Abstract
Objectives: Tenofovir DF (TDF) remains one of the preferred backbone agents for naïve HIV patients starting antiretroviral treatment (ART). The impact of TDF on renal function and metabolic parameters may vary by anchor agent. We investigated the impact of TDF in combination with 3 different integrase inhibitors on tubular and glomerular function, and metabolic parameters in ART-naïve patients.
Methods: Sixty patients with normal renal function were randomised (20 per arm) to TDF/emtricitabine (FTC) plus either raltegravir (RAL) (400 mg b.d.), dolutegravir (DTG) or elvitegravir/cobicistat (EVG/c) for 48 weeks.
Results: 57 patients completed the study. Significant increases in RBP/creatinine ratio at week 24 were seen in all arms [RAL +4.7 μg/mmol (CI 0.43 to 8.98, p = 0.032); DTG +4.96 μg/mmol (CI 0.77 to 9.15, p = 0.021); EVG/c +6.95 μg/mmol (CI 2.53 to 11.36, p = 0.002)], although this was not sustained to week 48 in the RAL arm. Similar changes across the arms were observed for urinary α1microglobulin (RAL +6.20 mg/L, p = 0.030; DTG +6.30 mg/L, p = 0.025; EVG/c +8.15 mg/L, p = 0.003). Urinary β2microglobulin significantly increased at week 24 with DTG and EVG/c but remained unchanged in the RAL arm. Glomerular filtration measured with CKD-EPI creatinine-cystatin C increased significantly in the RAL arm at week 24 through 48 but declined modestly in other two arms. Total and LDL cholesterol decreased in the RAL arm, but increased in the EVG/c arm, with no significant changes in the DTG arm. Weight increased significantly from baseline with DTG but not RAL or EVG/c.
Conclusion: INSTIs in combination with TDF/FTC impact differently on tubular microproteinuria, eGFR, metabolic markers and weight. Use of TDF/FTC with RAL had the least tubular effects and the most favorable metabolic profile.
Introduction
Life-long administration of antiretroviral therapy (ART) is hindered by treatment limiting toxicities and adverse effects.Citation1 Informed choice of individual components of the ART regimen can result in reduction of potential toxicity and ultimately improve patient’s care and quality of life.
Current national and international guidelinesCitation2–4 recommend as initial regimen the use of an integrase inhibitors (INSTI) plus 1 or 2 NRTIs.
The co-formulated nucleotide/nucleoside analogue combination Tenofovir Disoproxil Fumarate (TDF) and emtricitabine (FTC) is commonly used in first line and subsequent therapy regimens. Generic formulations are widely available. It is recommended in WHO guidelines and widely used in environments where limited safety monitoring is available.
Tenofovir is excreted from the kidney by glomerular filtration and via active secretion at the proximal tubules of the kidney. The renal effects of TDF range from no apparent impact through chronic subclinical tubular dysfunction to proximal renal tubular dysfunction (PRTD) or Fanconi-like syndrome, has been extensively studied and documented in the last decades.Citation5–13
An alternative formulation of tenofovir as an alafenamide salt (TAF) has lower impact than TDF on eGFR, urinary albumin and microproteins loss, bone density loss and bone turnover markers,Citation14,Citation15 but a less favorable effect on lipids and a higher cost than generic TDF.Citation16 In HIV negative subjects receiving TDF for hepatitis B or as part of HIV pre-exposure prophylaxis, weight loss is observed in the first year of therapy, an effect not seen with TAF.Citation17 TAF has also been implicated in weight gain.Citation18–20
The potential nephrotoxicity of TDF varies according to the accompanying drugs: studies with TDF used in combination with a pharmacoenhancer (ritonavir or cobicistat) show a higher incidence of renal injury.Citation21–23 Tubular dysfunction with TDF generally precedes the decline in glomerular filtration rate (GFR), suggesting that tubular markers are more sensitive than estimated GFR calculated by serum creatinine in screening for such nephrotoxicity.Citation9–13
The practical measurement and cut-off for clinical significance of isolated markers of tubular dysfunction, in the short and long term, remains unclear and there is a need for further investigation of the role of tubular markers such as Retinol Binding Protein (RBP), Beta-2 microglobulin (β2) and alpha-1 microglobulin (α1), that might help to identify early tubular cell toxicity and predict the clinical outcome in HIV-infected patients on treatment with drugs with nephrotoxic potential.
We studied the effect of TDF combined with either of three integrase inhibitors raltegravir (RAL), dolutegravir (DTG) or elvitegravir/cobicistat (EVG/c) on renal function in antiretroviral naive patients, focusing on low molecular weight tubule resorbed proteins, and metabolic and inflammatory markers.
Methods
Study design and participants
The study was a single-centre, open-label, randomised, phase IV, pilot, 48 weeks study at Chelsea and Westminster Hospital, London, UK.
Eligible subjects at screening were ART-naive HIV-1 infected patients, aged 18 years or above, with a viral load >1000 copies/mL, any CD4+ count, and with no known or established renal disease or abnormality (estimated glomerular filtration rate using MDRD formula >60 ml/min).
Subjects were excluded if they had baseline resistance to TDF, FTC or known baseline resistance to integrase inhibitors. Other exclusion criteria were HIV-2 infection, AIDS diagnosis (excluding patients with stable cutaneous Kaposi’s Sarcoma), diabetes, use of high protein training supplements (e.g. creatine), concomitant therapy with medications disallowed as per SPC for the study drugs (including nephrotoxic medicinal products). acute viral hepatitis, or pregnancy or breastfeeding.
