractures and osteonecrosis of the jaw, as well as a clinical examination of the oral cavity; those with poor dentition were referred on for preventive dentistry.
Study visits occurred at baseline, week 24 and week 48 for all participants, and included an assessment of overall health, concomitant medications and adverse events; questionnaires regarding study drug adherence, tolerability and acceptability (as applicable); pill count for study drug only (as applicable); and bloodwork for CD4 cell count, plasma HIV RNA, and routine hematology and biochemistry. Those in the treatment arms also underwent a study visit four weeks after initiating study drug (i.e. at week 4 for the CTA and week 28 for the DTA) for early assessment of study drug adherence, tolerability and acceptability.
All participants also underwent dual energy X-ray absorptiometry (DXA) for measurement of bone mineral density within 2 weeks of week 0, 24, and 48 at the Centre of Excellence in Skeletal Health Assessment at Toronto General Hospital. These measurements were performed on the Hologic Discovery A (Hologic, Inc., Massachusetts, USA) using standard methods at the lumbar spine and proximal femur, with T-scores calculated using sex-specific reference standards. Technologists followed standard protocols from the manufacturers with attention to accurate positioning and appropriate body size scaling, and were blinded to study arm.
Statistical considerations
The sample size of 30 participants for this pilot study was selected based on feasibility considerations and its high likelihood of providing a reasonable preliminary estimate for our primary outcomes measures of interest: the difference in the change in BMD from baseline to 48 weeks at both the hip and lumbar (L1–L4) spine, δ, the within-group standard deviation of the change at 48 weeks σ, and the intra-patient correlation coefficient (ICC) among repeated measurements of BMD. In the primary analysis, we descriptively summarized the changes and percent changes in BMD for the two alendronate/vitamin D arms and the control arm. ICC was estimated from linear mixed effects regression using BMD from baseline and weeks 24 and 48. Variables included in the regression model were baseline BMD, a participant-specific random effect term, and a variable representing the absence, presence, or post-presence of active treatment at each time point in the model to account for any treatment effect on BMD.
The BMD data at week 48 for the two alendronate/vitamin D arms were pooled and compared against the control arm. We performed this analysis using linear mixed effects regression modeling, adjusting for baseline BMD values and including a participant-specific random effect term to account for repeated measurements. Time was modeled as a categorical variable in these analyses to allow the treatment effect to potentially differ across the two time points. Importantly, as a pilot trial, this study was not intended to have sufficient power to detect a statistically or clinically significant difference in the change in BMD between study arms. We also conducted exploratory subgroup analyses according to underlying ART regimen. For this analysis, an interaction term between ART regimen and study arm was added to the regression model.
To assess the impact of the timing of alendronate/vitamin D therapy, secondary analyses compared the percent changes in BMD from baseline to 48 weeks at both the proximal femur and lumbar (L1–L4) spine between the CTA and DTA groups. Linear mixed effects regression was performed. In the context of this being a small pilot study, for this analysis we were interested only in whether there was a clear signal of potential difference in this outcome between arms, as might be represented in a difference that reached the traditional significance threshold of α = 0.05, recognizing the potential for type II error.
We assessed the feasibility of a larger trial by documenting the proportion of approached patients who declined or agreed to participate in the trial, the reasons for declining among those who declined, and the time required to accrue the full sample size of 30 participants. To assess the acceptability of, safety of and adherence to study drug, we used descriptive statistics to summarize the results of the acceptability questionnaires, adverse event assessments, adherence questions and pill counts.
Results
Between 04/2014 and 03/2017, we approached 40 individuals regarding study participation and screened 35, of which 30 were deemed eligible and randomized; of these, one had initiated tenofovir disoproxil fumarate/emtricitabine/efavirenz (Atripla®) before the protocol was amended to exclude this ART regimen, leaving 29 included in the present analyses. Given the presence of other competing clinical trials at the study sites during the recruitment period, we considered this enrollment rate and modest rate of screen failures to mean that a larger, multicentre trial with a similar design would be feasible.
