Abstract
Background: Evidence from clinical practice on the effects of switching from emtricitabine/tenofovir disoproxil fumarate (F/TDF) to emtricitabine/tenofovir alafenamide (F/TAF)-based triple-therapy (TT) regimens on renal parameters is limited.
Objective: This retrospective analysis evaluated the effects on renal function of switching from F/TDF to F/TAF-based TT regimens with no change in third agent among people living with HIV (PLWH).
Methods: Data were from a multicenter Spanish PLWH cohort. Patients with a baseline estimated glomerular filtration rate (eGFR-EPI) measurement, ≥1 follow-up measurement, ≥30 days treatment with F/TAF, and who switched from F/TDF to F/TAF with no change in third agent were included. Multivariate mixed linear models were used to evaluate change from baseline over time in eGFR-EPI. eGFR-EPI changes before and after switch were analyzed in a matched patient subgroup.
Results: Overall, 340 patients were included. Mean (95% CI) eGFR-EPI in patients with baseline eGFR-EPI <90 ml/min/1.73m2 (n = 125) was 79.6 (78.0; 81.2) ml/min/1.73m2 at baseline and 81.3 (79.9; 82.7) ml/min/1.73m2 at 12 months after switch. In the patient-matched subgroup (n = 175), median annual eGFR-EPI declined −4.24 ml/min/1.73m2 while on F/TDF and increased +0.93 ml/min/1.73m2 after switch to F/TAF (P < 0.0001). In patients with baseline eGFR-EPI <90 ml/min/1.73m2, median annual eGFR-EPI increased +4.19 mL/min/1.73m2 after switch (P < 0.0001).
Conclusion: Switching from F/TDF to F/TAF-based TT regimens while maintaining the same third agent numerically improved eGFR-EPI in PLWH with baseline eGFR-EPI <90 mL/min/1.73m2. eGFR-EPI improved significantly when comparing progression while on F/TDF vs progression after switch, confirming beneficial renal effects of switching to F/TAF in a clinical practice setting.
Introduction
Tenofovir disoproxil fumarate (TDF) is a prodrug of the HIV nucleotide reverse transcriptase inhibitor tenofovir and has been commonly used in antiretroviral therapy (ART) for many years. Although TDF is better tolerated than older nucleoside reverse transcriptase inhibitors, its primary limitations are renal toxicity and decreases in bone mineral density.Citation1 Use of TDF in patients with HIV is associated with an increased risk of chronic kidney disease and proximal tubular dysfunction, a decline in estimated glomerular filtration rates (eGFR), and changes in urinary markers of renal function.Citation2–5 An observational study found that the incidence of moderate or severe TDF-associated renal toxicity (defined as eGFR-EPI <60 ml/min) in Spain was 29.2 cases per 1000 person-years.Citation6
Another approved prodrug of tenofovir, tenofovir alafenamide (TAF), is associated with a significantly lower plasma concentration of the active metabolite tenofovir (TFV) compared to TDF and it is now widely used.Citation7–10 A pooled analysis of 26 trials indicated that compared with those receiving TAF-containing regimens, participants receiving TDF-containing regimens reported higher cumulative incidences of proximal renal tubulopathy (0.34% versus 0%, P < 0.001) and discontinuations due to renal adverse events (0.47% versus 0.05%, P < 0.001).Citation11 In the clinical trial setting, switching from emtricitabine/tenofovir disoproxil fumarate (F/TDF) to emtricitabine/tenofovir alafenamide (F/TAF)-based triple therapy (TT) regimens has been shown to improve renal parameters.Citation12–16 The lower renal toxicity associated with F/TAF compared with F/TDF is thought to be related to the significantly lower plasma concentration of TFV with TAF, since TFV-associated renal toxicity is concentration dependent.Citation8,Citation17,Citation18
Clinical practice data regarding the impact of switching from F/TDF to F/TAF on renal outcomes among people living with HIV (PLWH) are lacking globally. Only one other study in a clinical practice setting has been conducted, and a significant increase in eGFR-EPI (75.4 to 87.1 mL/min/1.73 m2) was observed 12 months after the switch in patients with baseline eGFR-EPI <90 mL/min/1.73 m2.Citation19 Other clinical practice data are limited to case reports and one case series.Citation20–24 The case series of 10 patients with F/TDF-induced renal toxicity who switched to F/TAF-based regimens reported no change in median eGFR-EPI (61 mL/min at baseline) after the switch.Citation21 It was speculated that the lack of improvement in eGFR-EPI may be due to the irreversible renal damage or other comorbidities such as hypertension that also contributed to the kidney disease. In addition to comorbidities, other factors such as sodium and potassium intake can influence changes in eGFR-EPI and contribute to the eGFR-EPI variation observed in the general population.Citation25 Such variations may explain some of the discrepancies among the clinical practice data for eGFR-EPI changes after switching from F/TDF-based to F/TAF-based regimens.