All subjects signed a written informed consent prior to study participation. The study was conducted in compliance with the principles of the Declaration of Helsinki and approved by the research ethics committee, the UK regulatory authority and medicines and health care products regulatory agency (MRHA) and registered with the EudraCT trials database under number 2014-004578-40 and under ClinicalTrials.gov NCT number: NCT02351908.
An open-label randomisation at a 1:1:1 ratio was performed using a computer generated list that was blinded to all study site personnel. Open label drug was dispensed as either TDF/FTC (in Truvada® co-formulation) plus RAL 400 mg b.d. or plus DTG 50 mg o.d., or with EVG/cobicistat (in Stribild® co-formulation).
There were 6 study visits following screening: baseline, week 4, 12, 24, 36, and 48.
The primary endpoint was change in RBP/creatinine ratio with each regimen over 24 weeks. Secondary endpoints were: change in RBP/creatinine ratio over 12 and 48 weeks; change in eGFR (calculated with C-G, MDRD, and CKD-EPI with both creatinine and creatinine-cystatin-C); changes in plasma urate, β2- and α1-microglubulin urinary excretion, urinary albumin and protein/creatinine ratio, urinary cystatin-C/creatinine ratio, fractional phosphate excretion and rates of normoglycemic glycosuria for each arm throughout the study.
Other secondary endpoints were: safety and tolerability, antiretroviral efficacy throughout the study; change from baseline in CD4 + count and CD4+/CD8 + ratio; changes in total cholesterol, HDL and LDL; change from baseline in insulin, fasting glucose and homeostatic model assessment-insulin resistance (HOMA-IR) at week 24, change from baseline in the inflammatory markers hsCRP, d-dimer and interleukin-6 (IL-6).
Study assessments
Blood samples were collected at each study visit for HIV-1 RNA viral load, lymphocyte subsets, biochemistry and haematology markers [see supplementary material]. All samples were collected after fasting (8 hrs) except for the screening visit.
At each visit concomitant medication review was performed.
Kidney and metabolic function measurements
Glomerular filtration rate using creatinine and cystatin was calculated at all study visits. eGFR was expressed as ml/min/1.73m2 and calculated according to the Cockcroft-Gault (C-G) formula, Modification of Diet in Renal Disease (MDRD) formula and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula with creatinine and creatinine-cystatin-C. [see supplementary material]
Urine samples were collected at each visit for assessment of: dipstick macro analysis, proteins and albumin, creatinine, albumin, cystatin-C, glucose and urine spot phosphate. In addition the following renal markers were measured: RBP/creatinine ratio (using nephelometric assay, Siemens BNII nephelometer), α1 and β2-microglobulin. Additional determinations of renal function were made from urine samples and blood samples at each study visit: protein/Cr, albumin/Cr; cystatin-C/Cr and fractional phosphate excretion (FEPi) [see supplementary material].
Statistical analysis
This was a pilot study design using a novel primary and some secondary endpoints mainly used as descriptive safety endpoints in clinical trials. We took into consideration the results of the ASSERT studyCitation24 together with the descriptive analysis from studies of TDF/FTC + Integrase inhibitors StartMRK,Citation25 SPRING-2Citation26 and Stribild developmental studies.Citation27 Small sample studiesCitation28–30 have been previously shown to provide adequate statistical and predictive power when using urinary tubular markers in people living with HIV (PLWH): twenty subjects per arm were considered sufficient to detect potentially meaningful differences in renal safety.
Study data were summarised using intention to treat (ITT) analysis, including all randomised subjects who received at least one dose of study medication, and where the last observation was carried forward if data were unavailable at the time point. Between randomised groups comparisons of quantitative data were performed using one way ANOVA where data were parametric, or Kruskal-Wallis test where data where non parametric, while qualitative data was compared by study arms using Chi-squared test and, where appropriate, Yates’ correction was applied. In addition, longitudinal analysis was performed using MIXED procedure in SAS to fit values of renal function markers from all study time points as a dependent variable. Independent variables included the fixed effects of the intervention arms, study visit time points and intervention by study time point interaction. A covariance matrix was used to model the within patient errors. Further multivariable analyses were conducted, adjusting for other time varying co-variables assumed to have potential confounding and or residual effect on the outcome variable over time. All data analyses were performed in SAS V 9.4 and all p-values presented were two tailed.
The longitudinal change in RBP/creatinine ratio in each arm over 12, 24 and 48 weeks was determined using the MIXED procedure in SAS and the changes from baseline were estimated together with 95% CI and are presented graphically. The proportion of patients with viral load <40 copies/mL at weeks 4 and 12, 24, 36 and 48 was analysed using McNemars chi-squared test. Changes from baseline in T cell counts and laboratory parameters, including urinary markers and fractional phosphate loss, were analysed using the paired t-test where data were geometrically distributed or Wilcoxon signed rank test where data were hypergeometrical. Changes from baseline in inflammatory markers were assessed using time-weighted differences in average.
Results
Study population
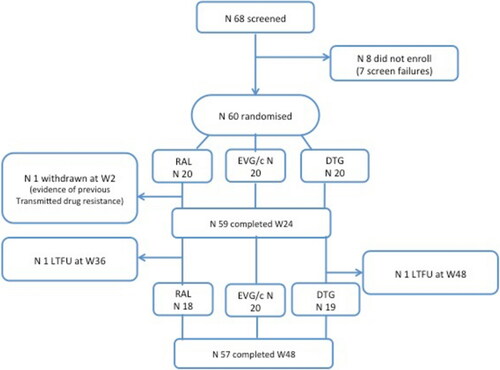
A total of 68 patients were screened, of whom 8 did not enrol (7 screen failures). Sixty patients were randomised, 20 in each study arm ().
Baseline demographics () were similar between treatment arms, although subjects in the DTG arm were slightly younger than in the other two arms.