Individuals who declined to participate and those who were excluded (n = 11) were similar in sex and age to that of included participants, who were mostly (97%) male with median (interquartile range, IQR) age of 33.2 (26.4, 42.0) years. The majority of participants initiated ART with TDF/FTC/ELV/c (72%) with the remainder initiating ABC/3TC/DTG (28%). Just under half of participants were White (45%), while 28% were Black and 28% other ethnicities. A minority (24%) of participants had at least one current or prior osteoporosis risk factor, most commonly current smoking (21%), although the ten year risk of a major osteoporotic fracture according to the FRAX tool was low for all participants. Median CD4 count and CD4 percent at baseline were 388 (303, 525) cells/mm3 and 24% (18%, 29%) and median plasma HIV RNA was 4.45 (2.26, 4.84) log10 copies/mL. Other demographic and clinical characteristics at baseline are shown in , and were generally well-balanced between the three randomization arms.
Table 1 Demographic and clinical characteristics of participants at baselineTable Footnotea
A summary of the baseline BMD values at the lumbar spine, femoral neck and total hip for the three study arms is shown in , along with the mean (SD) changes seen at 24 and 48 weeks, expressed as both absolute values and as percent changes compared to baseline. The mean BMD at each time point for each group is shown by anatomic site in . The ICC among repeated measurements of BMD was 0.978 at the lumbar spine, 0.964 at the femoral neck, and 0.967 at the total hip. Results from the linear mixed effects regression models are shown in ; these models provide a more appropriate comparison of the change in BMD between study arms since they adjust for baseline values. No participant experienced a fracture during the study.
Figure 1 Mean BMD over time by study group and anatomic site. Note: The sample size varied slightly across time points, with 27, 24 and 23 participants at baseline, week 24 and week 48 respectively. Thus, there may be small discrepancies between the BMD trend exhibited in the figure and the mean changes shown in , which was computed based on participants with both baseline and follow-up data.
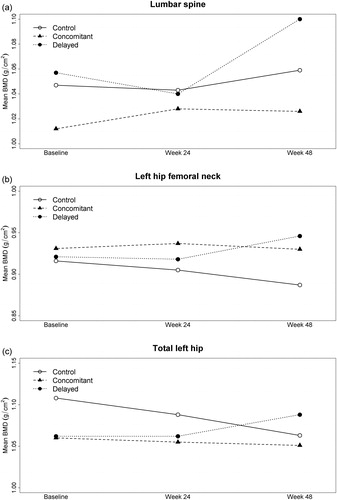
Table 2 Baseline mean (SD) BMD values and mean (SD) change from baseline in BMD at 24 and 48 weeks, by study armTable Footnotea
Table 3 Linear mixed regression results showing differences in BMD at weeks 24 and 48 between study arms
As seen in , when comparing the changes in BMD seen at week 48, we observed a more compelling suggestion of difference between study arms when comparing the combined active arms versus control, than when comparing the DTA and CTA to each other. These results suggest that the timing of anti-resorptive therapy was a less important driver of BMD than whether therapy was used at all, although the direction of difference between the two active treatment arms at week 48 may have slightly favored the DTA over the CTA. There was no evidence of a difference in these results according to ART regimen (data not shown). Taken together, these preliminary findings suggest that further investigation into the merits of the delayed approach is warranted.
The acceptability of various aspects of the study drug, including common potential side effects and the specific dosing requirements for alendronate was generally high. The proportion of participants reporting that they were either “minimally” or “not at all bothered” by study drug-related stomach upset, fasting post-dosing, remaining upright post-dosing, and following the medication routine overall was 94%, 89%, 100% and 100% respectively. The proportion reporting being “quite satisfied” or “very satisfied” by the frequency of study drug administration, mode of administration, convenience of administration, and requirement of taking the drug in addition to ART was 83%, 83%, 89%, and 72% respectively. When asked whether they would prefer to take this medication together with the first or second six months of ART, most participants (7/10) in the CTA preferred the first six months, while those in the DTA were evenly divided; 29% of participants across both treatment arms indicated that they would not choose one option over the other.