The objective of this analysis was to evaluate the effects on renal function of switching from an F/TDF to an F/TAF-based regimen with no change in third agent among PLWH in a large Spanish cohort.
Materials and methods
A retrospective analysis was performed using data from the VACH cohort. The VACH cohort is a prospectively recruited Spanish cohort of 14,833 HIV-infected adult patients from 23 investigational centers across Spain with the enrolment since 1997. Demographic data, HIV risk factors, Centers for Disease Control and Prevention (CDC) stage according to 1993 definitions, HIV-1 treatment initiation date, the specific antiretroviral regimens used, the date of change of every drug and the reasons for change, CD4 cell count, plasma HIV RNA levels, blood cell counts and blood chemistry tests are prospectively collected in an electronic case record form according to standardized criteria. Additional details on the data collection in VACH have been previously described.Citation26 All patients participating in the VACH cohort gave their consent to the use of anonymous data for epidemiologic studies. The study was reviewed and approved by Ethics Review Board of Cantabria.
Patient populations
The primary analyses were performed on all patients meeting the selection criteria and in subgroups of patients with eGFR-EPI at baseline of <90 and ≥90 mL/min/1.73 m2. To evaluate annual eGFR-EPI changes before and after the switch from F/TDF to F/TAF, analyses were also performed in a matched subgroup of patients with eGFR-EPI data during both the F/TDF and the F/TAF regimens (patient-matched subgroup).
Patient selection criteria
Patients at least 18 years of age who switched from F/TDF to F/TAF with no change in the third agent and who had eGFR-EPI data at the time of switch (baseline) from F/TDF to F/TAF up to March 2018 were included. Patients were also required to have been treated with F/TAF for at least 30 days and to have at least one follow-up eGFR-EPI measurement after the first month on F/TAF. To be included in the patient-matched subgroup, patients were also required to have at least one eGFR-EPI measurement at F/TDF start.
Data collection
Demographic characteristics extracted from the database were age, sex, race/ethnicity, weight, and body mass index. Clinical characteristics extracted from the database were the third agent of the therapeutic regimen, risk group of HIV transmission, CD4+ count, CD8+ count, HIV RNA copies, number of previous regimens, time since the first ART regimen, presence of comorbidities (e.g. diabetes, arterial hypertension, HCV, previous cardiovascular disease), and eGFR-EPI. If multiple follow-up eGFR-EPI measurements were available, the measurement closest to the relevant timepoint was selected. Dropouts and treatment changes were also collected. Estimated GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (eGFR-EPI) equation and updated for age during the course of the study.
Endpoints
Baseline was defined as the time of switch from F/TDF to F/TAF. The primary endpoint analyzed was the change from baseline in eGFR-EPI over time. Median annual eGFR-EPI changes before and after the switch from F/TDF to F/TAF were analyzed in the patient-matched subgroup.
