Abstract
Background
Chronic sleep disruption can have significant negative health effects and prior studies suggest that people with HIV (PWH) have disproportionately higher rates of sleep problems.
Methods
We evaluated baseline sleep of sedentary, older adults (50–75 years) with (n = 28) and without HIV (n = 29) recruited into a 24-week exercise study. Subjective sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI); objective sleep parameters were assessed using wrist-worn actigraphy. Regression models were used to investigate changes in outcomes.
Results
Fifty-seven participants completed the intervention. At baseline, PWH had significantly lower sleep efficiency (88.7 [95% CI 86, 91]%) compared to controls (91.8 [95% CI 91, 93]%; p = 0.02); other sleep measures indicated poorer sleep among PWH but did not reach statistical significance (p ≥ 0.12). Overall, sleep outcomes did not significantly change with the exercise intervention (all p > 0.05). In adjusted analyses, PWH demonstrated a decrease in total sleep time (–22.1 [–43.7, –0.05] p = 0.045) and sleep efficiency (–1.3 [–2.5, –.01], p = 0.03) during the 24 weeks of exercise; these differences were attenuated and no longer significant after adjusting for exercise intensity. At the completion of the intervention, compared to controls, PWH had significantly poorer sleep by PSQI score (2.2 [0.6, 3.8]; p = 0.006) and sleep efficiency (–2.8 [–5.4,–0.2]%; p = 0.04).
Conclusions
In this study, sleep disturbance was more prevalent in sedentary older PWH compared to uninfected controls. An exercise intervention had minimal effect on sleep impairments among PWH nor controls. Among older adults, interventions beyond cardiovascular and resistance exercise may be needed to significantly alter subjective and objective sleep outcomes.
Keywords:
Background
The average adult spends one-third of life sleeping, and the characteristics and quality of that sleep are an indicator of overall health.Citation1 Unfortunately, sleep disorders are quite common, with an estimated prevalence ranging from 25 to 30% among the U.S. adult population.Citation1,Citation2 Chronic sleep impairment is associated with obesity, diabetes, cardiovascular disease, and overall mortality.Citation3,Citation4 Furthermore, with increasing age, total sleep time shortens; the ability to maintain sleep worsens from birth to older adulthood; and less time is spent in deep sleep, particularly among older adults (>60 years).Citation5
As HIV has been transformed into a chronic disease over the past three decades, those aging with HIV are often faced with a greater burden of co-morbidities in comparison to their uninfected peers.Citation6,Citation7 A potential contributor or consequence of this comorbid disease burden is a high prevalence of self-reported poor sleep quality, with rates as high as nearly 70% among people with HIV (PWH), and even higher rates among PWH with cognitive issues.Citation8,Citation9 In a meta-analysis of 16 studies, sleep disturbance was present in 61% PWH but only 10% among persons without HIV.Citation10 Additional studies have demonstrated the potential implications of poor sleep: long-term sleep problems among PWH have been associated with greater inflammation, and poorer mental and physical health.Citation4,Citation11–13
Among older PWH with multiple co-morbidities and a high burden of concomitant medications, there is a pressing need for non-pharmacologic interventions to improve sleep. Strategies to treat sleep disturbances have been employed with limited success such as Cognitive Behavioral Stress Management, establishing a regular routine, or counseling for feelings of stigma, isolation, and depression.Citation14,Citation15 Among older adults without HIV, regular, consistent exercise has been shown to improve both subjective (e.g. Pittsburgh Sleep Quality Index [PSQI]) and objective (e.g. actigraphy) measures of sleep quality.Citation16–19 Various types of exercise regimens (i.e. aerobic exercise, moderate strength training, community-based regimens, etc.) have been demonstrated to improve mood, mental acuity, delay onset of comorbidities, and improve physical functioning which may indirectly improve sleep quality.Citation17,Citation20,Citation21 Among PWH, greater physical activity has been associated with less insomnia,Citation22 and decreased anxietyCitation23 but whether an exercise intervention can improve sleep is unknown. Here, we hypothesized that PWH would have poorer overall subjective and objective sleep in comparison to older adults without HIV, and that an exercise intervention would attenuate these sleep differences.
