Abstract
Background: GEMINI-1 and GEMINI-2 (ClinicalTrials.gov, NCT02831673 and NCT02831764, respectively) are double-blind, multicenter, phase III studies that demonstrated the non-inferiority of once-daily dolutegravir + lamivudine to dolutegravir + tenofovir disoproxil fumarate/emtricitabine in achieving HIV-1 RNA <50 copies/mL at 48, 96, and 144 weeks in treatment-naive adults with HIV-1 infection.
Objective: We present a post hoc analysis of the impact of treatment adherence on Week 48 virologic response.
Methods: Adherence was estimated using pill counts and categorized as ≥90% vs <90%. Unadjusted treatment differences with exact 95% CIs were derived for the proportion of participants with HIV-1 RNA <50 copies/mL within each adherence category, using Snapshot algorithm and last available on-treatment viral load through Week 48.
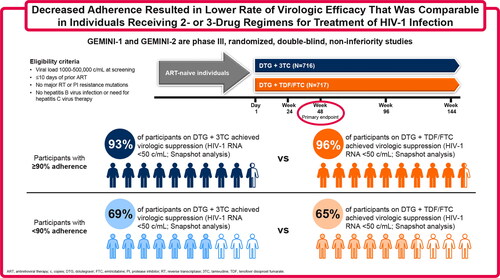
Results: In each treatment group, 5% of participants had <90% adherence (dolutegravir + lamivudine group, 35/716; dolutegravir + tenofovir disoproxil fumarate/emtricitabine group, 34/717). The proportion of participants with HIV-1 RNA <50 copies/mL (Snapshot) at Week 48 in the <90% adherence group was 69% in the dolutegravir + lamivudine group and 65% in the dolutegravir + tenofovir disoproxil fumarate/emtricitabine group (analysis by last on-treatment viral load: 91% and 85%, respectively). Corresponding proportions in the ≥90% adherence group were 93% and 96% (analysis by last on-treatment viral load: 97% and 99%, respectively).
Conclusions: Decreased adherence resulted in lower Week 48 virologic efficacy outcomes that were comparable between treatment groups. These results indicate that the robust antiviral activity and regimen forgiveness of dolutegravir + lamivudine is similar to dolutegravir-containing 3-drug regimens (see graphical abstract).
Graphical Abstract
Introduction
As individuals with HIV-1 require lifelong pharmacotherapy, 2-drug regimens (2DRs) can provide effective treatment options that reduce potential treatment-associated toxicity.Citation1 Week 48 primary analyses of the GEMINI-1 and GEMINI-2 trials demonstrated that the once-daily 2DR dolutegravir + lamivudine was non-inferior to the standard 3-drug regimen (3DR) dolutegravir + tenofovir disoproxil fumarate/emtricitabine in achieving HIV-1 RNA <50 copies/mL (US Food and Drug Administration [FDA] Snapshot algorithm) in treatment-naive adults (2DR, 91%; 3DR, 93%).Citation2 In the subsequent Week 96 and 144 analyses, sustained non-inferiority of dolutegravir + lamivudine vs dolutegravir + tenofovir disoproxil fumarate/emtricitabine was reported, demonstrating durable efficacy.Citation3,Citation4
High adherence to antiretroviral therapy (ART) is associated with increased virologic suppression rates, although the minimum adherence level necessary to ensure durable virologic suppression is unclear.Citation5,Citation6 A systematic review found that thresholds of >90% and >95% were consistently associated with virologic suppression but that findings were inconsistent at lower thresholds.Citation5 Another analysis found that the estimated adherence level necessary to achieve virologic suppression varied by regimen type and was lower than the conventionally reported >90% or >95%.Citation7 This concept of regimen “forgiveness,” or the ability to maintain virologic suppression with suboptimal adherence, is an important measure of regimen potency and durability.
This post hoc analysis evaluated the impact of treatment adherence on virologic suppression at Week 48 in the GEMINI studies.
