?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose: The purpose of this review is to evaluate the effect of resistance training (RT) as a unique intervention on muscle strength, body composition, and immune-inflammatory markers in people living with HIV (PLHIV).
Methods: The searches were conducted in seven databases and included published randomized clinical trials that assessed the effect of RT vs. no exercise on muscle strength, body composition, and immune-inflammatory markers in PLHIV until June 2021. Random effects meta-analyses of mean differences (MD) and their 95% confidence intervals (CI) were performed, and the effect size was estimated by Hedges’ g test.
Results: Seven RCTs were included (n= 258 PLHIV) and the study duration lasted between six and 24 weeks. In comparison to no exercise, RT improved muscle strength in bench press (MD 10.69 kg, 95%IC 3.44 to 17.93, p= 0.004, g =2.42) and squat (MD 22.66 kg, 95%IC 7.82 to 37.50, p= 0.003, g = 3.8) exercises, lean body mass (MD 2.96 kg, 95%CI 0.98 to 4.94, p= 0.003, g = 1.99), fat body mass(MD −2.67 kg; 95%CI −4.95 to −0.39, p= 0.02, g=-0.99), body fat percentage (MD −3.66%, 95%CI −6.04 to −1.29, p= 0.003, g=-1.99) and CD4+ cells count(MD 100.15 cells/mm3, 95%CI 12.21 to 188.08, p = 0.03, g = 2.91) in PLHIV. There was no effect of RT on IL-6 (MD −1.18 pg/mL, 95%CI −3.71 to 1.35, p = 0.36, g = 0.001) and TNF-α (MD −4.76 pg/mL, 95%CI −10.81 to 1.29, p = 0.12, g=-1.3) concentrations in PLHIV. Conclusions: RT as a unique intervention improves muscle strength, body composition and CD4+ count cells in PLHIV.
Introduction
People living with HIV (PLHIV) have significantly increased life expectancy after the highly antiretroviral therapy (HAART) era and nowadays the infection is considered a chronic disease.Citation1 However, HIV infection and HAART use are related to a large number of immune-metabolic and morphologic alterations such as chronic inflammation, dyslipidemia, diabetes, cardiovascular diseases, lipodystrophy, and wasting muscle.Citation2–7 Furthermore, has been reported that PLHIV presents lower physical capacities (e.g. muscle strength and/or cardiorespiratory fitness) compared to the general population which contributes to poor aging and quality of life.Citation8 Thereby, interventions to reduce and/or prevent the side effects of infection and HAART use became necessary for PLHIV.
In this scenario, exercise training plays a key role in the chronic management of PLHIV. Specifically, resistance training (RT) is characterized by using a resistance apparatus such as gym equipment, free weights, and elastic tapes against the target muscle group contraction.Citation9 Furthermore, there is a consensus that RT programs enhance muscle mass, muscle strength, and improves immune-metabolic markers in the general population.Citation9
Although there was an increase in studies involving PLHIV and exercise training, the results obtained by RT programs as unique intervention remain inconsistent.Citation10–16 The previous meta-analysis evaluated the effects of RT in PLHIV however were included results obtained by studies with combined exercise training (aerobic and resistance training in the same session) which could be a confusion factor for outcomes.Citation17–21 Although these previous meta-analyses found benefits for PLHIV it is important to evaluate the effects of RT program as a unique intervention for this population. This systematic review and meta-analysis aimed to determine the effects of RT on muscle strength, body composition, and immune-inflammatory markers in PLHIV when compared with no exercise in randomized controlled trials (RCTs).
Methods
This systematic review and meta-analysis were conducted following the recommendations outlined in Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statementsCitation22 and registered in the PROSPERO database (CRD42020182343).