Pill counts suggested that adherence to study drug was very good, with the median (IQR) proportion of study drug doses taken in the two treatment arms being 100% (87.5%, 100%). Self-reported adherence data at both the 4 and 24 week marks post-alendronate/vitamin D initiation visits were consistent with this very high level of adherence (full data not shown).
A total of 46 adverse events occurred in 24/29 (83%) participants during the study, all of which were mild, except for seven of moderate intensity (two in each of the two active arms, three in the control arm), none of which resulted in changes to study drug dosing. There were no serious adverse events. The majority of adverse events were deemed unrelated (91.3%) or unlikely to be related (2.2%) to study drug, while one episode each of nausea and insomnia in the CTA was deemed definitely and probably related, respectively, and one episode of headache in the DTA was deemed possibly related.
Discussion
In this pilot, dual-centre, open-label randomized trial of immediate or delayed alendronate/vitamin D for mitigating bone loss during ART initiation, we generated preliminary estimates for the difference in BMD at week 48 achieved at the lumbar spine, femoral neck and total hip, between the combined treatment and control arms, and the within-group standard deviation of the change at 48 weeks. The ICC for repeated measurements of BMD within individual participants was very high, between 0.964–0.978 for these three anatomical sites. These parameter estimates suggest that for a larger, definitive 1:1 randomized trial comparing six months of this therapy against no intervention in a similar population, the number of participants needed per study arm would be in the range of 58–429 to detect a minimum difference of 1–2% between study arms, assuming α = 0.05 and 80% power ().
Table 4 Estimated sample size requirements for a 1:1 randomized controlled trial of alendronate/vitamin D during antiretroviral initiation (for α = 0.05 and 80% power)
An important consideration when assessing the potential need for such treatment is the underlying ART regimen being used, since changes in BMD at 48 weeks differ widely. In particular, in a systematic review of randomized trials among treatment-naïve HIV-positive adults, we determined that tenofovir disoproxil fumarate has been associated with larger declines in BMD at 48 weeks compared to alternative agents in this class, such as abacavir, with a mean difference of −1.62% (95%CI = −2.30,−0.95%) at the lumbar spine, −1.75% (95%CI = −2.08,−1.42%) at the total hip, and −1.26% (95%CI = −2.15,−0.38%) at the femoral neck.Citation39 In our study, roughly three quarters versus one quarter of participants were using these agents, and exploratory analyses suggested that the magnitude and standard deviation of the differences in BMD achieved with alendronate/vitamin D were roughly comparable.
A major recent development regarding bone health in the context of ART is the availability of tenofovir alafenamide (TAF), which has been approved for use in several single tablet regimens for initial treatment of HIV in adults. This prodrug is metabolized to its active form, tenofovir diphosphate, primarily within leukocytes, resulting in lower circulating levels of the drug in plasma than are seen with TDF.Citation40 A benefit of this difference is that patients experience smaller changes in BMD at 48 weeks with TAF than TDF, estimated at −1.30% rather than −2.86% at the spine, and −0.66% rather than −2.95% at the hip.Citation41 Analogously, patients switching from TDF to TAF-containing regimens in the context of virologic suppression have modest increases in BMD after 48 weeks, with a difference of roughly +2.00% at the spine and +1.81% at the hip compared to those who remain on TDF.Citation42 In light of these salubrious effects, the relative importance of using bone anti-resorptive therapy when starting a TAF-containing regimen may arguably be lessened. Conversely, if short-term bone anti-resorptive therapy in the context of TDF use achieves benefits similar to long-term TAF use, the former may be more cost-effective. Overall, we posit that the predictable and incremental insult to bone density seen with ART initiation, burden of osteoporosis risk factors in people living with HIV (24% in our sample), long life expectancy for modern cohorts of antiretroviral-treated individuals,Citation43 and historically high prevalence of osteoporosis and fractures in the context of HIV suggest that there may be overall benefits to long term bone health with pre-emptive anti-resorptive therapy.