Table 1 Baseline characteristics
Most subjects were male (95%), caucasian (76.7%) with a median BMI of 23 kg/m2 (IQR 22.1–26.4). There were no differences in HIV RNA, CD4+ count or any other study parameter between arms.
At week 2 one patient was withdrawn from the RAL arm due to newly available evidence of transmitted drug resistance on a historical viral genotype performed in a different site, two years prior to study entry.
Two further patients were lost to follow up, one at week 36 in the RAL arm, and one at week 48 in the DTG arm.
Virologic efficacy was similar across treatment arms through week 48.
At week 24 the proportion of patients with an undetectable HIV RNA (<40 copies/mL plasma) by snapshot analysis was: RAL: 19/20 (95%); DTG: 18/20 (90%); EVG/c: 16/20 (80%); and at week 48 was RAL: 17/20 (85%); DTG: 17/20 (85%); EVG/c: 17/20 (85%).
A similar rise in CD4+ cell count and percentage was seen in all treatment arms by week 48. Median changes in CD4+ count over 48 weeks were: +164 cells/mcl with RAL, +141cells/mcl with DTG and +116 cells/mcl with EVG/c; changes in CD4+/CD8+ ratio were similar across all treatment arms: median values RAL +0.4, DTG +0.2, EVG/c +0.4;
Renal markers
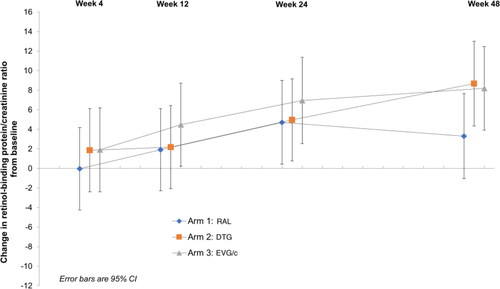
At week 24 there were significant increases from baseline in RBP/creatinine ratio in all three study arms: RAL +4.7 μg/mmol (CI 0.43 to 8.98, p = 0.032); DTG +4.96 μg/mmol (CI 0.77 to 9.15, p = 0.021); EVG/c +6.95 μg/mmol (CI 2.53 to 11.36, p = 0.002).
These changes were sustained at week 48 in the DTG and EVG/c arm, but not in the RAL arm: RAL +3.3 μg/mmol (CI −1.05 to 7.65, p = 0.138); DTG + 8.67 μg/mmol (CI 4.35 to 13, p < 0.001); EVG/c +8.19 μg/mmol (CI 3.92 to 12.45, p < 0.001) ().
Similarly there was a significant increase in urinary α1-microglobulin in all three treatment arms at week 24 compared with baseline (RAL +6.20 mg/L, p = 0.030; DTG +6.30 mg/L, p = 0.025; EVG/c +8.15 mg/L, p = 0.003); this increase was maintained with DTG and EVG/c at week 48, whereas the effect was not sustained for the RAL arm +2.84 mg/L (p = 0.327), DTG +6.12 mg/L (p = 0.031), EVG/c +8.55 mg/L (p = 0.002).
Urinary β2-microglobulin significantly increased at week 24 in the DTG (+434.4 ug/L p = 0.035) and EVG/c arms (+894.1 ug/L, p < 0.001), whilst remained unchanged in the RAL arm (+31.03 ug/L, p = 0.875). At week 48 values in all arms were similar to baseline (RAL: −46.56ug/L, p = 0.832; DTG: +26.18 ug/L, p = 0.897; EVG/c: +77.12 ug/L, p = 0.715). Changes in urinary tubular markers are summarized in .
Table 2 Urinary changes in tubular markers from baseline at W12, W24 and W48
Albumin/creatinine ratio saw only minimal changes in all three arms for the duration of the study, with the exception of the DTG arm at week 48 (+ 1.10 mg/mmol, CI 0.23 to 1.98, p = 0.014).
A significant increase in urine protein/creatinine ratio (UPCR) was observed only in the EVG/c arm at week 4 (+3.9 mg/mmol, CI 0.27 to 7.54, p = 0.03) and at week 48 (+5.98 mg/mmol, CI 2.46 to 9.45, p = 0.001); no significant changes were observed in the DTG arm (at week 48:+1.68 mg/mmol, CI −1.69 to 5.06, p = 0.392) and in the RAL arm (at week 48: −0.77 mg/mmol, CI −4.44 to 2.88, p = 0.677).
Similarly, over the weeks of the study a progressive increase in urinary cystatin/creatinine ratio was observed in the EVG/c arm (at week 48: +4.3ug/mmol, CI 1.15 to 7.46, p = 0.008); this effect was not observed in the other two arms.
There were no significant changes across treatment arms for urinary glucose and urinary phosphate. Fractional phosphate excretion increased at week 12 and 24 in both RAL (week 24: +2.93%, CI 0.26 to 5.61, p = 0.032) and DTG arms (+2.91%, CI 0.09 to 5.72, p < 0.001), but it was not sustained at week 48.
We analysed various estimates of glomerular function (C-G, MDRD, CKD-EPI with creatinine and CKD-EPI with creatinine-cystatin C).
Determination of eGFR with C-G, MDRD and CKD-EPI with creatinine showed a decline in all three arms, which was statistically significant throughout all weeks for the EVG/c and DTG arms, and only at week 36 and 48 for the RAL arm, albeit a significantly lesser decline compared to the other 2 arms ().
Figure 3 Estimates of glomerular filtration with C-G, MDRD, CKD-EPI (creatinine) and CKD – EPI (creatinine and cystatin) at week 12, 24 and 48.
