Abstract
Background: Treatment during acute or early human immunodeficiency virus (HIV)-1 infection is associated with immunologic and virologic benefits.
Objective: To evaluate darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) efficacy/safety among patients with acute or early HIV-1 infection who rapidly initiate treatment.
Methods: DIAMOND (ClinicalTrials.gov Identifier: NCT03227861), a phase 3 study, evaluated the efficacy/safety of D/C/F/TAF 800/150/200/10 mg in rapid initiation. Adults aged ≥18 years began D/C/F/TAF within 14 days of diagnosis, prior to the availability of screening/baseline laboratory results. In this subgroup analysis, virologic response (HIV-1 RNA <50 copies/mL) was assessed at Week 48 by intent-to-treat FDA snapshot (ITT-FDA snapshot) and observed (excluding patients with missing data) analyses in patients with acute (HIV-1 antibody negative and HIV-1 RNA positive/p24 positive) or early (HIV-1 antibody positive and suspected infection ≤6 months before screening/baseline) infection.
Results: Among 109 patients, 13 had acute and 43 had early HIV-1 infection. High rates of virologic response were demonstrated at Week 48 by ITT-FDA snapshot (acute: 10/13 [76.9%]; early: 37/43 [86.0%]) and observed (acute: 10/11 [90.9%]; early: 37/38 [97.4%]) analyses. No patients discontinued or required regimen change due to baseline resistance or lack of efficacy, or developed protocol-defined virologic failure. Through Week 48, 7 (53.8%) acute and 22 (51.2%) early infection patients had a D/C/F/TAF-related adverse event (AE); none had a D/C/F/TAF-related grade 4 or serious AE.
Conclusions: High rates of viral suppression during acute/early infection were achieved with D/C/F/TAF rapid initiation, no treatment-emergent resistant mutations were observed, and D/C/F/TAF was safe and well tolerated.
Introduction
During the period soon after human immunodeficiency virus (HIV)–1 infection, markedly elevated viral loads are observed in blood and genital secretions. Higher viral loads increase the risk of transmission despite the fact that patients may not be symptomatic during this initial phase of infection.Citation1,Citation2 Although clinical trial data are limited, there is evidence that patients who initiate antiretroviral therapy (ART) during this time have improved immunologic and virologic outcomes relative to patients who delay starting therapy.Citation3–10 Additionally, the risk of transmission decreases for patients who achieve HIV-1 RNA <200 copies/mL, a threshold recognized by multiple organizations, including Prevention Access Campaign’s Undetectable = Untransmittable, as that at which individuals are unable to sexually transmit HIV-1 to uninfected partners.Citation11,Citation12
HIV-1 is challenging to eradicate due, in part, to the development of latent viral reservoirs. Immediately after transmission (ie, during acute infection)Citation1,Citation13 and within 6 months of transmission, as HIV-1 antibodies become detectable (ie, during early infection),Citation1 HIV-1 may be more responsive to ART because reservoirs have not yet been created.Citation2,Citation14 The US Department of Health and Human Services (DHHS) guidelines recommend immediate initiation of ART after HIV-1 diagnosis to increase treatment uptake, promote linkage to care, reduce the time to viral suppression, and improve the rate of virologic suppression among patients with HIV-1.Citation1 However, despite these recommendations, some clinicians may be hesitant to rapidly initiate ART due, in part, to concerns regarding the patient’s adherence to and tolerability of treatment. Per US DHHS guidelines, in the setting of acute or early (sometimes referred to as “recent”) HIV-1 infection, darunavir-based regimens are recommended due to the slow emergence of resistance and uncommon transmission of clinically significant resistance to protease inhibitors.Citation1 Guidelines also support bictegravir- and dolutegravir-based regimens as options during these phases of infection; however, integrase inhibitor–containing regimens have been associated with greater weight gain than other regimens, including those with boosted protease inhibitors, among treatment-naïve patients, and there are limited data on integrase inhibitors for patients rapidly initiating therapy after diagnosis.Citation1
DIAMOND, a phase 3, prospective study of rapid initiation with a single-tablet regimen (STR), assessed the efficacy and safety of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) 800/150/200/10 mg in treatment-naïve patients newly diagnosed with HIV-1.Citation15 Overall, a high proportion of patients (84% [intent-to-treat (ITT) US Food and Drug Administration (FDA) snapshot] and 96% [observed]) achieved HIV-1 RNA <50 copies/mL at Week 48; no patients discontinued or required regimen change due to baseline resistance or lack of efficacy, or developed protocol-defined virologic failure (PDVF); and treatment was well tolerated. Reported here is an evaluation of the efficacy and safety of D/C/F/TAF among those who rapidly initiated treatment during acute or early HIV-1 infection. Given the high barrier to resistance of darunavir, good tolerability, and potential to improve treatment adherence as an STR,Citation1,Citation16,Citation17 D/C/F/TAF may be an ideal regimen in certain conditions, such as acute and early HIV-1 infection, when more urgent ART initiation is warranted and resistance testing results are pending.