Statistical analysis
Descriptive summaries were presented for baseline demographics and clinical characteristics. Comparisons between categorical groups were done with use of χ2 and Fisher's exact tests. Student's t test was used for continuous variables. All P values were 2-tailed. To evaluate change from baseline over time in eGFR-EPI, multivariate mixed linear models were used with adjustment (at time of switch) for age, sex, HIV-RNA level, CD4+ count, baseline eGFR-EPI, time on previous F/TDF regimen prior to switch, time on F/TAF, time on ART, and time from HIV diagnosis as well as an interaction term between baseline eGFR-EPI and time on F/TAF. Predicted eGFR-EPI values were obtained by applying the multivariate mixed linear model regression to each patient’s covariate values and averaging over the subgroup of interest.
For the patient-matched subgroup analysis, annualized median eGFR-EPI change while on F/TDF versus while on F/TAF was compared by Wilcoxon matched pairs signed rank test.
Results
In total, 340 patients were included; 81% were male and 35% were ≥50 years of age (). The baseline median eGFR-EPI was 95.7 ml/min/1.73 m2 and 37% of patients had eGFR-EPI <90 mL/min/1.73 m2. The median time on ART, on the previous F/TDF TT regimen, and on the F/TAF TT regimen was 8.5, 1.4, and 1.0 years, respectively. Patients with baseline eGFR-EPI <90 mL/min/1.73 m2 were older and had a lower CD4 nadir compared with patients with baseline eGFR-EPI ≥90 mL/min/1.73 m2 (). Most of the treatment switches were from E/C/F/TDF to E/C/F/TAF ().
Table 1 Patient demographics and clinical characteristics
Table 2 Distribution of regimen switches
Change of eGFR-EPI over time in the F/TAF regimen (adjusted for characteristics at the time of switch)
In the overall study population, the multivariate mixed model showed that age and baseline eGFR-EPI negatively impacted the change in eGFR after the switch from F/TDF, while longer time on F/TAF had the opposite effect of increasing eGFR-EPI change from baseline (with a lower positive impact for those with lower baseline eGFR-EPI as indicated by the interaction term) (P < 0.0001 for all). Sex, HIV RNA level, CD4+ counts, time on F/TDF prior to switch, time on ART, and time from diagnosis were not significantly associated with the change in eGFR-EPI ().
Table 3 Mixed linear model results for adjusted eGFR-EPI change from baseline over time, overall and by baseline eGFR-EPI class*
The impact of covariates was directionally identical in the subgroup analyses of patients with baseline eGFR-EPI above or below 90 ml/min/1.73 m2. Statistical significance was also consistent with findings observed in the overall study population with the exception of age for which a null impact could not be ruled out in the eGFR-EPI < 90 ml/min/1.73 m2 subgroup.
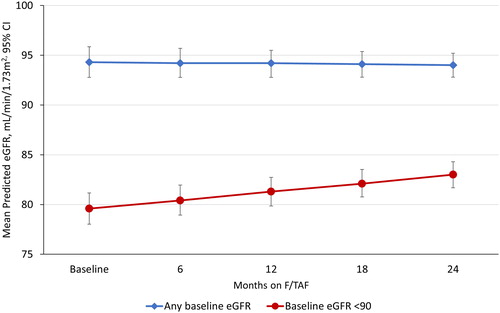
Predicted mean (95% CI) eGFR-EPI values were 94.3 (92.8; 95.9) at baseline and 94.2 (92.8; 95.5) ml/min/1.73 m2 at 12 months after the switch from F/TDF to F/TAF in the overall study population, and 79.6 (78.0; 81.2) and 81.3 (79.9; 82.7) ml/min/1.73 m2, respectively, in the subgroup of patients with a baseline eGFR-EPI <90 ml/min/1.73 m2 (). At 24 months after the switch, the predicted eGFR-EPI was 94.0 (92.8; 95.2) ml/min/1.73 m2 in the overall study population and 83.0 (81.7; 84.3) ml/min/1.73 m2 in the subgroup of patients with a baseline eGFR-EPI <90 ml/min/1.73 m2 ().