Materials and methods
As previously described,Citation24 the Exercise for Healthy Aging Study enrolled PWH and HIV-uninfected controls from the Denver metropolitan area from April 2014 to May 2017 (Clinical Trials NCT02404792), with the primary outcomes comparing response of physical function and inflammation to moderate or high intensity exercise by HIV serostatus. All participants were aged 50 to 75 years, sedentary (<60 minutes of self-reported physical activity each week for 6 months preceding), had a body mass index (BMI) between 20 and 40 kg/m2, and had no contraindications to initiating a moderate or high-intensity exercise regimen (e.g. severe mobility limitation, unstable angina, supplemental oxygen requirement, uncontrolled hypertension). PWH were on stable antiretroviral therapy (ART) with no HIV-1 RNA >200 copies/mL for a minimum of two years, and a CD4+ T-cell count > 200 cells/µL. The study procedures were reviewed and approved by the Colorado Multiple Institutional Review Board. Informed consent was obtained from all participants.
Intervention
Supervised exercise sessions were conducted three times per week at the University of Colorado Exercise Research Laboratory. For the first 12 weeks, all participants (PWH and uninfected controls) were prescribed moderate-intensity endurance and resistance exercise. Following a two-week low-intensity exercise acclimation (20–30 minutes of treadmill walking and three sets of eight repetitions on four machine exercises), cardiovascular exercise intensity increased to 40–50% of baseline VO2 max and duration increased by five minutes/week to achieve 50 minutes/session by 12 weeks; resistance exercise increased to 60–70% of 1-RM, with target weight loads adjusted every three weeks as needed. At week 12, VO2 max measurements were repeated and participants were randomized to either continue moderate-intensity exercise or advance to high-intensity (60–70% of week 12 VO2 max and >80% 1-RM) for an additional 12 weeks. Randomization assignment was balanced by HIV serostatus, gender, and age with block sizes that were blinded to the investigative team. Adherence to the intervention was calculated as the attended exercise sessions/expected exercise sessions.
Subjective sleep measures
Measures of sleep were pre-specified, exploratory outcomes of the study. The Munich Chronotype Questionnaire is designed to assess the human circadian clock rhythm or “chronotype.”Citation25 The Munich Chronotype Questionnaire estimates chronotype based on the midpoint between self-reported sleep onset and wake up time. The chronotype is calculated as mid-sleep on free days corrected for sleep debt on work days when work days differed from free days. This survey was performed at baseline only. The PSQI assesses sleep quality though 19 questions that investigate timing, disturbances, and other factors that may affect sleep over the preceding one month.Citation26 This survey was completed at baseline, week 12, and week 24.
Objective sleep measures
Wrist-worn actigraphy is a commonly used method of measuring sleep and is considered highly sensitive (94%) in determining when a wearer is asleep and moderately specific (46%) in determining when they are awake.Citation27 The Actigraph Spectrum (Phillips Respironics Murrysville, PA, USA) used in this study measures the presence of light and movement of the wearer.Citation27 Participants were instructed to wear this device for 7 days at baseline, week 12, and week 24, which included 5 week days and 2 weekend days. The baseline measurement was performed prior to the exercise intervention; the midpoint and final actigraphy measures were collected during weeks when supervised exercise continued. We included actigraphy measures when available for at least 4 of the 7 days. The Actiware 6 software was used to analyze the wrist actigraphy data. Rest intervals were set based on self-reported sleep times. When self-report conflicted with the light and activity data, the latter were used to guide the setting of rest intervals. Nightly sleep variables extracted by the Actiware 6 Software included sleep onset time (clock time of the first epoch scored as sleep in each rest interval), wake time (clock time of the last epoch scored as sleep in each rest interval), total sleep time (number of minutes scored as sleep in each rest interval) and sleep maintenance efficiencyCitation28,Citation29 (proportion of time from sleep onset to wake in each rest interval, scored as sleep, expressed as a percentage) were extracted.