Prior studies have examined the use of vitamin D/calcium supplementation and of the intravenous bisphosphonate zoledronate for a similar purpose, and seen evidence of benefit.Citation37 Our study adds to this knowledge base by suggesting that an oral bisphosphonate may similarly warrant further study, based on our enrollment feasibility findings, high levels of study drug adherence, low burden of side effects and excellent acceptability. Our pilot findings regarding the optimal timing of bisphosphonate therapy further suggest that delaying this treatment until six months after ART initiation may be possible or even preferred, based on cumulative changes in BMD observed at 48 weeks. This approach may offer the advantage of minimizing pill burden immediately after a new HIV diagnosis is made, when there may be multiple other clinical priorities including HIV treatment initiation, antimicrobial prophylaxis against opportunistic pathogens, vaccinations, patient counseling for these issues, and linkages to other forms of care for syndemic conditions. While participants in the CTA of our study tended to prefer concomitant therapy, a fair proportion of participants overall indicated no clear preference regarding timing, and including a DTA in a definitive trial may therefore be worthwhile. However, with any bisphosphonate use must come careful consideration of their known potential toxicities, including common ones such as esophagitis and gastritis, and rare one such as osteonecrosis of the jaw and atypical femur fractures. Bisphosphonates are generally discouraged in women who are planning to become pregnant as they may have a negative effect on fetal bone development; as such, women of childbearing potential should use contraception while using these agents.
This study has limitations that warrant consideration. First, we included only two ART regimens in our study in order to minimize heterogeneity related to those drugs within the study and for feasibility reasons; these regimens were popular first-line options when the study was designed. Our estimates may have differed had we included additional regimens recommended in updated treatment guidelines such as those containing TAF, but the impact on our key question of interest regarding sample size for a definitive trial are unclear. Second, the trial was open-label. However, we blinded BMD technologists to study arms, and the objective nature of our primary outcomes renders it less likely that this design would have biased our findings. Third, because we lacked a biological marker of adherence, it is possible that some participants in the treatment arms ingested less drug than was intended. This possibility would have biased our findings toward the null, and generated a smaller standard deviation, resulting in a smaller estimate for the required sample size for a definitive trial. However, our pill count and self-reported data were both broadly consistent with high adherence. Fourth, although widely used, BMD is an imperfect marker of fracture risk, with standards derived from post-menopausal women and no clear fracture threshold.Citation44 In addition, we did not consider age, sex, ethnicity or other participant characteristics relevant to BMD in our regression models due to the modest sample size of this pilot study; analyses within a large-scale trial should take these covariates into account. Finally, we opted to study only six months of therapy rather than a longer period, reasoning that this duration would boost acceptability and be appropriate to implement if beneficial, given that bone density tends to improve on its own to some extent during the latter half of the first year of ART. It is possible that a longer period of alendronate/vitamin D would have resulted in larger bone benefits.
In conclusion, our pilot study suggests that alendronate/vitamin D supplementation during ART initiation can modestly mitigate the BMD losses seen during the first year of therapy with TDF/FTC/ELV/c or ABC/3TC/DTG, and that the timing of this bone anti-resorption therapy can be given either during the first or the second six months after starting treatment. Larger more definitive trials of such a strategy are warranted, and our parameter estimates to inform sample size calculations as well as good feasibility will be useful for informing its design.
Declaration of interests (past 3 years)
DHST’s institution has received investigator-initiated research grants from Gilead Sciences and Viiv Healthcare, and DHST is a site principal investigator for clinical trials sponsored by Glaxo Smith Kline. AMC has served as a consultant for Amgen, Alexion and Eli Lilly. SW has served on advisory boards, speaking engagements, meetings, symposiums and clinical studies for Viiv Healthcare, Glaxo Smith Kline, Merck and Gilead Sciences.
Additional information
Funding
References
- Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 1993;341(8837):72–75.
- Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS 2007;21(5):617–623.
- Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Santoro N, Schoenbaum EE. HIV infection and bone mineral density in middle-aged women. Clin Infect Dis. 2006;42(7):1014–1020.
- Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004;292(2):191–201.
- Nolan D, Upton R, McKinnon E, et al. Stable or increasing bone mineral density in HIV-infected patients treated with nelfinavir or indinavir. AIDS 2001;15(10):1275–1280.
- Amiel C, Ostertag A, Slama L, et al. BMD is reduced in HIV-infected men irrespective of treatment. J Bone Miner Res. 2003;19(3):402–409.