Materials and methods
Participants and study design
GEMINI-1 (NCT02831673) and GEMINI-2 (NCT02831764) are identically designed, double-blind, phase III, non-inferiority trials conducted at 192 centers in 21 countries to evaluate the efficacy and safety of dolutegravir + lamivudine vs dolutegravir + tenofovir disoproxil fumarate/emtricitabine in treatment-naive adults with HIV-1.Citation2 Detailed methodology has been described.Citation2 Treatment-naive (≤10 days of prior ART) adults (aged ≥18 years) with HIV-1 and screening viral load between 1000 and 500,000 copies/mL were randomized 1:1 to receive either a once-daily 2DR of dolutegravir 50 mg + lamivudine 300 mg or a once-daily 3DR of dolutegravir 50 mg + tenofovir disoproxil fumarate 300 mg/emtricitabine 200 mg. Participants were stratified by HIV-1 RNA (≤100,000 or >100,000 copies/mL) and CD4+ cell count (≤200 or >200 cells/mm3).
GEMINI-1 and GEMINI-2 were performed in accordance with International Conference on Harmonization Good Clinical Practice, following the principles of the Declaration of Helsinki, with informed consent obtained before study procedure initiation.
Adherence and efficacy assessments
Plasma for HIV-1 RNA was quantified at baseline and Weeks 4, 8, 12, 16, 24, 36, and 48 using the Abbott RealTime HIV-1 assay. Association between adherence and proportion of participants with virologic suppression (HIV-1 RNA <50 copies/mL) was evaluated at Week 48 using (1) FDA Snapshot algorithmCitation8 and (2) last available on-treatment viral load by the end of the Week 48 analysis window (assessment of virologic response not accounting for discontinuations for non-virologic reasons).
Percent adherence included all treatment interruptions, irrespective of whether the interruption was due to adverse events or laboratory abnormalities, which was allowed according to the protocol. A sensitivity analysis was performed that excluded treatment interruptions due to adverse events or laboratory abnormalities. Percent adherence was calculated as 100 × (number of pills taken by Week 48/number of pills prescribed by Week 48). All participants received open-label dolutegravir and double-blind background agent (lamivudine or tenofovir disoproxil fumarate/emtricitabine). The number of pills taken was estimated using pill count data as the difference between the number of pills dispensed and the number of pills returned. Participants were stratified by ≥90% vs <90% adherence. For participants meeting confirmed virologic withdrawal (CVW) criteria, adherence was derived for the time window between the scheduled visit preceding the suspected virologic withdrawal (SVW) time point and the SVW visit itself. Criteria for CVW were defined as a second and consecutive HIV-1 RNA value meeting any of the following: decrease from baseline in plasma HIV-1 RNA <1 log10 copies/mL, unless HIV-1 RNA <200 copies/mL, by Week 12; confirmed plasma HIV-1 RNA ≥200 copies/mL at or after Week 24; or plasma HIV-1 RNA ≥200 copies/mL after previous confirmed suppression to HIV-1 RNA <200 copies/mL. Participants who met CVW criteria discontinued the study.Citation2
Statistical analysis
All participants receiving ≥1 dose of study medication were included in the intention-to-treat–exposed (ITT-E) population, which was used for the pre-planned primary efficacy analysis and the current post hoc analysis. Using both the FDA SnapshotCitation8 endpoint and last available on-treatment viral load through the Week 48 analysis window, differences in virologic suppression rates between treatments within each adherence category were reported with exact unadjusted 95% CIs using the Clopper-Pearson method.Citation9 Participants in the ITT-E population who met CVW criteria by Week 48 were also analyzed.
To evaluate extent of missing data in the adherence calculation, the number of dispensing records with complete adherence data was calculated; dispensing records covered a duration of 4 to 12 weeks, depending on study time point. Additionally, the mean percentage of days unaccounted for, derived as the sum of the length of dispensing periods for which no returned pill amount could be obtained for the randomized treatment divided by the total number of days from the start of the first dispensing period to the end of the last dispensing period, was reported.