Eligibility criteria
Articles were eligible for inclusion if they were RCTs involving PLHIV (18-60 years old) undergoing HAART (for at least 2 years) in which RT was carried out as a unique exercise intervention, performed two times a week and for four weeks at least, compared with no exercise (control group) and where outcomes were: (1) muscle strength (assessed via one-repetition maximum) in the bench press and/or squat exercise, (2) body composition measured by lean body mass (LBM), fat body mass (FBM) and/or body fat percentage (BF%) (assessed via dual-energy X-ray absorptiometry, bioelectrical bioimpedance or skinfold thickness), lymphocyte TCD4+ cell counts (via flow cytometric), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) (via enzyme-linked immunosorbent assay or cytometric bead array). Studies were ineligible if involving anabolic steroids or nutritional supplementation, reviews, and systematic reviews, and those that investigated the RT acute effects.
Search strategy
Searches were performed in PubMed, Europe PMC, Web of Science, Scielo, SPORTDiscuss, PEDro and Cochrane databases without language restriction until June 2021. A Medical Subject Heading (MeSH) search was performed using the keywords ‘resistance exercise’ and ‘HIV’. Thus, the following search strategy was used to search articles: (‘Resistance training’ OR ‘Strength Training’ OR ‘Weight-Lifting Strengthening Program’ OR ‘Weight-Lifting Strengthening exercise’ OR ‘Weight-Bearing Strengthening Program’ OR ‘Weight-Bearing Strengthening exercise’) AND (‘HIV’ OR ‘people living with HIV’ OR ‘seropositive people’).
Studies selection
In the initial screening, two independent authors (HRZ and LTPL) performed the search and screened the titles, abstracts, or full texts from database records. The duplicates and the studies that did not meet the eligibility criteria were removed. In the case of disagreements between the authors a third author was included (AG).
Data extraction
Two researchers (VLS and WFS) independently extracted data using a customized spreadsheet. After the data extraction, there was a conference of the data, and a third researcher (ELM)was consulted in case of disagreement. The data extracted from the article were (1) number of initial participants involved in the study; (2) number of participants that finished the intervention; (3) training protocol, including week frequency, number of exercises, volume, and intensity; (4) outcomes per arm. Quantitative data from pre- and post-training intervention were extracted from text, tables, and figures of published articles as mean ± standard deviation (SD), and in instances where results were presented as the standard error of the mean (SEM), it was converted to SD.
Quality assessment
Two researchers (VLS and WFS) independently assessed the quality of the included studies using the risk of bias 2.Citation23 The tool consists of five distinct domains and leads to judgments of “low risk of bias”, “high risk of bias” and/or “some concerns”.
Rating quality of evidence
The Grading of Recommendations Assessment Development and Evaluation (GRADE), which evaluated the risk of bias, consistency; directness; precision; and publication bias, was used to determine the quality of evidence per outcome.Citation24 The quality of evidence was graded from ‘high quality’ to ‘very low quality’ according to the criteria.
Statistical analysis
Meta-analyses were performed using Review Manager 5.3 for Windows. The delta (post-pre) values were calculated for all outcomes and the SD change was calculated according to the equation where the imputed correlation coefficient is 0.50.Citation25 Effects on outcomes are presented as mean differences (MD) and their 95% confidence intervals (CI). Study heterogeneity was assessed using Q and I2 statistics. The heterogeneity thresholds for I2 were 25% (low), I2 = 50% (moderate), and I2 = 75% (high) .Citation26 Furthermore, the mean effect size was estimated by Hedges’ g test. Due to anticipated study heterogeneity, random-effects models were used. Publication bias was examined visually via funnel plots and statistically (p < 0.10) using Egger’s.