Methods
Study design and population
DIAMOND (ClinicalTrials.gov Identifier: NCT03227861), a phase 3, prospective, single-arm, open-label, multicenter study, evaluated the efficacy and safety of D/C/F/TAF rapid initiation over 48 weeks. Detailed methods have been publishedCitation15 and are summarized in the Supplementary Materials and Supplementary Figure S1.
For this subgroup analysis, acute infection was defined as HIV-1 antibody negative and HIV-1 RNA positive/p24 positive.Citation1,Citation13 Early infection was defined as HIV-1 antibody positive and suspected infection ≤6 months before screening/baseline (based on the definition of “recent” infection in US DHHS guidelines)Citation1; to classify a patient as having early infection, investigators must have confirmed evidence of a negative HIV-1 antibody test within 6 months of the most recent HIV-1 antibody test prior to the screening/baseline visit.
DIAMOND was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by an institutional review board and all contributing sites that required local institutional review board approval. Written informed consent was provided by all participants.
Analyses
The primary objective was the proportion of patients who had virologic response (HIV-1 RNA <50 copies/mL) at Week 48 (ITT-FDA snapshot). Adherence was evaluated by pill count at predefined time points, and cumulatively from baseline to Week 48. Safety was assessed by adverse events (AEs) and discontinuations due to protocol-defined safety stopping rules.
Statistical analyses
Analyses were performed on all patients who received ≥1 dose of study drug (ITT population). Observed values were used in descriptive statistics; missing values were not imputed. Descriptive statistics with 95% exact confidence intervals were used to account for variability in the small sample sizes.
Results
Study population
Of the 109 patients enrolled in DIAMOND, 13 had acute and 43 had early HIV-1 infection (see Supplementary Table S1 for baseline patient demographic and clinical characteristics). Among patients with available genotype data from screening/baseline (n = 53), all exhibited full genotypic susceptibility to darunavir and tenofovir. Two (5.0%) patients with early infection had emtricitabine resistance (1 had M184M/I and 1 had M184M/V).
Patient disposition
Among the 13 patients with acute infection, 11 (84.6%) completed the study and 2 discontinued early; 1 patient discontinued due to protocol-defined safety stopping criteria (aspartate aminotransferase and/or alanine aminotransferase elevations ≥2.5 times the upper limit of normal at screening/baseline) and 1 patient was lost to follow-up at Week 36. Of the 43 patients with early infection, 38 (88.4%) completed the study and 5 discontinued early; 2 were lost to follow-up (1 at Week 8 and 1 at Week 12), 1 discontinued due to an AE, 1 withdrew consent, and 1 discontinued for other reasons (switch to a different ART regimen due to D/C/F/TAF food requirement).