Results
Study participants
Overall, 1433 participants were randomized in GEMINI-1 and GEMINI-2 and received ≥1 dose of study medication (2DR, N = 716; 3DR, N = 717). Demographics and baseline characteristics were well balanced between treatment groups.Citation2 Median age (range) was 32 (18–72) and 33 (18–70) years in the dolutegravir + lamivudine and dolutegravir + tenofovir disoproxil fumarate/emtricitabine groups, respectively, and women composed 16% and 14% of each group, respectively. Most participants in each treatment group were White (2DR, 68%; 3DR, 70%).
Baseline median (range) HIV-1 RNA was 4.43 (1.59–6.27) log10 copies/mL in participants receiving dolutegravir + lamivudine, and 20% of participants in this group had baseline HIV-1 RNA >100,000 copies/mL. Baseline values were similar in the dolutegravir + tenofovir disoproxil fumarate/emtricitabine group (median [range] HIV-1 RNA 4.46 [2.11–6.37] log10 copies/mL; 21% with HIV-1 RNA >100,000 copies/mL). Both treatment groups had similar baseline CD4+ cell counts (median [range], 427.0 [19–1399] and 438.0 [19–1497] cells/mm3 in the 2DR and 3DR groups, respectively) and similar and small proportions of participants with CD4+ cell count ≤200 cells/mm3 (9% and 8%, respectively).
Adherence
For the open-label dolutegravir treatment, complete pill count data were available for 92% of participants in each treatment group (2DR, 662/716; 3DR, 663/717). For the double-blind treatment (lamivudine or tenofovir disoproxil fumarate/emtricitabine), complete pill count data were available for 94% in each treatment group (2DR, 670/716; 3DR, 676/717). Mean percentage of days with no accountability information with ≥1 missing record was 17% in the 2DR group and 13% in the 3DR group.
In each treatment group, 5% of participants had <90% adherence (2DR, 35/716; 3DR, 34/717). Baseline HIV-1 RNA and CD4+ cell counts were comparable across adherence categories in both treatment groups ().
Table 1. Baseline characteristics by adherence category in GEMINI-1 and GEMINI-2 (ITT-E population).
Efficacy by adherence
The proportion of participants with HIV-1 RNA <50 copies/mL at Week 48 was lower in those with <90% adherence compared with those with ≥90% adherence, regardless of regimen (). In the Snapshot analysis, the unadjusted difference (95% CI) in proportion of participants achieving HIV-1 RNA <50 copies/mL at Week 48 between the dolutegravir + lamivudine and dolutegravir + tenofovir disoproxil fumarate/emtricitabine groups was −2.6% (−7.9%, 2.7%) in the ≥90% adherence group and 3.9% (−20.4%, 26.2%) in the <90% adherence group (). Based on a last on-treatment viral load analysis, the unadjusted difference (95% CI) between the dolutegravir + lamivudine and dolutegravir + tenofovir disoproxil fumarate/emtricitabine groups was −1.3% (−6.7%, 4.1%) in the ≥90% adherence group and 6.1% (−17.6%, 28.8%) in the <90% adherence group.
Figure 1. (A) Proportion of participants with HIV-1 RNA <50 copies/mL at Week 48 using the FDA Snapshot algorithm and last on-treatment viral load by adherence category, (B) unadjusted treatment differences (95% CI) between groups, and (C) Snapshot outcomes by adherence category. AE, adverse event; ART, antiretroviral therapy; 2DR, dolutegravir + lamivudine; 3DR, dolutegravir + tenofovir disoproxil fumarate/emtricitabine; FDA, US Food and Drug Administration.