Results
Search results
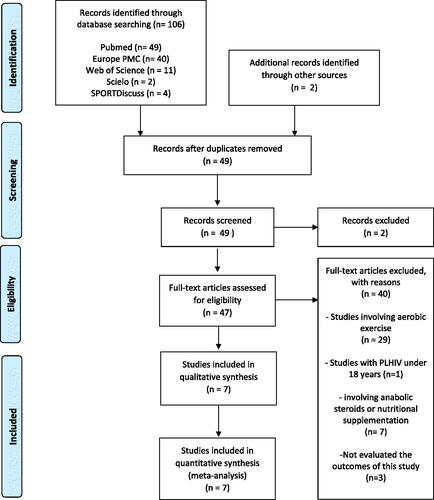
The initial database search found 106 articles and 2 articles were included from other sources. A total of 57 articles were removed due to duplicate records and two articles were excluded from records screened. Forty-seven studies were assessed for eligibility which 40 were excluded based on (a) studies involving aerobic exercise in the training protocol (n= 29); (b) intervention with PLHIV under 18 years (n= 1); (c) study involving anabolic steroid or nutritional supplementation (n= 7); (e) not evaluated the outcomes target of the present study (n= 3). Finally, seven RCTs (n= 258) were included in the meta-analysis.Citation10–16 The PRISMA flowchart is demonstrated in .
Descriptive characteristics of the studies
Included RCTs are summarized in . Four studies were conducted in Brazil,Citation10,Citation12–14 one in the United States of America,Citation15 one in IranCitation11 and one in Zimbabwe.Citation16 Two RCTs involved the same sampleCitation12,Citation14 and therefore a total of 261 PLHIV were recruited to RT programs and 258 (95 men and 163women) completed follow-up, representing a loss of 1.1%. Five studies reported the mean age of participants (41.04 ± 0.82 years).Citation11–15 Four studies reported the time of infection diagnosis (8.5 ± 2.2 years) .Citation11–14 All studies included participants under HAART use and presented undetectable viral load.
Table 1. Summary of included studies.
Four studies evaluated the muscle strength using the one-repetition maximum test in bench press exerciseCitation10,Citation12,Citation15,Citation16 while three studies also used the squat exercise.Citation10,Citation12,Citation16 Six RCTs estimate the body composition which five RCTs used skinfolds thicknessCitation10,Citation13–16 and one study used bioelectrical bioimpedance.Citation11 Three studies analyzed the CD4+ cells count, and all used the cytometric flow method.Citation10–12 Finally, two RCTs evaluated the concentrations of IL-6 and TNF-α using cytometric bead array methodCitation12,Citation15
Descriptive characteristics of interventions
Although all RT protocols were performed three times a week, the time of intervention varied between six weeks,Citation15 eight weeks,Citation11 12 weeksCitation12–14,Citation16 and 24 weeks.Citation10 One study performed circuit training with elastic tape and body weightCitation11 while six studies used machines.Citation10,Citation12–16 The intervention protocols are described in .
Quality assessment
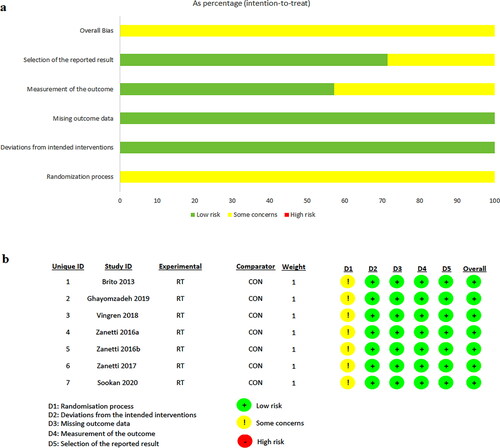
The risk of bias graph is demonstrated in . All studies presented “some concerns” in the randomization process but overall, the studies presented a low risk of bias.
Quality of evidence
The GRADE demonstrated a moderate quality of evidence for improvements of muscle strength in the bench press and squat exercise improvements on LBM, FBM and BF%. T CD4+ count cells, IL-6 and TNF-α ().
Table 2. Summary of grading of recommendations assessment development and evaluation (GRADE).