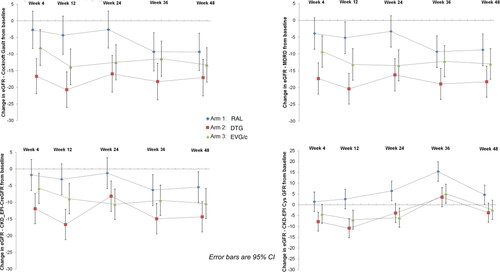
Glomerular filtration measured with CKD-EPI creatinine-cystatin C increased significantly in the RAL arm at week 24, 36 and 48 (respectively +6.39 ml/min, CI 1.88 to 10.90, p < 0.001;+15.36 ml/min, CI 10.85 to 19.87, p < 0.001;+4.58, CI 0.07 to 9.09, p = 0.047); cystatin-based CKD-EPI eGFR dropped in the other two arms throughout the study with differences in eGFR with CKD-EPI creatinine-cystatin C between RAL and the other 2 arms being statistically significant at week 12, 24, 36 and 48 ().
Metabolic and inflammatory markers
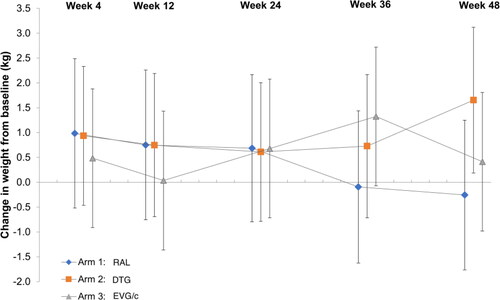
Over 48 weeks a significant weight gain was observed in the DTG arm only (+1.68Kg, SD 3.26, p = 0.049) ().
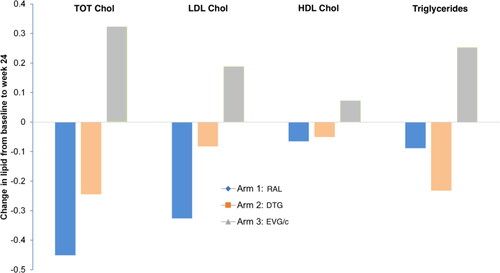
There were no significant changes in triglyceride levels in any arm over the study period. Total and LDL cholesterol decreased in the RAL arm but increased in the EVG/c arm. There were no significant changes in the DTG arm ().
HDL cholesterol values remained stable during the study, besides for week 48 with EVG/c, where it significantly increased by +0.92 mmol/L (CI 0.45 to 1.39, p < 0.001) from baseline.
The HOMA index was assessed at baseline and week 24: values in the RAL arm were significantly lower compared to baseline (−1.80 mmol/L, CI −3.25 to −0.36, p = 0.017), and were unchanged in the DTG and EVG/c arms (respectively: +0.11 mmol/L, CI −1.29 to 1.52, p = 0.874; −0.41, CI −1.84 to 1.01, p = 0.571). Similar results were observed for the fasting insulin resistance index.
Inflammatory markers remained mostly unchanged throughout the study. The only significant change from baseline was a reduction in plasma D-dimer concentration in RAL and EVG/c arms at week 24 (RAL: −116.8, CI −215.4 to −18.19, p = 0.021 and EVG/c: −113.95, CI −211.07 to −16.82, p = 0.022), which were both sustained at week 48 (RAL: −108.26, CI −208.41 to −8.10, p = 0.035; EVG/c: −118.95, CI −216.07 to −21.82, p = 0.017). There were no changes in CRP or IL-6 in any of the treatment arms throughout the study period.
Safety and tolerability
Seven ‘grade 3′ adverse events (AE) were reported. Three AE in the RAL arm were reported in 2 different patients: duodenal adenoma and worsening of epigastric pain in one patient (which subsequently had a serious adverse event described below), and acute gastroenteritis in the second one. In the DTG arm there were two AE in one patient (degenerative hearing disease diagnosis and worsening of tinnitus). In the EVG/c arm there was one report of cutaneous Kaposi sarcoma.
There were 6 Serious Adverse Events reported, occurring in 3 different patients (all in the RAL arm), none of which was attributed to study medication. These were the following: diarrhoea and abdominal pain due to acute gastroenteritis in a patient, which led to a brief hospitalization; vomiting, diarrhea and prolongation of hospitalization in a patient admitted for elective surgical resection of duodenal adenoma; acute hepatitis C infection in a third patient. There were no dropouts secondary to drug related adverse events.
Discussion
This study investigated the renal and metabolic profile of TDF/FTC given in combination with three different INSTIs in a randomised controlled trial, the first to study all 3 regimens directly and the first to use these novel endpoints. The study demonstrates similar viral efficacy but some differences in the renal and metabolic safety of the regimens.
It is well established that renal toxicity and declining GFR in PLWH can occur more frequently with some antiretroviral regimens: in the EuroSIDA Study each additional year of TDF, atazanavir, and ritonavir-boosted lopinavir was associated with 16%, 21%, and 8% higher incidence of CKD, respectively.Citation31 The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study reported similar findings.Citation32
Additional risk factors for declines in renal function include age, female sex, black race, smoking, dyslipidemia, diabetes, obesity, or Hepatitis C Virus (HCV) infection.Citation33,Citation34 ART associated renal toxicity can manifest with a range of clinical presentations, which vary from loss of low molecular weight proteins to albumin, and occurs in both the presence or absence of changes in glomerular filtration.