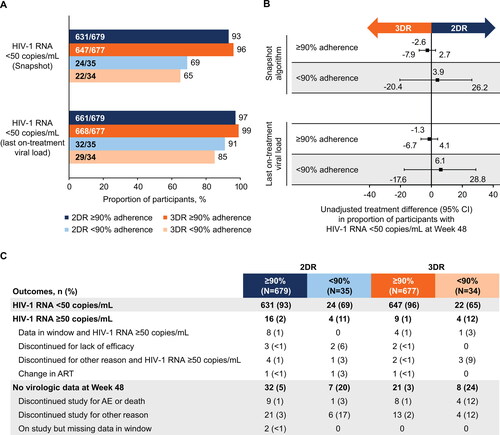
In both treatment groups, compared with participants with ≥90% adherence, higher proportions of participants with <90% adherence had HIV-1 RNA ≥50 copies/mL (11% vs 2% and 12% vs 1% for the 2DR and 3DR groups, respectively) or had no virologic data at Week 48 (20% vs 5% and 24% vs 3% for the 2DR and 3DR groups, respectively; ). Notably, of participants with no virologic data at Week 48, the proportion who discontinued study for other reasons was higher in those with <90% adherence vs ≥90% adherence in the 2DR (17% vs 3%) and 3DR groups (12% vs 2%).
A sensitivity analysis showed that excluding treatment interruptions due to adverse events or laboratory abnormalities in the adherence definition did not substantially affect the proportion of participants with HIV-1 RNA <50 copies/mL at Week 48 (Snapshot analysis) stratified by adherence level (2DR, 64% [18/28] and 3DR, 63% [17/27] with <90% adherence; 2DR, 93% [637/686] and 3DR, 95% [652/684] with ≥90% adherence).
CVW in adherence groups
Ten participants (2DR, 6; 3DR, 4) met CVW criteria,Citation2 all with ≥90% adherence between the visit preceding the SVW time point and SVW visit. None had treatment-emergent integrase strand transfer inhibitor or nucleoside reverse transcriptase inhibitor resistance mutations.
Discussion
In this study, adherence level had a similar impact on efficacy as assessed by virologic suppression for participants in both the dolutegravir + lamivudine and dolutegravir + tenofovir disoproxil fumarate/emtricitabine groups. Overall, response rates were high in participants with ≥90% adherence for both regimens based on Snapshot and last on-treatment viral load analyses. Response rates were lower using Snapshot in participants with <90% adherence but high when last on-treatment viral load was assessed; response rates were comparable between treatment groups in both analyses. The high rates of response across adherence categories by last on-treatment viral load analyses are supported by a real-world database analysis that suggests a ≥80% adherence level as a threshold for achieving virologic suppression on modern ART.Citation7
Snapshot outcomes assessment by adherence category in both treatment groups suggests that the drivers for the differences in virologic outcomes between adherence groups are the higher proportions of participants with HIV-1 RNA ≥50 copies/mL but also missing virologic data in the <90% adherence group, which includes loss to follow-up or withdrawal of consent. Frequency of CVWs was low across both treatment groups, with no apparent link to adherence level. However, adherence was measured for the entire period between study visits, which could reflect a variable duration of up to 12 weeks before SVW (depending on study visit time point), but adherence within the 2 weeks immediately preceding SVW would be most relevant for understanding the impact of adherence. Of note, plasma HIV-1 RNA trajectories in terms of level of rebound and re-suppression before and after CVW support the link between temporary non-adherence and CVW.Citation10
One limitation of this analysis is the small number of participants with <90% adherence, restricting ability to compare results between groups. Additionally, there is no standard for measuring adherence, and analyzing pill count (i.e. the difference between pills available and pills returned) may reflect lost or discarded medication as well as consumed medication, potentially leading to inaccurate adherence categorization.Citation11 Moreover, it is not possible to determine exactly when doses were missed using pill count data and correlate timing with virologic outcomes (i.e. CVW). For example, in this analysis, adherence was measured during study visits (up to 12 weeks apart) but not immediately before SVW. Despite this limitation, recording pill count is an objective adherence measurement that avoids issues associated with subjective measurements (e.g. participant self-reported questionnaires) or methodologies involving invasive procedures (e.g. serum drug concentration analyses).Citation12 There are also limitations inherent to exploratory post hoc analyses such as this one. However, these limitations were present in both treatment groups, therefore reducing their potential to influence treatment outcomes.