Efficacy
At Week 48, 10 (76.9%) acute and 37 (86.0%) early infection patients achieved HIV-1 RNA <50 copies/mL (ITT-FDA snapshot; ). Of the remaining 3 patients with acute infection, 1 had HIV-1 RNA ≥50 copies/mL (viral load = 102 copies/mL), 1 discontinued for reasons other than virologic failure (ie, safety stopping rules) with the last available HIV-1 RNA ≥50 copies/mL, and 1 did not have viral load data in the Week 48 window. Among the 6 patients with early infection who did not achieve HIV-1 RNA <50 copies/mL, 1 had HIV-1 RNA ≥50 copies/mL (viral load = 134 copies/mL), 2 discontinued for reasons other than virologic failure (ie, lost to follow-up and withdrawal of consent) with the last available HIV-1 RNA ≥50 copies/mL, and 3 did not have viral load data in the Week 48 window. According to the observed algorithm, 10 of 11 (90.9%) acute and 37 of 38 (97.4%) early infection patients had achieved HIV-1 RNA <50 copies/mL at Week 48 ().
Figure 1. Virologic response with D/C/F/TAF rapid initiation over time in patients with acute or early HIV-1 infection using a threshold of HIV-1 RNA <50 copies/mL and A) ITT-FDA snapshot analysis and B) observed analysis, and a threshold of HIV-1 RNA <200 copies/mL and C) ITT-FDA snapshot analysis and D) observed analysis.
D/C/F/TAF, darunavir/cobicistat/emtricitabine/tenofovir alafenamide; FDA, Food and Drug Administration; HIV-1, human immunodeficiency virus–1; ITT, intent-to-treat.
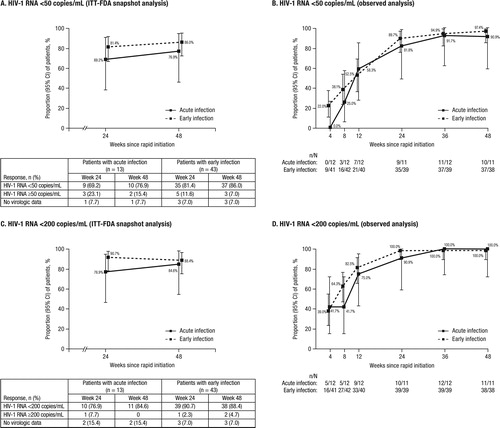
The threshold of HIV-1 RNA <200 copies/mL was reached by 11 (84.6%) acute and 38 (88.4%) early infection patients at Week 48 using ITT-FDA snapshot analysis (). According to the observed algorithm, the majority of patients in each subgroup had HIV-1 RNA <200 copies/mL by Week 12, and all patients achieved this by Week 36 ().
No patients discontinued or required regimen change due to baseline resistance or lack of efficacy, or developed PDVF. No patients met the criteria for postbaseline resistance testing.
Treatment adherence
Across both subgroups, the mean (standard deviation) cumulative adherence through Week 48 was 94.7% (11.8%). At each study visit, most patients with acute or early infection had adherence >95%. Adherence in both subgroups was similar to that observed in the overall DIAMOND study population (which had a mean [standard deviation] cumulative adherence of 95% [13%]).Citation18
Safety
The majority of AEs for patients with acute or early infection were grade 1 or 2 in severity, and no serious AEs were considered to be study drug–related (). The most common AEs related to study drug (occurring in ≥5% of the combined acute and early infection patients) were diarrhea, nausea, and vomiting. There were no cases of immune reconstitution inflammatory syndrome (IRIS), and no deaths were reported. No patients discontinued due to weight gain or gastrointestinal, central nervous system, renal, or bone AEs.
Table 1. Summary of AEs through Week 48.