As the major site for filtered protein reabsorption is in the proximal tubule, when there is a disruption in the tubular reuptake process an increment in urinary excretion of such proteins is seen, generally proportionate to the degree of damage.Citation35–37 While changes in urinary low molecular weight protein loss have not been correlated with clear clinical endpoints, they are generally viewed as evidence of tubular dysfunction and therefore a potential harbinger of more serious renal toxicity. Urinary levels of retinol binding protein, beta-2 microglobulin and alpha-1 microglobulin are considered sensitive biomarkers to detect TDF induced tubular injury in children and adults.Citation28–30,Citation38
Retinol binding protein is a low molecular weight protein freely filtered by the glomerulus and almost completely reabsorbed in the proximal tubule, without being secreted.Citation39 Retinol binding protein non-reabsorption is one of the most sensitive markers of early renal tubular damage,Citation40–43 and although not routinely used in clinical practice, an increase in urinary retinol binding protein has been demonstrated in proximal renal tubule disease in PLWH.Citation38,Citation44–47 It has been shown that in Fanconi syndrome retinol binding protein levels are about 104 above the upper limit of normal (the laboratory upper limit of normal for RBP/Cr being 2.93 μg/mmol).Citation36
Similarly urinary beta-2 microglobulin and alpha-1 microglobulin are considered useful screening markers for tubulopathy, including tenofovir-induced.Citation48
The primary endpoint of the study was to evaluate changes in RBP/creatinine ratio at 24 weeks: all three arms had significant increases in RBP/creatinine at this timepoint, but these changes were not sustained in the RAL arm at 48 weeks.
We would anticipate that the effects on tubular markers are principally driven by TDF, with toxicity being related to cumulative exposure. Elevations of plasma TDF by cobicistat would lead to more rapid cumulative exposure to TDF than with RAL. While no plasma interaction between TDF and DTG exists, we could speculate that the effects of DTG on some renal transporters may lead to greater tubular TDF exposure than with RAL. Finally, there is evidence to suggest untreated HIV infection may have an impact on tubular functionCitation49 and we might postulate that the restoration of tubular health seen with RAL at 48 weeks is a result of viral suppression, with overall less cumulative TDF exposure than in the other two arms.
Similarly there was an increase in urinary α1-microglobulin in all three arms at week 24, which was sustained with DTG and EVG/c at week 48, but not with RAL, whilst urinary β2microglobulin significantly increased at week 24 in the DTG and EVG/c arms, but did not significantly change in the RAL arm. These findings are suggestive of a preferred renal tubular safety profile of TDF when given with RAL.
Furthermore we observed an increase in UPCR only in the EVG/c arm, at week 4 and at week 48. Albumin/creatinine ratio saw only minimal changes in all three arms for the duration of the study, with the exception of the DTG arm at week 48. This in contrast with findings from other studies like SPRING-2 were no changes in albumin/creatinine ratio were seen in DTG and RAL arms.Citation26
Proximal tubulopathy secondary to TDF typically manifests with normoglycemic glycosuria and fractional phosphate loss, leading to calcium and phosphate dysregulation, and consequently osteomalacia and defective bone mineralization.Citation10 We did not observe significant changes across treatment arms in urinary glucose, urinary phosphate and fractional phosphate excretion.
True GFR can be determined from the renal clearance of blood markers that are maintained at stable plasma concentrations, are inert and freely filtered by glomeruli, while not being reabsorbed, secreted or metabolised. As no ideal endogenous marker has been identified, serum creatinine (SCr) is used to indicate renal function, with the caveat that changes in filtrate may not be apparent until severe tubular toxicity has been achieved. Estimation of creatinine clearance by the Cockcroft-Gault equation (C-G) is calculated with weight plus creatinine whilst the Modified Diet in Renal Disease (MDRD) and CKD-EPI equations do not require weight assessments.
Several ARVs may affect creatinine-based eGFR through inhibition of creatinine active transport in the tubule, which doesn’t reflect a true loss of renal function.
Amongst integrase inhibitors, RAL has not been associated with a significant effect on renal transporters.Citation50 On the contrary DTG inhibits the basolateral active transporter OCT2Citation51 and the pharmacoenhancer cobicistat inhibits MATE-1,Citation52 both drugs contributing to a reduced excretion of creatinine from the tubule and affecting values of glomerular filtrate, complicating the surveillance for nephrotoxicity in the context of HIV treatment and relevant co-morbidities. Moreover cobicistat inhibits PGP,Citation53 a transporter involved in TDF metabolism, resulting in higher systemic TDF exposure with increased risk of TDF renal toxicity.
Determination of eGFR with C-G, MDRD and CKD-EPI with creatinine showed an expected decline in all three arms, which was statistically significant throughout all study weeks for the EVG/c and DTG arms, and only at week 36 and 48 for the RAL arm, albeit significantly less compared to the other 2 arms.
The changes in estimates of GFR based on C-G, MDRD and CKD-EPI with creatinine formulas observed for the DTG and EVG/c arms were expected and can be partially attributed to the inhibition of tubular transporters OCT-2 and MATE-1 by DTG and cobicistat respectively. No patients had grade 3 or 4 increases in creatinine and none in either group discontinued because of renal events.
Cystatin C is an alternative filtration marker for estimating GFR, which is less affected by muscle mass and diet than creatinine,Citation54 but also less subject to the effects of age, sex, and race. However GFR estimates based on equations that use cystatin C as the sole filtration marker are not more accurate than creatinine-based estimates, whilst there is evidence that the estimate of GFR based on a combined creatinine–cystatin C equation performs better than equations based on either of these markers alone.Citation55 A study conducted to evaluate the true impact of DTG on GFR, measured by iohexol plasma clearance, found no effect of DTG on GFR.Citation56
We observed a significant improvement in glomerular filtration measured with CKD-EPI creatinine-cystatin C in the RAL arm from week 24 to 48, whilst it declined in the other two arms throughout the study. In particular we were surprised to observe a small but statistically significant decline from baseline in the DTG arm.