Figure 1 Mean predicted eGFR-EPI values* after switching from F/TDF to F/TAF (N = 331). eGFR-EPI, estimated glomerular filtration rate; F/TAF, emtricitabine/tenofovir alafenamide; F/TDF, emtricitabine/tenofovir disoproxil fumarate.
*Predicted values were obtained by applying the fitted multivariate models to each patient’s covariate values and averaging over the group of interest. The following formulas were applied:
For all patients:
eGFR-EPI = 30.69 + 0.03* (days on TAF) – 0.25* Age – 0.21* (eGFR-EPI switch) −0.0003* (eGFR-EPI switch)*(days on TAF)
For patients with eGFR-EPI at time of switch <90 mL/min/1.73 m2:
eGFR-EPI = 17.91 + 0.05* (days on TAF) – 0.13* Age – 0.23* (eGFR-EPI switch) −0.0006* (eGFR-EPI switch)*(days on TAF)
For patients with eGFR-EPI at time of switch ≥90 mL/min/1.73 m2:
eGFR-EPI = 32.73 + 0.04* (days on TAF) – 0.33* Age – 0.19* (eGFR-EPI switch) −0.0004* (eGFR-EPI switch)*(days on TAF)
Patient-matched eGFR-EPI over time
In the patient-matched subgroup that had eGFR-EPI information from start of the F/TDF TT regimen through switch to the F/TAF TT regimen (n = 175), there was a statistically significant change in eGFR-EPI progression from before to after the switch (P < 0.0001; ). Median annual eGFR-EPI while on F/TDF declined −4.24 ml/min/1.73 m2, whereas median annual eGFR-EPI after switch to F/TAF increased +0.93 ml/min/1.73 m2 (P < 0.0001). In patients with baseline eGFR-EPI <90 ml/min/1.73 m2, median annual eGFR-EPI while on F/TDF declined −8.24 ml/min/1.73 m2, whereas median annual eGFR-EPI after switch to F/TAF increased +4.19 mL/min/1.73 m2 (P < 0.0001; ).
Table 4 Subgroup analysis of patient-matched annual eGFR-EPI change
Discussion
In this cohort of PLWH, switching from F/TDF to F/TAF-based TT regimens while maintaining the same third agent improved eGFR with baseline eGFR-EPI <90 mL/min/1.73 m2. Furthermore, eGFR-EPI significantly improved when comparing the progression of individual patients while on F/TDF to progression after the switch to F/TAF. The median annual change in eGFR-EPI declined on F/TDF but increased on F/TAF, in a sample with a median follow-up time on F/TAF of 1 year. Together these data suggest a beneficial effect on renal safety of a switch from F/TDF to F/TAF-based TT regimens on eGFR-EPI in this cohort.
Similar to the results found in the current Spanish cohort, in a study conducted in a German cohort of PLWH there was a significant increase in eGFR-EPI (75.4 to 87.1 mL/min/1.73 m2) 12 months after switching from a F/TDF to F/TAF-based TT regimen in patients with baseline eGFR-EPI <90 mL/min/1.73 m2.Citation19 The percentage of patients in the overall study cohort with normal eGFR-EPI (≥90 mL/min/1.73 m2) increased from 42% at baseline to 62% at 12 months. In both studies, the results indicate that patients with baseline eGFR-EPI <90 ml/min/1.73 m2 experience more benefit when switching from F/TDF to F/TAF-based regimens compared with those who had normal baseline eGFR-EPI, although the studies were not designed to compare differences between these groups. For patients in the Spanish cohort with eGFR-EPI <90 ml/min/1.73 m2, the positive association between time on F/TAF and eGFR-EPI change shown in the mixed linear model highlighted the benefits of F/TAF for long-term treatment, especially considering the natural eGFR decline with age. A small retrospective study found no significant change in eGFR-EPI overall after a switch from F/TDF- to F/TAF-based regimens, but lower pre-switch eGFR-EPI was a strong predictor of post-switch eGFR-EPI improvement.Citation18 These data suggest that an eGFR-EPI <90 ml/min/1.73 m2 may be one indicator for considering a switch from a F/TDF-based regimen to a F/TAF-based regimen; however, other markers of renal function may be impacted in patients with normal eGFR-EPI.
