Abstract
Background/Aims: Switching from a three-drug regimen (3DR: boosted darunavir [bDRV] and two nucleoside reverse transcriptase inhibitors [NRTIs]) to a two-drug regimen (2DR: bDRV and dolutegravir [DTG]) demonstrated non-inferiority with regard to viral suppression in people living with HIV (PLWH) in the DUALIS study. This sub-analysis focuses on changes in metabolic and renal parameters when sparing the NRTI backbone.
Methods: DUALIS was a randomized, open-label, multicenter (27) phase 3-trial. Participants were virologically suppressed (HIV-RNA < 50 copies/mL) on 3DR for at least 24 weeks. Subjects were either switched to DTG 50 mg + bDRV 800 mg (with ritonavir 100 mg) (2DR) or continued their regimen consisting of two NRTIs in combination with ritonavir-bDRV (3DR) once daily. Data of metabolic and renal parameters at baseline and week 48 were compared.
Results: The LDL-fraction increased by + 13.3 (−3.0 to +31.3) mg/dL on 2DRs and was stable (−14.0 to +18.0 mg/dL) on 3DRs (p < 0.0010).
PLWH gained +2.0 (−0.2 to +4.0) kg and +0.2 (−1.9 to +2.1) kg in body weight on 2DRs and 3DRs, respectively 3 (p = 0.0006).
The MDRD eGFR decreased by −7,8 (−17.4 to −0.3) mL/min/1.73m2 and 0.4 (−8.8 to +5.7) mL/min/1.73m2 on 2DRs and 3DRs, respectively (p = 0.0002), while serum levels of cystatin C were stable in both arms (2DR: −0.1 to +0.1 mg/L; 3DR: 0.0 to +0.1 mg/L).
Conclusions: While being non-inferior in terms of viral suppression, sparing the NRTI backbone showed a non-favorable profile in metabolic or renal parameters over 48 weeks.
Introduction
Today, people living with HIV (PLWH) should receive antiretroviral therapy (ART) as early as possible. ART results in lower rates of acquired immune deficiency syndrome (AIDS) and an increased quality of life.Citation1–3 The use of nucleoside reverse transcriptase inhibitors (NRTIs) within ART is recommended but can be associated with adverse side effects. Renal toxicity, cardiovascular disease, and bone-health are of particular concern,Citation4 potentially resulting in a switch of ART. Thus, it is worth evaluating NRTI-sparing ART regimens.
Within the DUALIS study, virologically suppressed PLWH were switched to a two-drug regimen (2DR) consisting of dolutegravir (DTG) and boosted darunavir (bDRV) and were compared to PLWH continuing a three-drug regimen (3DR), consisting of bDRV in combination with two NRTIs. Non-inferiority of the 2DR arm in maintaining viral suppression, with no treatment-emergent resistance and similar rates of adverse events compared to the continued 3DR arm, was demonstrated in the DUALIS study.Citation5 Data from other studies confirmed these findings, even in acute HIV-infection.Citation6–8
Here, we present a post-hoc sub-analysis of the DUALIS study exploring metabolic and renal parameters.
Methods
Study design and participants
The DUALIS study was a randomized, open-label, multicenter (27 centers), noninferiority phase IIIb trial including 263 PLWH. Subjects were ≥18 years of age with documented HIV-1 infection, who had been virologically suppressed (plasma HIV RNA <50 copies/mL) for at least 6 months before screening (one blip of HIV RNA <200 copies/mL was accepted) and were on a stable, once-daily antiretroviral therapy consisting of bDRV in combination with two NRTIs (emtricitabine/tenofovir disoproxil [FTC/TDF], emtricitabine/tenofovir alafenamide [(FTC/TAF], or abacavir/lamivudine [ABC/3TC]). PLWH with documented major resistance to darunavir or to integrase strand transfer inhibitors, replicative hepatitis B surface antigen–positive hepatitis B infection, any evidence of active AIDS-defining disease (according to the Centers for Disease Control and Prevention stage CCitation9) estimated glomerular filtration rate (eGFR) <50 mL per minute, alanine aminotransferase above a 5-fold increase of the upper limit of normal, and any evidence of unstable liver disease or severe hepatic impairment were excluded.
For 1:1 randomization, a sequence generated with nQuery Advisor 7.0 (Statsols, Cork, Ireland) was used. Subjects were randomized and were either switched to DTG 50 mg + bDRV 800 mg (with ritonavir 100 mg) once daily (2DR) or continued their regimen consisting of two NRTIs in combination with ritonavir-bDRV (3DR) once daily for a total study duration of 48 weeks. A switch of the NRTI backbone (e.g., to tenofovir alafenamide/emtricitabine) was permissible at any time at the discretion of the investigator. Both treatment groups were open label.