Measures of physical function
Physical function measures included a modified Short Physical Performance Battery (mSPPB), 10 time repeat chair stand, 400-m walk test, VO2 maximum (or peak), grip strength, and 1 repetition maximum, all obtained at baseline, week 12, and week 24, as previously described.Citation24
Statistical analysis
Differences in baseline demographics and outcomes were compared using t-tests for continuous measures and Fisher’s Exact test for categorical outcomes. Since non-parametric tests lack transitivity, we prefer a t-test over a non-parametric alternative when the sample size is near 30 per group.Citation30 Linear mixed models with a random intercept were used to assess the change from baseline to 24 weeks, among participants with data available at both baseline and 24 weeks. Multiple models were constructed: Model A) change in the sleep outcomes with the exercise intervention, B) the same model allowing the intervention effect to change with HIV serostatus (time/serostatus interaction), and C) model B adjusted for exercise intensity. The relationships between a change in sleep characteristics and change in physical function measures were explored using Spearman correlations with 95% CI calculated using the R function DescTools: SpearmanRho.Citation31 All comparisons assumed a significance level of 0.05 with no adjustment for multiple comparisons. However, all comparisons were reported, so that the reader can informally account for multiple comparisons.Citation32 All analyses were performed using SAS 9.4 or R 3.5.2 software.
Results
A total of 89 PLWH and HIV-uninfected controls underwent screening and 69 participants began exercise (32 PLWH, 37 HIV-uninfected controls). Twenty-eight (13 PLWH, 15 uninfected controls) participants were randomized to continue moderate-intensity exercise and 31 (15 PLWH, 16 uninfected controls) to advance to high-intensity exercise. Fifty-seven participants completed the intervention and were included in the analysis (): 28 (49%) participants were PWH, 29 (51%) were uninfected controls, and the majority of participants (n = 63) were male. Participants with and without HIV were similar in respect to gender, race/ethnicity, smoking status and alcohol use (p > 0.05). PWH and controls differed in employment, education, marijuana use, sleep medications (as reported on PSQI), and illicit substance use (p < 0.05). Although not statistically significant, controls were older (p = 0.09), had higher BMI (p = 0.06) and more comorbidities (p = 0.07) (). No participants had night-shift employment.
Table 1 Baseline participant characteristics
Table 2 Differences in subjective and objective sleep measures at baseline and at completion of the 24-week exercise intervention
Munich Chronotype Questionnaire data was available on 51 participants (exclusions due to the use of an alarm clock on free days or missing data; ). The distribution of these cases is skewed to the right, with the majority of cases being normal to earlier sleepers.
Figure 1 Munich Chronotype Questionnaire (MCTQ) showing the sleep type as defined by midsleep (sleep onset + sleep duration/2) for all participants (top), the people with HIV (lower-left) and uninfected controls (lower-right). The people with HIV had a larger proportion of abnormal sleep types compared to uninfected controls. Data not present for all participants due to missing data or the use of an alarm clock on all days.
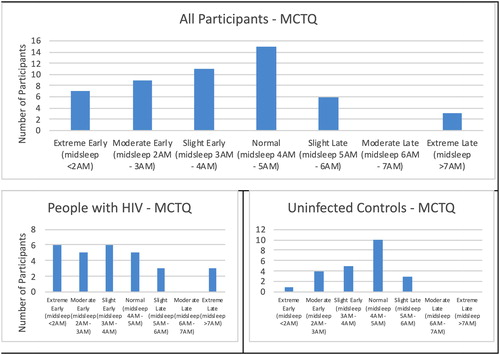
PSQI was available in 28 PWH and 29 uninfected controls at both baseline and week 24; actigraphy measures (TST, SE) were available in 20 PWH and 21 controls at both time points and were missing due to incomplete sleep data or participant refusal to wear the watch. Differences between those that completed or did not complete the sleep measures are presented in the Supplemental Table. At baseline, PWH had poorer sleep efficiency (88.7 [95% CI 86, 91]% vs 91.8 [95% CI 91, 93]%); p = 0.02) compared to uninfected controls. Subjective sleep (PSQI) was poorer and total sleep time less among PWH but differences were not statistically significant (p ≥ 0.15); .
Change in sleep characteristics with the exercise intervention
For participants completing at least 1 exercise session, the median adherence during the first 12 weeks was 91.7% (IQR 86.1, 94.4%) among PWH and 88.9% (83.3, 94.4) among controls. For participants completing at least 1 exercise session after 12 weeks, adherence from weeks 13–24 was 88.9% (73.6, 88.9%) among PWH and 77.8% (73.6, 88.9%) among controls. Adherence was similar between the high-intensity (83.3 [77.1, 88.9%] for weeks 13–24) and moderate-intensity arms (83.3 [68.1, 88.9%]). Among those with sleep outcomes available at both visits, 54% of PWH and 66% of controls had <90% exercise adherence (p = 0.52).