The generalizability to PLWH is a strength of this study as the VACH cohort includes PLWH with comorbidities and disease characteristics that may exclude them from clinical trials. Another strength is the patient-matched subgroup analysis, where the eGFR-EPI before and after regimen switch was analyzed with each patient serving as his/her own comparator. This eliminates confounding from differences between treatment groups, such as the presence of cardiovascular disease, which can have an impact on renal function. Limitations of the study include those inherent to observational studies, including potentially heterogeneous data collection among the various physicians and missing data for some of the variables. Moreover, while confounding factors have been adjusted for in the regression model, confounding by indication remains a possibility.
The results of the current study of PLWH support clinical trial data indicating that switching from F/TDF-based regimens to F/TAF-based regimens has a beneficial effect on renal dysfunction. Switching reversed the decline in eGFR-EPI associated with TDF in patients with baseline eGFR-EPI <90 mL/min/1.73 m2.
Acknowledgments
This research was funded by Gilead Sciences Europe, Ltd. Medical writing and editorial assistance were provided by Erin P. Scott, PhD, of Maple Health Group and was funded by Gilead Sciences Europe, Ltd.
Disclosure statement
R. Teira, J. Muñoz, P. Galindo, M.D. Merino, B. de la Fuente, M.A. Sepúlveda, P. Domingo, J. García, M. Castaño, E. Ribera, P. Geijo A. Romero, J. Peraire, E. deig, B. Roca, E. Martínez, V. Estrada, M. Montero, J. Berenguer and N. Espinosa declare at least one of the following: having performed consultancy, or having received research grants, or having received financial compensation for scientific talks, or having collaborated in the preparation of educational material, or having received travel grants for attending scientific congresses, from at least one of the following: Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, MSD, ViiV Healthcare o AbbVie. H. Diaz-Cuervo is a Gilead Sciences Europe, Ltd employee, F. Aragão received consultancy fees from Maple Health Group, LLC, a company hired by Gilead Sciences Europe, Ltd to perform the analysis.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, RT. The data are not publicly available due to restrictions [e.g. their containing information that could compromise the privacy of research participants].
Additional information
Funding
References
- Van Rompay KKA, Durand-Gasselin L, Brignolo LL, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother. 2008;52(9):3144–3160.
- Ascher SB, Scherzer R, Estrella MM, et al. Association of urinary biomarkers of kidney injury with estimated GFR decline in HIV-infected individuals following tenofovir disoproxil fumarate initiation. Clin J Am Soc Nephrol. 2018;13(9):1321–1329.
- Jotwani V, Scherzer R, Estrella MM, et al. Brief report: Cumulative tenofovir disoproxil fumarate exposure is associated with biomarkers of tubular injury and fibrosis in HIV-infected men. J Acquir Immune Defic Syndr. 2016;73(2):177–181.
- Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. Aids. 2012;26(7):867–875.
- Horberg M, Tang B, Towner W, et al. Impact of tenofovir on renal function in HIV-infected, antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2010;53(1):62–69.
- Quesada PR, Esteban LL, García JR, et al. Incidence and risk factors for tenofovir-associated renal toxicity in HIV-infected patients. Int J Clin Pharm. 2015;37(5):865–872.
- Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2014;67(1):52–58.
- Podany AT, Bares SH, Havens J, et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. Aids. 2018;32(6):761–765.
- Surial B, Cavassini M, Calmy A, Swiss HIV Cohort Study, et al. Rates and predictors of switching to tenofovir alafenamide-containing ART in a nationwide cohort. BMC Infect Dis. 2019;19(1):834.