Discussion
Rapid initiation has been shown to improve retention in care, reduce time to virologic suppression, and decrease morbidity and mortality.Citation19–24 For these reasons, US DHHS guidelines recommend all patients diagnosed with HIV-1 immediately initiate ART.Citation1 Furthermore, the guidelines emphasize the importance of rapid initiation for patients with acute or early HIV-1 infection due, in part, to the sudden onset of viremia associated with these early phases.Citation1 Prompt ART initiation during these beginning phases of infection minimizes the risk of transmission and poses benefits to virus eradication efforts by rapidly reducing viral load and limiting viral reservoir size.Citation1,Citation2
Among patients newly diagnosed with HIV-1 infection who rapidly started therapy with D/C/F/TAF in DIAMOND, a subset had acute or early infection. Virologic response rates were high despite some patients having high baseline viral loads. No patients discontinued or required regimen change due to baseline resistance or lack of efficacy, or met PDVF criteria. At Week 24, the majority of acute and early infection patients had HIV-1 RNA <200 copies/mL (ITT-FDA snapshot), the Undetectable = Untransmittable threshold.Citation11,Citation12 In this subset of patients who likely contribute disproportionately to HIV-1 transmission,Citation1,Citation2 achieving this threshold soon after infection is particularly significant. Additionally, despite the challenge of keeping patients in care and adherent to treatment, especially those who are newly diagnosed,Citation25,Citation26 a high proportion (84.6%–88.4%) of patients were retained in care through 48 weeks, none discontinued due to noncompliance, and most achieved adherence >95% at each study visit. These data indicate that patients recently diagnosed with HIV-1, including those with acute or early disease, can adhere to treatment to achieve favorable outcomes and, moreover, perceptions around poor adherence should not be a barrier to starting treatment. A patient’s ability to adhere to treatment may be influenced by tolerability of their regimen. D/C/F/TAF was well tolerated, and no study drug–related serious AEs were reported. Most AEs were grade 1 or 2 in severity, and no cases of IRIS were reported. Furthermore, in the overall DIAMOND population, patients showed high treatment satisfaction according to the HIV Treatment Satisfaction Questionnaire status version, which may have contributed to the high rates of retention and adherence.Citation27
Transmitted resistance is also a concern in rapid initiation scenarios, in which ART is started before the availability of resistance test results.Citation1 According to US DHHS guidelines, transmitted non-nucleoside reverse transcriptase inhibitor (NNRTI) and nucleos(t)ide reverse transcriptase inhibitor (N[t]RTI) resistance are the most common.Citation1 Among this subset of patients in DIAMOND, none required a change in ART based on receipt of screening/baseline resistance testing results, indicating D/C/F/TAF can be started with little or no concern of pre-existing resistance to regimen components. Additionally, no new resistance mutations were observed during the study, as no patients met PDVF criteria. These results are consistent with those from other darunavir-based studies, in which patients were able to achieve virologic response despite harboring baseline NNRTI and/or N(t)RTI resistance-associated mutations.Citation28
There are limited clinical trial data regarding the initiation of ART in acute or early HIV-1 infection.Citation1 However, in a retrospective case-note analysis of 113 patients in the United Kingdom diagnosed with acute HIV-1 infection, the time from diagnosis to ART initiation was 20 days (median), and high rates of virologic suppression were observed. The time to viral suppression, defined as HIV-1 RNA ≤200 copies/mL, was 74 days (median) and, at 24 weeks, 99% of patients had achieved viral suppression.Citation29 These results from a real-world setting indicated that most patients with acute HIV-1 infection found rapid initiation of treatment to be acceptable and nearly all were able to achieve virologic suppression after 24 weeks. Moreover, the results are consistent with DIAMOND, in which 90.9% of patients with acute infection and 100.0% of those with early infection achieved HIV-1 RNA <200 copies/mL by Week 24 (observed).
In summary, findings from this subset of DIAMOND patients with acute or early HIV-1 infection and high baseline viral loads demonstrated that a high proportion of patients achieved virologic suppression and were retained in care. No new resistance mutations were observed and D/C/F/TAF demonstrated good tolerability and safety, reinforcing the recommendation of using a darunavir-based regimen for rapid initiation.
Supplemental Material
Download MS Word (47 KB)Acknowledgments
Medical writing support was provided by Caryn Gordon, PharmD, and Courtney St. Amour, PhD, of MedErgy.
Disclosure statement
This study was supported by Janssen Scientific Affairs, which was involved in the study design, analysis and interpretation of results, manuscript preparation, and publication decisions. K. Dunn, R. Rogers, R.B. Simonson, P.T. Kassam, and S. Seyedkazemi are employees of Janssen Scientific Affairs, LLC, and stockholders of Johnson & Johnson. D. Luo, S. Sheng, and H. Hardy are employees of Janssen Research & Development, LLC; D. Luo and H. Hardy are stockholders of Johnson & Johnson.