In unadjusted comparisons, PSQI was not significantly different between PWH and uninfected controls (p = 0.16, ). Between baseline and week 24, in PSQI there was a non-significant improvement (decrease) from 5.9 to 5.3 in average PSQI score (p = 0.25) in uninfected controls and a corresponding non-significant increase from 7.1 to 7.2 (p = 0.80) in PWH, resulting in significantly higher (worse) scores in the PWH group vs. controls at week 24 (p = 0.02). PWH had less total sleep time and lower sleep efficiency at week 24 compared to baseline, although these changes did not reach statistical significance (p ≥ 0.06); no significant differences were seen among controls (p ≥ 0.71). The differences between PWH and controls at week 24 in total sleep time and mean sleep efficiency were greater than at baseline but were not statistically significant (p ≥ 0.06). Average bedtime and wake time, as reported by actigraphy, were similar in both groups following the exercise intervention (Supplemental Table).
In unadjusted mixed model analyses (, Model A), the exercise intervention resulted in no significant changes in subjective and objective measures of sleep (p ≥ 0.09). When the HIV serostatus/time interaction was added (model B), PWH had significantly lower total sleep time (p = 0.045) and sleep efficiency (p = 0.03), although the change with the exercise intervention was not significantly different between groups for any measure (p ≥ 0.16). The week 24 measures were significantly different by HIV serostatus for PSQI and sleep efficiency: PWH had poorer subjective sleep by PSQI (2.2, p = 0.006) and lower sleep efficiency (–2.8%, p = 0.038) with the intervention, compared to controls. Similar findings were observed in the final model (C) incorporating exercise intensity: neither group had significant improvement in sleep measures with the exercise intervention. Notably, after adjusting for high intensity exercise, the differences in sleep measures with the intervention among PWH were attenuated and no longer significant, however those with HIV still had significantly poor PSQI and sleep efficiency at week 24 compared to controls.
Table 3 Change in subjective and objective sleep parameters from baseline to week 24 in unadjusted and adjusted models
Lastly, we explored the correlation between changes in sleep characteristics and measures of physical function (VO2 maximum, 400-m walk, chair rise, and strength measuresCitation24). A decrease in PSQI (improved sleep) correlated with greater improvements in lateral pulldown strength (r = −0.36, p = 0.007) in the overall cohort. Similar effects were seen in subgroups by HIV serostatus and exercise intensity, reaching significance only in PWH and those randomized to moderate intensity exercise (p < 0.05). An increase in total sleep time was correlated (r = 0.52) with an increase in the time needed to walk 400 meters (p < 0.001), with significant, strong correlations among those randomized to moderate intensity exercise (r = 0.67, p = 0.006). Lastly, improved sleep efficiency was associated with longer time to complete the 400-m walk time among the overall cohort, among controls, and among moderate-intensity exercisers; similarly, improved sleep efficiency was associated with less improvement in strength by lateral pulldown among controls, PWH, and moderate-intensity exercisers.
Discussion
To the best of our knowledge, this study includes the largest number of participants over the longest duration to investigate whether an exercise intervention can improve sleep quality, using both objective and subjective sleep measures, in either PWH or uninfected older adults. First, we found that, at baseline, older PWH reported poorer sleep quality, and had poorer objective sleep efficiency. After 24 weeks of exercise, neither group had significant improvements in self-reported or objective sleep measures. Importantly, the decreased total sleep time and decreased sleep efficiency seen among PWH with the intervention were both attenuated after adjusting for high intensity exercise (, model C). Furthermore, times for sleep or wake minimally changed, indicating no major alterations in sleep habits (Supplemental Table).
Multiple prior studies have also shown poor sleep quality among PWH. Compared to the existing literature, our participants had similar sleep quality (PSQI of 7.1) compared to a larger (n = 290) cohort of adults with HIV (PSQI 7.4),Citation33 and better quality than two other studies where the average PSQI scores were 9.2 and 12.3.Citation34,Citation35 Few studies in HIV have also utilized actigraphy for more objective sleep measuresCitation36–39: our participants with HIV had similar sleep efficiency (88%) to a large cohort of adults with HIV reporting long or typical sleep duration (89% among those with >8 hours and 82% among those sleeping 6–8 hours) but considerably better sleep efficiency than those with < 6 hours of sleep (64%).Citation33 In contrast to our participants, this cohort was considerably younger (mean age 45), 30% were not on ART, and based on the date of publication (2012), the majority of participants were likely taking efavirenz,Citation33 a regimen that has been strongly linked to sleep disturbances.