- Li X, Brown TT, Ho KS, Witt MD, Phair J, Jacobson LP. Recent trends and effectiveness of antiretroviral regimens among men who have sex with men living with HIV in the United States: The Multicenter AIDS Cohort Study (MACS) 2008–2017. Open Forum Infect Dis. 2019;6(9):ofz333–ofz.
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. Aids. 2019;33(9):1455–1465.
- Orkin C, Molina J-M, Negredo E, et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5:e23–e34.
- Daar ES, DeJesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e347–e56.
- DeJesus E, Haas B, Segal-Maurer S, et al. Superior efficacy and improved renal and bone safety after switching from a tenofovir disoproxil fumarate- to a tenofovir alafenamide-based regimen through 96 weeks of treatment. AIDS Res Hum Retrovirus. 2018;34(4):337–342.
- Orkin C, DeJesus E, Ramgopal M, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide coformulated with rilpivirine and emtricitabine in virally suppressed adults with HIV-1 infection: a randomised, double-blind, multicentre, phase 3b, non-inferiority study. Lancet HIV. 2017;4(5):e195–e204.
- Gallant JE, Daar ES, Raffi F, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3(4):e158-65–e165.
- Baxi SM, Scherzer R, Greenblatt RM, et al. Higher tenofovir exposure is associated with longitudinal declines in kidney function in women living with HIV. Aids. 2016;30(4):609–618.
- Turner D, Drak D, O’Connor CC, Templeton DJ, Gracey DM. Renal function change after switching tenofovir disoproxil fumarate for tenofovir alafenamide in the HIV-positive patients of a metropolitan sexual health service. AIDS Res Ther. 2019;16(1):40.
- Rieke A, Jessen H, Pauli R, Waizmann M, Heuchel T, Postel N., editors. Real-world effects of treatment with emtricitabine/tenofovir alafenamide versus emtricitabine/tenofovir disoproxil fumarate-based regimens in people living with HIV in a clinical cohort in Germany. HIV Glasgow; 2018 October 28–31; Glasgow, UK.
- Mothobi NZ, Masters J, Marriott DJ. Fanconi syndrome due to tenofovir disoproxil fumarate reversed by switching to tenofovir alafenamide fumarate in an HIV-infected patient. Ther Adv Infect Dis. 2018;5(5):91–95.
- Walti LN, Steinrucken J, Rauch A, Wandeler G. Tenofovir alafenamide in multimorbid HIV-infected patients with prior tenofovir-associated renal toxicity. Open Forum Infect Dis. 2018;5(11):ofy275.
- Gallagher A, Quan D, Gracey DM. Improvement in renal function and resolution of proteinuria in an HIV-infected patient switched from tenofovir disoproxil fumarate to tenofovir alafenamide. Intern Med J. 2017;47(7):826–827.
- Mikula JM, Manion MM, Maldarelli F, et al. Tenofovir alafenamide as part of a salvage regimen in a patient with multi-drug resistant HIV and tenofovir-DF-associated renal tubulopathy. Antivir Ther. 2016;21(6):553–558.
- Karris MY. Short communication: Resolution of tenofovir disoproxil fumarate induced fanconi syndrome with switch to tenofovir alafenamide fumarate in a HIV-1 and hepatitis B coinfected patient. AIDS Res Hum Retrovirus. 2017;33(7):718–722.
- Deriaz D, Guessous I, Vollenweider P, et al. Estimated 24-h urinary sodium and sodium-to-potassium ratio are predictors of kidney function decline in a population-based study. J Hypertens. 2019;37(9):1853–1860.
- Suarez-Lozano I, Fajardo JM, Garrido M, et al. Epidemiological trends of HIV infection in Spain: preventative plans have to be oriented to new target populations (Spanish VACH Cohort). Aids. 2002;16(18):2496–2499.