Meta-analysis results
Muscle strength
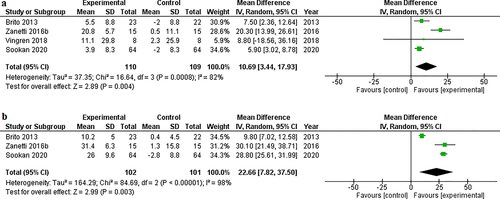
RT program improved muscle strength in bench press (MD 10.69 kg, 95%IC 3.44 to 17.93, p= 0.004, I2= 82%, g= 2.42) () and squat (MD 22.66 kg, 95%IC 7.82 to 37.50, p= 0.003, I2= 98%, g = 3.8) ().
Body composition
RT program increased LBM (MD 2.96 kg, 95%CI 0.98 to 4.94, p= 0.003, I2= 0%, g = 1.99) (), and reduced FBM (MD −2.67 kg, 95%CI −4.95 to 0.39, p= 0.02, I2= 25%, g= −0.99) () and BF% (MD −3.66%, 95%CI −6.04 to −1.29, p= 0.003, I2= 28%, g= −1.99] ().
Immune-inflammatory markers
RT program improved CD4+ cells count (MD 100.15 cells/mm3, 95%CI 12.21 to 188.08, p= 0.03, I2= 0%, g = 2.91) (). However, there were no difference for IL-6 (MD −1.18 pg/dL, 95%IC −3.71 to 1.35, p= 0.36, I2= 93%, g= −0.001) () and TNF-α (MD −4.76 pg/dL, 95%IC −10.81 to 1.29, p= 0.12, I2= 68%, g= −1.3) ().
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis that evaluated the effect of RT as a unique intervention for PLHIV. The main results of the present study suggest a beneficial effect of the RT intervention on muscle strength, body composition, and CD4+cell count among PLHIV. These results should be interpreted with caution once all studies included clinical stable volunteers with undetectable viral load and HAART use. In addition, the results obtained by this systematic review and meta-analysis were evidenced by RCTs with moderate quality of evidence.
The meta-analysis found beneficial effects of RT on overall and by subgroup muscle strength increase in PLHIV, despite the significant heterogeneity observed among studies. Muscle strength is an important marker associated with the ability to perform daily activities and a mortality predictor.Citation27,Citation28 It has been reported that PLHIV has a lower muscle strength compared to the non-infected population with up to 57% lower strength in lower limbs.Citation29 In addition, this meta-analysis revealed that RT is an effective intervention for enhancing the body composition in PLHIV and the findings reflect the large benefits such as the lower risk of mortality and higher cardiorespiratory fitness.Citation30 The HIV-related wasting muscle is a well-established phenomenon and is determined by HIV infection, HAART use, and high levels of physical inactivity.Citation31,Citation32 Previous studies reported that these factors are associated with chronic inflammation and muscle structure abnormalities which are responsible for wasting muscle in PLHIV.Citation15,Citation29 Metabolic diseases such as insulin resistance and diabetes mellitus 2 are associated with lower LBM therefore RT could be proposed as a key role for prevention or mitigation of these conditions.Citation33–35
A promising clinical finding of this meta-analysis was the increase in CD4+ cell count after RT program in PLHIV. TCD4+ lymphocytes play a central role in regulating immune functions by activating B cells in the productions of antibodies and improve cellular immune response to antigens. In this scenario, HIV preferentially infects activated CD4+cells resulting in the loss of HIV-specific immune response, recall antibody response and, non-specific immune response in the AIDS stage.Citation36 It has been widely reported the potential of exercise to modulate immune responses in the human being environment. For example, resistance exercise-induced effects on CD4+ T activation were observed following an acute RT section and results in a greater susceptibility to HIV‐1 infection in vitro.Citation36 Of course, these results should be observed with caution, as the participants were on HAART use and with a controlled viral load. Furthermore, the studies used for this analysis were classified as of moderate quality, which limits the accuracy of these results.