Although prior studies have explored the relationship between sleep and physical function cross-sectionally, we are unaware of existing studies exploring the relationship between changes in sleep and function in the setting of an exercise intervention. In contrast to a hypothesized improvement in both sleep parameters and function, while improved self-reported sleep was associated with improved strength, many of the measures had an unexpected relationship: improved sleep efficiency correlating with less improvement in physical function or strength. Similarly, longer sleep time was significantly associated with slower (longer) time to complete 400-m. The correlations were greatest for cardiovascular measures among controls and moderate intensity, while both controls, PWH, and moderate-intensity groups had weaker strength with improved sleep efficiency. The correlations in the moderate intensity group only suggest that perhaps greater exercise intensity can overcome any sleep-influenced changes in physical function. However, these findings could also be the results of Type I error with multiple exploratory analyses, or may reflect a significant underlying sleep pathology less amenable to the effects of exercise.
While prior studies have investigated the effects of exercise upon sleep and cognitive behavior among PWH or people without HIV, we are unaware of any studies that compare the effects of routine, supervised exercise on sleep by HIV serostatus. Similar to PWH, sleep complaints are highly prevalent among all older adults. Prior studies in populations without HIV have demonstrated the beneficial effect of exercise upon sleep: King et al found significantly less time in stage 1 sleep, more time in stage 2, and less awakening using polysomnography and significant improvements in PSQI in a population (n = 66) of older adults (mean age 62) with moderate sleep complaints, following a moderate intensity exercise intervention.Citation17 Reid et al found that moderate aerobic physical activity was able to significantly improve subjective sleep quality in a largely female population (n = 17, mean age 62) with insomnia.Citation20 Additional studies have demonstrated the effectiveness of exercise on sleep in a similarly aged populations with baseline sleep complaints.Citation19,Citation40 Only one study that we are aware of has explored the impact of supervised exercise interventions on sleep quality and quantity of PWH. McDermott et. al. investigated the effects of a 16-week aerobic exercise regimen among PWH, with a primary outcome of cognitive function.Citation41 In the McDermott study (5 exercisers, 6 controls with no exercise), a small improvement in sleep quality was observed among both exercisers (PSQI change −0.5) and controls (PSQI change –0.5).Citation41
Several limitations may explain the lack of a beneficial effect of exercise on sleep quality and objective sleep measures. First, we had a relatively small sample size and few women. As this was an exploratory endpoint of a study focused on physical function, we did not recruit participants based on sleep complaints thus some participants may have had limited room for improvement. Similarly, we were not specifically powered for sleep outcomes, and may have been underpowered, although our sample size was similar or larger than other exercise studies. Many of our participants already used medications to aid with sleep, or psychotropic medications that may impact sleep, particularly among the PWH. Similarly, our population could have had undiagnosed sleep impairments requiring medical interventions, such as obstructive sleep apnea, although when we included BMI in the current model there was no significant impact, suggesting that obesity-related sleep apnea is not likely contributing (results not shown). Regardless, underlying intrinsic or pharmacologic sleep disturbance may not be overcome with exercise alone. The type or duration of exercise (walking and resistance exercise) may not have influenced sleep quality as much other types of exercise, such as mindfulness-based exercises (yoga or tai chi), or group activities. In the Reid study, older adults with insomnia showed significant improvements in sleep following an aerobic exercise regimen, however, this study had more frequent exercise and provided sleep hygiene education.Citation20 Napping may also play an important role on sleep, especially with increasing age. Unfortunately, we did not collect or examine shorter periods of sleep throughout the day to explore the effect of napping on nocturnal sleep. Our participants enrolled in the RCT with the intention of completing 24 weeks of an exercise intervention and may have had less fatigue than their non-exercising peers. Similarly, those missing assessments at the 24 week time period did differ from those with complete data in some characteristics (Supplemental Table), and have had better sleep and therefore felt less inclined to participate in the sleep evaluations. Our participants were sedentary prior to enrolling; the effects of exercise on sleep may require an adjustment period, and perhaps a longer exercise intervention may have had greater impact. While existing literature has delved into the impacts of regular physical activity upon sleep, generally showing a positive association, many studies focus on chronic behaviors, and few have focused on interventions among people with poor sleep.Citation42
In conclusion, while exercise has been demonstrated to be effective at improving sleep in older adults, we failed to find a significantly beneficial effect of an exercise intervention on objective sleep parameters or subjective sleep quality. Instead, HIV serostatus was associated with poorer sleep quality across multiple outcomes and those with HIV had significantly poorer subjective sleep and lower sleep efficiency following the intervention, compared to controls. Poor subjective sleep quality implies that an individual may experience tiredness upon waking and throughout the day.Citation43 This poor perceived sleep quality may serve as a barrier to both initiating and maintaining exercise, particularly among older adults with HIV. Additional interventions to effectively improve sleep quality are needed and may improve exercise initiation and long-term maintenance.