These findings are again suggestive of a more neutral role of RAL given in combination with TDF on renal function, compared to DTG and EVG/c.
Our study also looked at differences in metabolic profiles of these three regimens.
Patients in the RAL arm had lower total and LDL cholesterol at all weeks, whilst the opposite effect was seen in the EVG/c arm. There were no significant changes in the DTG arm.
HDL cholesterol did not appear to be affected by study drugs, with the exception of EVG/c at week 48. No significant changes in triglyceride levels were observed.
TDF is known to have a small lipid lowering effect, with an 8% decline observed in total and LDL cholesterol over 2 weeks in healthy volunteers.Citation57
Our findings are in keeping with the evidence of RAL and DTG as lipid neutral agents,Citation58,Citation59 whereas EVG/c exerts a modestly unfavorable effect on lipid profile as previously described also in other studies.Citation60
Concerns have been raised regarding weight gain with INSTI drugs. Large cohort studies in naïve subjects such as the NA-Accord studyCitation61 have suggested this effect is real and may be greatest with DTG compared with other INSTIs. The most recent ADVANCE trialCitation19 also found that weight gain was more common among study participants on the DTG-based regimens compared to the EFV-based regimen, with the highest weight gain among those receiving DTG/FTC/TAF. In our small study, only DTG was associated with significant weight gain from baseline, albeit the changes were only modest.
HOMA index values and fasting insulin resistance index in the RAL arm were significantly lower compared to baseline, whilst were unchanged in the DTG and EVG/c arms suggesting that impact of insulin resistance on INSTI weight gain effect needs to be further explored.
Finally, INSTI drugs have been linked to reduction in levels of inflammatory and coagulation biomarkers, with less immune activation both in naïve and experienced patients.Citation25,Citation62,Citation63
For instance in the Spiral study, a switch from PI-based therapy to a RAL-containing regimen in patients with suppressed viremia, a decrease in inflammatory biomarkers, insulin resistance, and hypercoagulability was observed.Citation64
We did not observe any significant changes in inflammatory markers throughout the study, with the exception of a reduction in plasma D-dimer concentration in RAL and EVG/c arms at week 24, sustained at week 48. There were no changes in CRP or IL-6 in any of the treatment arms.
Our study has a number of limitations: the small number of patients and the fact that the study participants were almost all young white men, at low risk for TDF nephrotoxicity, may limit generalization of results.
Moreover, the relatively short duration of follow-up may not fully inform on the potential further impact on tubular and metabolic markers with the different agents over time. Tubular markers like urinary beta-2 microglobulin in PLWH started on treatment with TDF were found to increase significantly within the first 12 to 24 weeks in two studies;Citation28,Citation65 Nishijima et al.Citation65 suggested that an increase of urinary beta-2 microglobulin above 1700 ug/L within 180 days of starting treatment with TDF can predict renal dysfunction with drop in eGFR. Furthermore, in two randomized trials, urine levels of retinol binding protein and β2-microglobulin were 50% higher after 48 weeks among TDF versus non-TDF users.Citation24,Citation35
The meta-analysis by Hill et al.Citation21 suggested renal adverse events were seen more commonly over less than 3 years follow-up with boosted TDF than with non-boosted regimens. However, in the D:A:D studyCitation32 renal events with TDF accumulated over time, and eGFR decrease below 70 and 60 ml/min or TDF discontinuations continued throughout the follow-up duration (median 4.5 years). Thus, further differences between regimens may be discerned over longer follow-up, which is challenging to perform in a pilot study (as it would be unlikely to be able to maintain sufficient numbers for a longer follow-up duration), and best done in cohort analyses. Hence larger and longer studies would be needed to confirm our findings.
Conclusions
Choice of INSTI with TDF/FTC may impact the renal and metabolic safety profile of the regimen in treatment naive subjects. Raltegravir, which has no substantial impact on renal transporters that may impact TDF exposure or elimination, has a preferred safety profile with TDF/FTC relative to DTG or EVG/c.
Supplemental Material
Download MS Word (108.6 KB)Additional information
Funding
References
- Chawla A, Wang C, Patton C, Murray M, Punekar Y, de Ruiter A, et al. A review of long-term toxicity of antiretroviral treatment regimens and implications for an aging population. Infect Dis Ther. 2018;7(2):183–195.
- BHIVA guidelines for the treatment of HIV-1-positive adults with ART 2015. Interim update. https://www.bhiva.org/file/RVYKzFwyxpgiI/treatment-guidelines-2016-interim-update.pdf. Updated 2016. Accessed August 8, 2021.
- Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Updated March 17, 2019. Accessed August 8, 2021.
- Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection (WHO/CDS/HIV/18.5). https://apps.who.int/iris/bitstream/handle/10665/277395/WHO-CDS-HIV-18.51-eng.pdf?ua=1. Published 2018. Updated March 17, 2019.
- Viread summary of product characteristics. https://www.medicines.org.uk/emc/product/1615/smpc. Published 2021. Accessed August 8, 2021.
- Verhelst D, Monge M, Meynard J-L, Fouqueray B, Mougenot B, Girard P-M, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002;40(6):1331–1333.
- Karras A, Lafaurie M, Furco A, Bourgarit A, Droz D, Sereni D, et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis. 2003;36(8):1070–1073.
- Créput C, Gonzalez-Canali G, Hill G, Piketty C, Kazatchkine M, Nochy D. Renal lesions in HIV-1-positive patient treated with tenofovir. Aids. 2003;17(6):935–937.
- Horberg M, Tang B, Towner W, Piketty C, Kazatchkine M, Nochy D. Impact of tenofovir on renal function in HIV-infected, antiretroviral-naive patients. JAIDS J Acquir Immune Defic Syndr. 2010;53(1):62–69.