Our analyses revealed no statistically significant effects of the RT on IL-6 and TNF- levels in PLHIV. The meta-analysis by Ibeneme et al found similar results when including articles that used combined or isolated exercise in PLHIV. It is worth noting that both meta-analyses were conducted with a small number of articles and, thus, conclusions should be taken with caution in this direction.Citation21
The present study presents some limitations. First, it is important to focus that RT as a unique intervention for PLHIV is scarce and, therefore, the included studies showed diversified protocols using different times of intervention, volume and intensities which could interfere in the main outcomes. Second, only two studies evaluated inflammatory biomarkers (IL-6 and TNF-α) which could underestimate the anti-inflammatory effect of exercise. Third, most variables evaluated in the present study showed high heterogeneity (I2> 75%) due to the influence of clinical and methodological variability however the problem was solved by the funnel plots.
The present systematic review and meta-analysis clarify that RT should be included in the healthcare programs for PLHIV. Furthermore, the study evidences the scarcity of RCTs involving RT and their variables (e.g., volume, intensity, frequency, periodization, time of intervention) which could be investigated for new studies. Another important point that needs attention is the additional effect of exercise on HAART side effects such as dyslipidemia, diabetes, mitochondrial dysfunction, cardiovascular diseases and mortality.
Conclusion
In conclusion, based on the findings of this meta-analysis, RT may be useful in the management of HIV/HAART-related morphological and immune disorders due to improvement in body composition, muscle strength and CD4+ cell count.
References
- Mahungu TW, Rodger AJ, Johnson MA. HIV as a chronic disease. Clin Med (Lond). 2009;9(2):125–128.
- Esser S, Helbig D, Hillen U, Dissemond J, Grabbe S. Side effects of HIV therapy. J Dtsch Dermatol Ges. 2007;5(9):745–754.
- Zanetti HR, Mendes EL, Palandri Chagas AC, et al. Triad of the ischemic cardiovascular disease in people living with HIV? Association between risk factors, HIV infection, and use of antiretroviral therapy. Curr Atheroscler Rep. 2018;20(6):30.
- Beraldo RA, Santos APD, Guimaraes MP, et al. Body fat redistribution and changes in lipid and glucose metabolism in people living with HIV/AIDS. Rev Bras Epidemiol. 2017;20(3):526–536.
- Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165(10):1179–1184.
- Calza L, Masetti G, Piergentili B, et al. Prevalence of diabetes mellitus, hyperinsulinaemia and metabolic syndrome among 755 adult patients with HIV-1 infection. Int J STD AIDS. 2011;22(1):43–45.
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155.
- Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses. 2006;22(11):1113–1121.
- American College of Sports M. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009;41(3):687–708.
- Brito CJ, Mendes EL, Ferreira AP, De Paula SO, Nóbrega OT, Córdova C. Impacto do treinamento resistido na força e hipertrofia muscular em HIV-soropositivos. Motriz: Rev Educ Fis. 2013;19(2):313–324.
- Ghayomzadeh M, SeyedAlinaghi S, Shamsi MM, et al. Effect of 8 weeks of hospital-based resistance training program on TCD4+ cell count and anthropometric characteristic of patients with HIV in Tehran, Iran: A randomized controlled trial. J Strength Cond Res. 2019;33(4):1146–1155.
- Zanetti HR, Cruz L. G d, Lourenço CLM, Neves F. d F, Silva-Vergara ML, Mendes EL. Non-linear resistance training reduces inflammatory biomarkers in persons living with HIV: A randomized controlled trial. Eur J Sport Sci. 2016;16(8):1232–1239.
- Zanetti HR, Lucas G, Cruz d, et al. Does nonlinear resistance training reduce metabolic syndrome in people living with HIV? A randomized clinical trial. J Sports Med Phys Fitness. 2017;57(5):678–684.
- Zanetti HR, da Cruz LG, Lourenço CLM, et al. Nonlinear resistance training enhances the lipid profile and reduces inflammation marker in people living with HIV: A randomized clinical trial. J Phys Act Health. 2016;13(7):765–770.