- Fausto A, Bongiovanni M, Cicconi P, et al. Potential predictive factors of osteoporosis in HIV-positive subjects. Bone 2006;38(6):893–897.
- Grund B, Peng G, Gibert CL, et al. Continuous antiretroviral therapy decreases bone mineral density. AIDS 2009;23(12):1519–1529.
- Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006;20(17):2165–2174.
- Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93(9):3499–3504.
- Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS 2012;26(7):825–831.
- Young B, Dao CN, Buchacz K, Baker R, Brooks JT, the HIV Outpatient Study (HOPS) Investigators. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000-2006. Clin Infect Dis. 2011;52(8):1061–1068.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215–1220.
- Ross PD, He YF, Davis JW, Epstein RS, Wasnich RD. Normal ranges for bone loss rates. Bone Miner. 1994;26(2):169–180.
- Fakruddin JM, Laurence J. HIV-1 Vpr enhances production of receptor of activated NF-kappaB ligand (RANKL) via potentiation of glucocorticoid receptor activity. Arch Virol. 2005;150(1):67–78.
- Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003;278(48):48251–48258.
- van Vonderen MG, Lips P, van Agtmael MA, et al. First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS 2009;23(11):1367–1376.
- Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51(5):554–561.
- Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 2009;23:817–824.
- Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010;182(17):1864–1873.
- Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(5):651–681.
- McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51(8):937–946.
- McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007;21(18):2473–2482.
- Mondy K, Powderly WG, Claxton SA, et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr. 2005;38(4):426–431.
- Guaraldi G, Orlando G, Madeddu G, et al. Alendronate reduces bone resorption in HIV-associated osteopenia/osteoporosis. HIV Clin Trials. 2004;5(5):269–277.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148(3):197–213.
- McClung M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. A double-blind, randomized, controlled trial. Alendronate Osteoporosis Prevention Study Group. Ann Intern Med. 1998;128(4):253–261.
- Hosking D, Chilvers CE, Christiansen C, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998;338(8):485–492.
- Ravn P, Bidstrup M, Wasnich RD, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med. 1999;131(12):935–942.
- McClung MR, , Wasnich RD, Hosking DJ, et al. Prevention of postmenopausal bone loss: six-year results from the Early Postmenopausal Intervention Cohort Study. J Clin Endocrinol Metab.2004;89(10):4879–4885.
- Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med. 1998;339(5):292–299.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44(1):202–211.
- Stoch SA, Saag KG, Greenwald M, et al. Once-weekly oral alendronate 70 mg in patients with glucocorticoid-induced bone loss: a 12-month randomized, placebo-controlled clinical trial. J Rheumatol. 2009;36(8):1705–1714.
- Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146(6):416–424.
- Klotz LH, McNeill IY, Kebabdjian M, Zhang L, Chin J. A phase 3, double-blind, randomised, parallel-group, placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: the cancer and osteoporosis research with alendronate and leuprolide (CORAL) study. Eur Urol 2012;
- Overton ET, Chan ES, Brown TT, et al. Vitamin D and calcium attenuate bone loss with antiretroviral therapy initiation: a randomized trial. Ann Intern Med. 2015;162(12):815–824.
- Ofotokun I, Titanji K, Lahiri CD, et al. A single-dose zoledronic acid infusion prevents antiretroviral therapy-induced bone loss in treatment-naive HIV-infected patients: a phase IIb trial. Clin Infect Dis. 2016;63(5):663–671. Epub 2016 May 18.
- Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1):1. 10.1186/471-2288-10-1.
- Baranek B, Wang S, Cheung AM, Mishra S, Tan D. What does HIV pre-exposure prophylaxis (PrEP) do to bone health? A systematic review and meta-analysis. Paper presented at: 27th Canadian Conference on HIV/AIDS Research Poster CSP701; 2018; Vancouver, Canada.
- Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615.
- Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015;385(9987):2606–2615. Epub 2015 Apr 15.
- Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52. Epub 2015 Nov 2.
- Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med. 2017;18(4):256–266. Epub 2016 Aug 31.
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312(7041):1254–1259.