- Casado JL. Renal and bone toxicity with the use of tenofovir: understanding at the end. AIDS Rev. 2016;18(2):59–68.
- Ezinga M, Wetzels JF, Bosch ME, van der Ven AJ, Burger DM. Long-term treatment with tenofovir: prevalence of kidney tubular dysfunction and its association with tenofovir plasma concentration. Antivir Ther. 2014;19(8):765–771.
- Jotwani V, Scherzer R, Estrella MM, Jacobson LP, Witt MD, Palella FJ, et al. HIV infection, tenofovir, and urine α1-microglobulin: a cross-sectional analysis in the multicenter AIDS cohort study. Am J Kidney Dis. 2016;68(4):571–581.
- Nishijima T, Mutoh Y, Kawasaki Y, Tomonari K, Kikuchi Y, Gatanaga H, et al. Cumulative exposure of TDF is associated with kidney tubulopathy whether it is currently used or discontinued. Aids. 2018;32(2):179–188.
- Wohl D, Oka S, Clumeck N, Clarke A, Brinson C, Stephens J, et al. Brief report: a randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial HIV-1 treatment: week 96 results. JAIDS J Acquir Immune Defic Syndr. 2016;72(1):58–64.
- Gupta SK, Post FA, Arribas JR, Eron JJ, Wohl DA, Clarke AE, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate. AIDS. 2019;33(9):1455–1465.
- Wassner C, Bradley N, Lee Y. A review and clinical understanding of tenofovir: tenofovir disoproxil fumarate versus tenofovir alafenamide. J Int Assoc Provid AIDS Care. 2020;19:2325958220919231.
- Glidden DV, Mulligan K, McMahan V, Anderson PL, Guanira J, Chariyalertsak S, et al. Metabolic effects of preexposure prophylaxis with coformulated tenofovir disoproxil fumarate and emtricitabine. Clin Infect Dis. 2018;67(3):411–419.
- Milinkovic A, Berger F, Arenas-Pinto A, Mauss S. Reversible effect on lipids by switching from tenofovir disoproxil fumarate to tenofovir alafenamide and back. AIDS. 2019;33(15):2387–2391.
- Venter WDF, Sokhela S, Simmons B, Moorhouse M, Fairlie L, Mashabane N, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7(10):e666–e676. Oct
- Sax PE, Erlandson KM, Lake JE, Mccomsey GA, Orkin C, Esser S, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71(6):1379–1389.
- Hill A, Hughes SL, Gotham D, Pozniak AL. Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety? J Virus Eradic. 2018;4(2):72–79.
- Kalayjian RC, Lau B, Mechekano RN, Crane HM, Rodriguez B, Salata RA, et al. Risk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine care. AIDS. 2012;26(15):1907–1915.
- Goicoechea M, Liu S, Best B, Sun S, Jain S, Kemper C, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy . J Infect Dis. 2008;197(1):102–108.
- Moyle GJ, Stellbrink H-J, Compston J, Orkin C, Arribas JR, Domingo P, et al. 96-week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz, in antiretroviral-naive, HIV-1-infected adults: ASSERT study. Antivir Ther. 2013;18(7):905–913.
- Rockstroh JK, Lennox JL, DeJesus E, Saag MS, Lazzarin A, Wan H, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1–infected patients: 156-week results From STARTMRK. Clin Infect Dis. 2011;53(8):807–816.
- Raffi F, Rachlis A, Stellbrink H-J, Hardy WD, Torti C, Orkin C, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–743. Mar
- Zolopa A, Sax PE, DeJesus E, Mills A, Cohen C, Wohl D, et al. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection. JAIDS J Acquir Immune Defic Syndr. 2013;63(1):96–100.
- Kinai E, Hanabusa H. Renal tubular toxicity associated with tenofovir assessed using urine-beta 2 microglobulin, percentage of tubular reabsorption of phosphate and alkaline phosphatase levels. Aids. 2005;19(17):2031–2033.
- Papaleo A, Warszawski J, Salomon R, Jullien V, Veber F, Dechaux M, et al. Increased beta-2 microglobulinuria in human immunodeficiency virus-1-infected children and adolescents treated with tenofovir. Pediatr Infect Dis J. 2007;26(10):949–951.
- Gatanaga H, Tachikawa N, Kikuchi Y, Teruya K, Genka I, Honda M, et al. Urinary β2-microglobulin as a possible sensitive marker for renal injury caused by tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2006;22(8):744–748.
- Mocroft A, Kirk O, Reiss P, de Wit S, Sedlacek D, Beniowski M, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24(11):1667–1678.
- Ryom L, Mocroft A, Kirk O, Worm SW, Kamara DA, Reiss P, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207(9):1359–1369.
- Rasch MG, Engsig FN, Feldt-Rasmussen B, Kirk O, Kronborg G, Pedersen C, et al. Renal function and incidence of chronic kidney disease in HIV patients: a Danish cohort study. Scand J Infect Dis. 2012;44(9):689–696.
- Mocroft A, Lundgren JD, Ross M, Law M, Reiss P, Kirk O, et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med. 2015;12(3):e1001809.
- Vrouenraets SME, Fux CA, Wit FWNM, Garcia EF, Furrer H, Brinkman K, et al. Persistent decline in estimated but not measured glomerular filtration rate on tenofovir may reflect tubular rather than glomerular toxicity. Aids. 2011;25(17):2149–2155.
- Burling KA, Cutillas PR, Church D, Lapsley M, Norden AGW. Analysis of molecular forms of urine retinol-binding protein in Fanconi syndrome and design of an accurate immunoassay. Clin Chim Acta. 2012;413(3-4):483–489.