Supplemental Material
Download MS Word (17.9 KB)Conflicts of interest
KME has received research funding to the University of Colorado from Gilead Sciences, and has served on advisory boards for ViiV and Gilead Sciences. HJB is a consultant for Natrol, LLC, who manufacture dietary supplements.
Additional information
Funding
Notes on contributors
Brian Hixon
Brian Hixon completed this work as part of his masters in public health dissertation.
Helen J. Burgess
Helen J. Burgess is a Professor and Co-Director of the Sleep and Circadian Research Laboratory at the University of Michigan. Her research focuses on sleep and circadian rhythms, HIV, chronic pain, alcohol use disorders, and mental health.
Melissa P. Wilson
Melissa P. Wilson is a clinical data analyst at the University of Colorado with research interests in electronic medical record data and informatics.
Samantha MaWhinney
Samantha MaWhinney is a Professor of Biostatistics and Informatics with expertise in HIV, clinical trials, and models accounting for drop-out.
Catherine M. Jankowski
Catherine M. Jankowski is an Associate Professor and exercise physiologist with research expertise on maintenance and promotion of physical function during aging and chronic disease.
Kristine M. Erlandson
Kristine M. Erlandson is an Associate Professor, board certified in Internal Medicine and Infectious Diseases, whose research and clinical practice focuses on maintenance of physical function with aging in HIV or with other chronic infections.
References
- Suni E. How much sleep do we really need? [cited August 6, 2020]. Available from: https://www.sleepfoundation.org/articles/how-much-sleep-do-we-really-need.
- Taibi DM. Sleep disturbances in persons living with HIV. J Assoc Nurses AIDS Care. 2013;24(1 Suppl):S72–S85.
- Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261.
- Wirth MD, Jaggers JR, Dudgeon WD, et al. Association of markers of inflammation with sleep and physical activity among people living with HIV or AIDS. AIDS Behav. 2015;19(6):1098–1107.
- Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1–11.
- Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59(12):1787–1797.
- Sigel K, Park L, Justice A. HIV and cancer in the Veterans Health Administration System. Semin Oncol. 2019;46(4–5):334–340.
- Faraut B, Malmartel A, Ghosn J, et al. Sleep disturbance and total sleep time in persons living with HIV: a cross-sectional study. AIDS Behav. 2018;22(9):2877–2887.
- Ren J, Zhao M, Liu B, et al. Factors associated with sleep quality in HIV. J Assoc Nurses AIDS Care. 2018;29(6):924–931.
- Chaponda M, Aldhouse N, Kroes M, Wild L, Robinson C, Smith A. Systematic review of the prevalence of psychiatric illness and sleep disturbance as co-morbidities of HIV infection in the UK. Int J STD Aids. 2018;29(7):704–713.
- Rogers BG, Lee JS, Bainter SA, Bedoya CA, Pinkston M, Safren SA. A multilevel examination of sleep, depression, and quality of life in people living with HIV/AIDS. J Health Psychol. 2020;25(10–11):1556–1566.
- Ning C, Lin H, Chen X, et al. Cross-sectional comparison of various sleep disturbances among sex- and age-matched HIV-infected versus HIV-uninfected individuals in China. Sleep Med. 2020;65:18–25.
- Gomez-Olive FX, Rohr JK, Roden LC, Rae DE, von Schantz M. Associations between sleep parameters, non-communicable diseases, HIV status and medications in older, rural South Africans. Sci Rep. 2018;8(1):17321.