- Vingren JL, Curtis JH, Levitt DE, et al. Adding resistance training to the standard of care for inpatient substance abuse treatment in men with human immunodeficiency virus improves skeletal muscle health without altering cytokine concentrations. J Strength Cond Res. 2018;32(1):76–82.
- Sookan T. Effects of a resistance training programme in people living with HIV in Zimbabwe. Sport Sciences for Health 2020;16:551–560.
- Poton R, Polito M, Farinatti P. Effects of resistance training in HIV-infected patients: A meta-analysis of randomised controlled trials. J Sports Sci. 2017;35(24):2380–2389.
- O'Brien K, Tynan AM, Nixon S, Glazier RH. Effects of progressive resistive exercise in adults living with HIV/AIDS: systematic review and meta-analysis of randomized trials. AIDS Care. 2008;20(6):631–653.
- O'Brien KK, Tynan AM, Nixon SA, Glazier RH. Effectiveness of Progressive Resistive Exercise (PRE) in the context of HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis. 2017;17(1):268.
- Pérez Chaparro CGA, Zech P, Schuch F, Wolfarth B, Rapp M, Heiβel A. Effects of aerobic and resistance exercise alone or combined on strength and hormone outcomes for people living with HIV. A meta-analysis. PLoS One. 2018;13(9):e0203384.
- Ibeneme SC, Irem FO, Iloanusi NI, et al. Impact of physical exercises on immune function, bone mineral density, and quality of life in people living with HIV/AIDS: a systematic review with meta-analysis. BMC Infect Dis. 2019;19(1):340.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
- Guyatt GH, Oxman AD, Vist GE, GRADE Working Group, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926.
- Higgins JPT, Altman DG, Gøtzsche PC, Cochrane Statistical Methods Group, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Volaklis KA, Halle M, Meisinger C. Muscular strength as a strong predictor of mortality: A narrative review. Eur J Intern Med. 2015;26(5):303–310.
- Macdonald H, Nettlefold L, Maan E, Côté H, Alimenti A. Muscle power in children, youth and young adults who acquired HIV perinatally. J Musculoskeletal & Neuronal Inter 2017;17(2):27.
- Gomes-Neto M, Rodriguez I, Ledo AP, Vieira JPB, Brites C. Muscle strength and aerobic capacity in HIV-infected patients: A systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2018;79(4):491–500.
- Tang AM, Jacobson DL, Spiegelman D, Knox TA, Wanke C. Increasing risk of 5% or greater unintentional weight loss in a cohort of HIV-infected patients, 1995 to 2003. J Acquir Immune Defic Syndr. 2005;40(1):70–76.
- Tran T, Guardigni V, Pencina KM, et al. Atypical skeletal muscle profiles in human immunodeficiency virus-infected asymptomatic middle-aged adults. Clin Infect Dis. 2018;66(12):1918–1927.
- Wasserman P, Segal-Maurer S, Rubin DS. High prevalence of low skeletal muscle mass associated with male gender in midlife and older HIV-infected persons despite CD4 cell reconstitution and viral suppression. J Int Assoc Provid AIDS Care. 2014;13(2):145–152.
- Hong S, Chang Y, Jung H-S, Yun KE, Shin H, Ryu S. Relative muscle mass and the risk of incident type 2 diabetes: A cohort study. PloS One. 2017;12(11):e0188650.
- Son JW, Lee SS, Kim SR, et al. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: findings from the KoGES. Diabetologia 2017;60(5):865–872.
- Yalamanchi SV, Stewart KJ, Ji N, et al. The relationship of fasting hyperglycemia to changes in fat and muscle mass after exercise training in type 2 diabetes. Diabetes Res Clin Pract. 2016;122:154–161.
- Holbrook AK, Peterson HD, Bianchi SA, et al. CD4+ T cell activation and associated susceptibility to HIV-1 infection in vitro increased following acute resistance exercise in human subjects. Physiol Rep. 2019;7(18):e14234.