- Tramonti G, Kanwar YS. Review and discussion of tubular biomarkers in the diagnosis and management of diabetic nephropathy. Endocrine 2013;43(3):494–503.
- del Palacio M, Romero S, Casado JL. Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev. 2012;14(3):179–187.
- Zeni L, Norden AGW, Cancarini G, Unwin RJ. A more tubulocentric view of diabetic kidney disease. J Nephrol. 2017;30(6):701–717.
- Bernard AM, Vyskocil AA, Mahieu P, Lauwerys RR. Assessment of urinary retinol-binding protein as an index of proximal tubular injury. Clin Chem. 1987;33(6):775–779.
- Post FA, Wyatt CM, Mocroft A. Biomarkers of impaired renal function. Curr Opin HIV Aids. 2010;5(6):524–530.
- Campbell LJ, Dew T, Salota R, Cheserem E, Hamzah L, Ibrahim F, et al. Total protein, albumin and low-molecular-weight protein excretion in HIV-positive patients. BMC Nephrol. 2012;13:85.
- Fiseha T, Gebreweld A. Urinary markers of tubular injury in HIV-infected patients. Biochem Res Int. 2016;2016:1501785.
- Hamzah L, Samarawickrama A, Campbell L, Pope M, Burling K, Walker-Bone K, et al. Effects of renal tubular dysfunction on bone in tenofovir-exposed HIV-positive patients. Aids. 2015;29(14):1785–1792.
- Szymańska BM, Marchewka Z, Knysz B, Piwowar AB. A panel of urinary biochemical markers for the noninvasive detection of kidney dysfunction in HIV-infected patients . Pol Arch Intern Med. 2019;129(7-8):490–498.
- Norden AGW, Lapsley M, Unwin RJ. Urine retinol-binding protein 4: a functional biomarker of the proximal renal tubule. Adv Clin Chem. 2014;63:85–122.
- Hall AM, Edwards SG, Lapsley M, Connolly JO, Chetty K, O’Farrell S, et al. Subclinical tubular injury in HIV-infected individuals on antiretroviral therapy: a cross-sectional analysis. Am J Kidney Dis. 2009;54(6):1034–1042.
- Takano M, Tanuma J, Tsukada K, Teruya K, Kikuchi Y, Nishijima T, et al. Urinary beta-2 microglobulin and alpha-1 microglobulin are useful screening markers for tenofovir-induced kidney tubulopathy in patients with HIV-1 infection: a diagnostic accuracy study. J Infect Chemother. 2013;19(5):850–857.
- Kabanda A, Vandercam B, Bernard A, Lauwerys R, van Ypersele de Strihou C. Low molecular weight proteinuria in human immunodeficiency virus-infected patients. Am J Kidney Dis. 1996;27(6):803–808.
- Rizk ML, Houle R, Chan GH, Hafey M, Rhee EG, Chu X. Raltegravir has a low propensity to cause clinical drug interactions through inhibition of major drug transporters: an in vitro evaluation. Antimicrob Agents Chemother. 2014;58(3):1294–1301.
- Lepist E-I, Zhang X, Hao J, Huang J, Kosaka A, Birkus G, et al. Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int. 2014;86(2):350–357.
- German P, Liu HC, Szwarcberg J, Hepner M, Andrews J, Kearney BP, et al. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. JAIDS J Acquir Immune Defic Syndr. 2012;61(1):32–40.
- Lepist E-I, Phan TK, Roy A, Tong L, MacLennan K, Murray B, et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56(10):5409–5413.
- Vinge E, Lindergård B, Nilsson–Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. 1999;59(8):587–592.
- Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29.
- Koteff J, Borland J, Chen S, Song I, Peppercorn A, Koshiba T, et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in healthy subjects. Br J Clin Pharmacol. 2013;75(4):990–996.
- Randell PA, Jackson AG, Zhong L, Yale K, Moyle GJ. The effect of tenofovir disoproxil fumarate on whole-body insulin sensitivity, lipids and adipokines in healthy volunteers. Antivir Ther. 2010;15(2):227–233.
- Patel DA, Snedecor SJ, Tang WY, Sudharshan L, Lim JW, Cuffe R, et al. 48-week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1–infected patients: a systematic review and network meta-analysis. PLoS One. 2014;9(9):e105653.
- Quercia R, Roberts J, Martin-Carpenter L, Zala C. Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks. Clin Drug Investig. 2015;35(3):211–219.
- Wohl DA, Cohen C, Gallant JE, Mills A, Sax PE, DeJesus E, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014;65(3):e118–e120.
- Bakal D, Coelho L, Luz P, Clark J, Veloso V, Lake J, et al. HIV-infected individuals using integrase inhibitors are at high risk of weight gain. Paper presented at: the 20th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV; 2018; New York.
- Hileman CO, Kinley B, Scharen-Guivel V, Melbourne K, Szwarcberg J, Robinson J, et al. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor–based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis. 2015;212(3):345–354.
- Eron JJ, Young B, Cooper DA, Youle M, DeJesus E, Andrade-Villanueva J, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. The Lancet. 2010;375(9712):396–407.
- Martínez E, D’Albuquerque PM, Llibre JM, Gutierrez F, Podzamczer D, Antela A, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS. 2012;26(18):2315–2326.
- Nishijima T, Kurosawa T, Tanaka N, Kawasaki Y, Kikuchi Y, Oka S, et al. Urinary β2 microglobulin can predict tenofovir disoproxil fumarate-related renal dysfunction in HIV-1-infected patients who initiate tenofovir disoproxil fumarate-containing antiretroviral therapy. AIDS. 2016;30(10):1563–1571.