Data availability statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Additional information
Funding
Notes on contributors
Keith Dunn
Keith Dunn, PharmD, BCPS, AAHIVE is Scientific Field Director, Infectious Diseases for Janssen Infectious Diseases & Vaccines. Dr. Dunn earned his PharmD at Northeastern University. He began caring for people living with HIV in 2008 through a 2-year residency in HIV at Boston University, which cared for >1,000 people living with HIV. As a Scientific Field Director at Janssen, he addresses data gaps identified by the HIV community and how novel treatment options for people living with HIV may meet patients’ needs. Most recently Keith has focused his research efforts to better understand the efficacy and safety of starting patients on ART rapidly, the cost implications of delaying treatment, and newly emergent CNS and metabolic concerns with ART.
Rachel Rogers
Rachel Rogers, PharmD, is an Associate Medical Director for Janssen Infectious Diseases & Vaccines. She has served in other roles across the Janssen Infectious Diseases & Vaccines franchise to support research, launch activities, and business partner collaborations. She received her PharmD at Rutgers University and completed post-graduate training at Duke University Hospital and an Infectious Diseases specialty residency at the South Texas Veterans Affairs (VA) Healthcare System. She remained at the VA in HIV/HCV clinical practice prior to joining Janssen.
Richard Bruce Simonson
Richard Bruce Simonson is a Director for Janssen Infectious Diseases & Vaccines. He has served other roles within Janssen across Infectious Diseases and other therapeutic areas; in these roles he has managed multiple phase 3 and phase 4 clinical trials.
Donghan Luo
Donghan Luo is an experienced statistician with over 20 years of clinical research experience in the pharmaceutical industry. He has authored and published over 30 clinical research manuscripts in the areas of infectious disease, diabetes, oncology, and cardiovascular disease. He had academic training in mathematics and statistics, with a PhD in Mathematics and MS in Biometrics and Statistics.
Shubin Sheng
Shubin Sheng, PhD, is Associate Director, Biostatistics at Cytel FSP-Janssen. He has more than 13 years of experience as a biostatistician in clinical trials and outcomes research. Prior to joining Cytel, he served as a Biostatistician III at Duke Clinical Research Institute (DCRI). He attended the University of Illinois at Urbana Champaign and received a PhD in Molecular Physiology and MS in Statistics.
Purnima T. Kassam
Purnima T. Kassam, PharmD, is an Associate Field Director, MSLs supporting the Janssen Infectious Diseases & Vaccines team. She earned her PharmD from the University of the Sciences in Philadelphia in 2005. In her current role in Medical Affairs, she supports healthcare professionals by addressing medical inquiries, collaborating with business partners, and supporting efforts in HIV clinical research.
Sareh Seyedkazemi
Sareh Seyedkazemi, PharmD, is Director of Scientific Communications at Janssen Infectious Diseases & Vaccines. In this role she is predominantly responsible for leading a team delivering on data dissemination efforts. She earned her PharmD degree from the University of Maryland, Baltimore and completed a PGY-1 residency at Mercy Hospital in Miami, FL. She joined Janssen Infectious Diseases & Vaccines in 2015.
Hélène Hardy
Hélène Hardy is a Senior Director in R&D at Janssen Pharmaceuticals. She serves as a Compound Development Team Leader at Janssen Research & Development, Pharmaceutical Companies of Johnson & Johnson in the Infectious Diseases & Vaccines therapeutic area. Her current research focuses on bacteriophage therapy, and she has been active in HIV drug development since 2010 via the tenure of various roles within the pharmaceutical industry.
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Updated 2019. Accessed January 12, 2021.
- Rutstein SE, Ananworanich J, Fidler S, et al. Clinical and public health implications of acute and early HIV detection and treatment: a scoping review. J Int AIDS Soc. 2017;20(1):21579.
- Hogan CM, Degruttola V, Sun X, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J Infect Dis. 2012;205(1):87–96.
- Grijsen ML, Steingrover R, Wit FW, et al. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: the randomized Primo-SHM trial. PLoS Med. 2012;9(3):e1001196.
- Hamlyn E, Ewings FM, Porter K, et al. Plasma HIV viral rebound following protocol-indicated cessation of ART commenced in primary and chronic HIV infection. PLoS One. 2012;7(8):e43754.