In conclusion, in the GEMINI studies, rate of virologic response at Week 48 was comparable between treatment groups in treatment-naive adults with <90% or ≥90% adherence. These results provide additional information about the robustness of dolutegravir + lamivudine as a 2DR compared with the 3DR dolutegravir + tenofovir disoproxil fumarate/emtricitabine and suggest similar regimen forgiveness and reassurance in the case of sporadic missed doses. Clinicians should continue to promote and support optimal adherence (ie, “every dose, every day”) for optimal virologic suppression rather than rely on a regimen’s perceived forgiveness.Citation13 This is essential for minimizing the risk of true virologic failure with resistance development and, importantly, reducing the risk of HIV transmission in people with intermittent periods of viremia.
Disclosure of interest
Mounir Ait-Khaled, Choy Man, Jörg Sievers, Andrew Zolopa, Brian Wynne, and Jean van Wyk are employees of ViiV Healthcare and may own stock in GlaxoSmithKline. Debbie Hagins has served as a consultant for Gilead, Merck, and ViiV Healthcare; received grant/research support from Gilead, Janssen, Merck, and ViiV Healthcare; served as a scientific research study investigator for Gilead; and served on advisory boards or review panels for Gilead, Merck, and ViiV Healthcare. Richard Grove is an employee of and may own stock in GlaxoSmithKline. Juan Sierra Madero, Vicente Estrada, Roberto Gulminetti, and Hung-Chin Tsai have nothing to disclose.
Acknowledgments
The authors thank the study participants; their families and caregivers; investigators and site staff who participated in the study; and the ViiV Healthcare, GlaxoSmithKline, Pharmaceutical Product Development, and Parexel study team members. Editorial assistance was provided under the direction of the authors by Aarthi Gobinath, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and funded by ViiV Healthcare.
Data availability statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Additional information
Funding
References
- Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs. 2017;7(3):113–114. PMID: 28932710.
- Cahn P, Sierra MJ, Arribas JR, GEMINI Study Team, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143–155. PMID: 30420123.
- Cahn P, Sierra MJ, Arribas JR, et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr. 2020;83(3):310–318. PMID: 31834000.
- Cahn P, Sierra Madero J, Arribas JR, et al. Durable efficacy of dolutegravir (DTG) plus lamivudine (3TC) in antiretroviral treatment–naive adults with HIV-1 infection—3-year results from the GEMINI studies. Poster Presented at: HIV Drug Therapy; October 5-8, 2020; Glasgow.
- Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Ppa. 2019;13:475–490. PMID: 31040651.
- Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017;390(10107):2073–2082. PMID: 28867499.
- Byrd KK, Hou JG, Hazen R, for the Patient-Centered HIV Care Model Team, et al. Antiretroviral adherence level necessary for HIV viral suppression using real-world data. J Acquir Immune Defic Syndr. 2019;82(3):245–251. PMID: 31343455.
- US Food and Drug Administration, Center for Drug Evaluation and Research. Human immunodeficiency virus-1 infection: developing antiretroviral drugs for treatment. Guidance for industry. Department of Health and Human Services. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-immunodeficiency-virus-1-infection-developing-antiretroviral-drugs-treatment. Updated November 2015. Accessed March 28, 2021.
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26(4):404–413.
- Underwood M, Wang R, Benson P, et al. DTG + 3TC vs DTG + TDF/FTC (GEMINI-1 & -2): confirmed virologic withdrawals through Week 96. Poster Presented at: Conference on Retroviruses and Opportunistic Infections, March 8-11, 2020; Boston, MA.
- Lehmann A, Aslani P, Ahmed R, et al. Assessing medication adherence: options to consider. Int J Clin Pharm. 2014;36(1):55–69. PMID: 24166659.
- Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92(2):117–122. PMID: 31086837.
- Centers for Disease Control and Prevention. Partnership for health - medication adherence. US Department of Health and Human Services. https://www.cdc.gov/hiv/effective-interventions/treat/pfh-ma/index.html?Sort=Priority%3A%3Aasc&Intervention%20Name=Partnership%20for%20Health%20-%20Medication%20Adherence. Updated July 21, 2020. Accessed March 28, 2021.