- McIntosh R, Antoni M, Seay J, et al. Associations among trajectories of sleep disturbance, depressive symptomology and 24-hour urinary cortisol in HIV + women following a stress management intervention. Behav Sleep Med. 2019;17(5):605–620.
- Vance DE, Cody SL, Yoo-Jeong M, Jones GL, Nicholson WC. The role of employment on neurocognitive reserve in adults with HIV: a review of the literature. J Assoc Nurses AIDS Care. 2015;26(4):316–329.
- Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002;2002(4):CD003404.
- King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277(1):32–37.
- Wang X, Youngstedt SD. Sleep quality improved following a single session of moderate-intensity aerobic exercise in older women: results from a pilot study. J Sport Health Sci. 2014;3(4):338–342.
- Varrasse M, Li J, Gooneratne N. Exercise and sleep in community-dwelling older adults. Curr Sleep Med Rep. 2015;1(4):232–240.
- Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11(9):934–940.
- Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58(3):157–163.
- Webel AR, Willig AL, Liu W, et al. Physical activity intensity is associated with symptom distress in the CNICS cohort. AIDS Behav. 2019;23(3):627–635.
- Heissel A, Zech P, Rapp MA, et al. Effects of exercise on depression and anxiety in persons living with HIV: a meta-analysis. J Psychosom Res. 2019;126:109823.
- Erlandson KM, MaWhinney S, Wilson M, et al. Physical function improvements with moderate or high-intensity exercise among older adults with or without HIV infection. AIDS. 2018;32(16):2317–2326.
- Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438.
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
- Slater JA, Botsis T, Walsh J, King S, Straker LM, Eastwood PR. Assessing sleep using hip and wrist actigraphy. Sleep Biol Rhythms. 2015;13(2):172–180.
- Patel SR, Weng J, Rueschman M, et al. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep. 2015;38(9):1497–1503.
- Shrivastava D, Jung S, Saadat M, Sirohi R, Crewson K. How to interpret the results of a sleep study. J Commun Hosp Intern Med Perspect. 2014;4(5):24983.
- Lumley T, Gillen DL. Characterising transitive two-sample tests. Stat Probab Lett. 2016;109:118–123.
- Signorell A, Aho K, Alfons A, et al. DescTools: tools for descriptive statistics. R package version 09934. [cited August 2, 2020]. Available from: https://cranr-projectorg/package=DescTools 2020.
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46.
- Lee KA, Gay C, Portillo CJ, et al. Types of sleep problems in adults living with HIV/AIDS. J Clin Sleep Med. 2012;08(01):67–75.
- Oshinaike O, Akinbami A, Ojelabi O, Dada A, Dosunmu A, John Olabode S. Quality of sleep in an HIV population on antiretroviral therapy at an urban tertiary centre in Lagos, Nigeria. Neurol Res Int. 2014;2014:1–6.
- Robbins JL, Phillips KD, Dudgeon WD, Hand GA. Physiological and psychological correlates of sleep in HIV infection. Clin Nurs Res. 2004;13(1):33–52.
- Taibi DM, Price C, Voss J. A pilot study of sleep quality and rest-activity patterns in persons living with HIV. J Assoc Nurses AIDS Care. 2013;24(5):411–421.
- Chen WT, Lee SY, Shiu CS, et al. Fatigue and sleep disturbance in HIV-positive women: a qualitative and biomedical approach. J Clin Nurs. 2013;22(9–10):1262–1269.
- Sabin CA, Harding R, Doyle N, et al. Associations between widespread pain and sleep quality in people with HIV. J Acquir Immune Defic Syndr. 2020;85(1):106–112.
- Campbell LM, Tang B, Watson CW, et al. Cannabis use is associated with greater total sleep time in middle-aged and older adults with and without HIV: a preliminary report utilizing digital health technologies. Cannabis. 2020;3(2):180–189.
- Ferris LT, Williams JS, Shen CL, O’Keefe KA, Hale KB. Resistance training improves sleep quality in older adults a pilot study. J Sports Sci Med 2005;4(3):354–360.
- McDermott A, Zaporojan L, McNamara P, et al. The effects of a 16-week aerobic exercise programme on cognitive function in people living with HIV. AIDS Care. 2017;29(6):667–674.
- Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24(2):355–365, xi.
- Harvey AG, Stinson K, Whitaker KL, Moskovitz D, Virk H. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep 2008;31(3):383–393.