- Fidler S, Porter K, Ewings F, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368(3):207–217.
- Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407(6803):523–526.
- Schuetz A, Deleage C, Sereti I, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10(12):e1004543.
- Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77(21):11708–11717.
- Nozza S, Poli A, Ripa M, et al. Efficacy of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate as treatment for primary or recent HIV infection. J Antimicrob Chemother. 2017;72(2):632–633.
- Prevention Access Campaign. Consensus statement. https://www.preventionaccess.org/consensus. Issued 2016. Accessed January 12, 2021.
- National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of HIV/AIDS Prevention. Evidence of HIV treatment and viral suppression in preventing the sexual transmission of HIV. https://www.cdc.gov/hiv/pdf/risk/art/cdc-hiv-art-viral-suppression.pdf. Published 2020. Accessed January 12, 2021.
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. Laboratory testing for the diagnosis of HIV infection: updated recommendations. http://dx.doi.org/10.15620/cdc.23447 Published 2014. Accessed February 11, 2021.
- Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191(9):1410–1418.
- Huhn GD, Crofoot G, Ramgopal M, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in a rapid initiation model of care for HIV-1 infection: primary analysis of the DIAMOND study. Clin Infect Dis. 2020;71(12):3110–3117.
- Lathouwers E, Wong EY, Luo D, Seyedkazemi S, De Meyer S, Brown K. HIV-1 resistance rarely observed in patients using darunavir once-daily regimens across clinical studies. HIV Clin Trials. 2017;18(5–6):196–204.
- Clay PG, Nag S, Graham CM, Meta NS. Analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine. 2015;94(42):e1677.
- Hardy H, Luo D, Israel D, Simonson RB, Dunn K. Treatment adherence and virologic response over 48 weeks among patients rapidly initiating darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in the DIAMOND study. Poster presented at: 14th International Conference on HIV Treatment and Prevention Adherence; June 17–19, 2019; Miami, FL.
- Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient's first clinic visit: the RapIT randomized controlled trial. PLoS Med. 2016;13(5):e1002015.
- Koenig SP, Dorvil N, Devieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med. 2017;14(7):e1002357.
- Labhardt ND, Ringera I, Lejone TI, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: the CASCADE randomized clinical trial. JAMA. 2018;319(11):1103–1112.
- Coffey S, Bacchetti P, Sachdev D, et al. RAPID antiretroviral therapy: high virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS. 2019;33(5):825–832.
- Halperin J, Butler I, Conner K, et al. Linkage and antiretroviral therapy within 72 hours at a federally qualified health center in New Orleans. AIDS Patient Care Stds. 2018;32(2):39–41.
- Colasanti J, Sumitani J, Mehta CC, et al. Implementation of a rapid entry program decreases time to viral suppression among vulnerable persons living with HIV in the southern United States. Open Forum Infect Dis. 2018;5(6):ofy104.
- Centers for Disease Control and Prevention. Understanding the HIV care continuum. https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-care-continuum.pdf. Published 2019. Accessed February 11, 2021.
- World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva: World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/255884/9789241550062-eng.pdf?sequence=1. Published 2017. Accessed December 22, 2020.
- Dunn K, Simonson RB, Luo D, et al. High levels of patient satisfaction and virologic suppression at 48 weeks in newly diagnosed black/African American individuals rapidly initiating darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in the DIAMOND study. Poster presented at: National Medical Association Annual Convention and Scientific Assembly; July 27–31, 2019; Honolulu, HI.
- Lathouwers E, Seyedkazemi S, Luo D, Brown K, De Meyer S, Wong EY. Pooled resistance analyses of darunavir once-daily regimens and formulations across 10 clinical studies of treatment-naive and treatment-experienced patients with human immunodeficiency virus-1 infection. HIV Res Clin Pract. 2020;21(2–3):83–89.
- Girometti N, Nwokolo N, McOwan A, Whitlock G. Outcomes of acutely HIV-1-infected individuals following rapid antiretroviral therapy initiation. Antivir Ther. 2017;22(1):77–80.