Written informed consent was obtained from all participants before enrollment. This study was approved by the Institutional Review Board of the Technical University of Munich, Munich, Germany (approval number: 162/15) and by the German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM), Bonn, Germany (approval number 4 040 568). The study was registered with EudraCT (number 2015-000360-34) and was performed in accordance with the Declaration of Helsinki.
Statistical analyses
The study population and statistical methods of the DUALIS study have been previously described.Citation5 We performed a post-hoc analysis on changes in metabolic and renal parameters (total cholesterol, low-density lipoproteins [LDL], high-density lipoproteins [HDL], MDRD eGFR, CKD-EPI Creatinine eGFR, CKD-EPI Creatinine-Cystatin eGFR, and serum levels of cystatin C). For protocol-requested laboratory parameters, the use of appropriate, decentralized, local, accredited laboratories was accepted. Additionally, we collected each participant’s body weight and calculated their body mass index (BMI). For undressed weighting, patients came in the morning without having breakfast before. All data are presented as median (interquartile range [IQR]). The two-sided Wilcoxon signed-rank test for paired samples was used to evaluate the significance of changes from baseline. The two-sided Mann-Whitney U test was used to compare continuous outcomes between the treatment groups. The exploratory level of significance was 5%. No adjustments were made for multiple testing.
Results
Overall, 263 subjects could be included. 90% were male (237), particularly men having sex with men (69%). Median age was 48 (39–54) with a median time since HIV-diagnosis of 7.2 years (4.3–12.3). 27% of participants had a prior infection with hepatitis B (65/243), 5% were positive for hepatitis C (13/260). At baseline, mean total cholesterol was 200.7 (93.0 to 382.9) mg/dl with a mean LDL-fraction of 129.3 (30.0 to 284.0) mg/dl and a mean HDL-fraction of 48.9 (16.0 to 131.0) mg/dl. Mean body weight was 79.9 (46.0 to 155) kg with a corresponding body mass index (BMI) of 25.1 (17.9 to 55.6) mg/m2. Regarding renal function, mean serum creatinine was 0.9 (0.6 to 1.5) mg/dl and mean cystatin C was 0.9 (0.6 to 1.6) mg/l. Mean estimated glomerular filtration rates (eGFR) vary between 94.2 (53.4 to 134.) ml/min/173m2 and 88.7 (46.0 to 128.4) ml/min/173m2 depending on different calculations. There were no substantial differences between the groups (2DR versus 3DR) after randomization. Prior to inclusion, ART-regimes were DRV/r either plus FTC/TDF (77%, n = 185) or plus FTC/TAF (24%, n = 24) or ABC/3TC (12%, n = 32). Baseline characteristics are summarized in .
Table 1: Baseline characteristics.
After 48 weeks, an increase of total cholesterol by + 20.0 (+3.0 to +35.5) mg/dL on 2DR versus 0.0 (−18.0 to +15.5) mg/dL on 3DR was found (p < 0.0010). Thereafter, different lipoprotein fractions were analyzed. In the 2DR arm, the LDL-fraction increased by + 13.3 (−3.0 to +31.3) mg/dL and the HDL-fraction by +4.9 (−1.0 to +10.5) mg/dL. In comparison, in the 3DR arm, the LDL-fraction was stable at +0.0 (−14.0 to +18.0) mg/dL (p = 0.0003), whereas the HDL-fraction decreased significantly by −1.0 (−5.0 to +4.0) mg/dL (p < 0.0010). Regarding the different examination points (weeks 12, 24 and 48), a continuous increase in the lipoprotein fractions was observed in the 2DR arm until week 24 and reached a plateau thereafter. In comparison, in the 3DR arm, the lipoprotein fractions were almost unchanged at any point in time ().
Figure 1. Metabolic changes. 2DR, dolutegravir + darunavir/ritonavir; 3DR, continuation of triple therapy of 2NRTI + darunavir/ritonavir; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
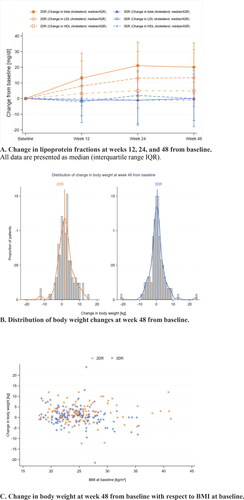
Over the 48 weeks, subjects in the 2DR arm gained +2.0 (−0.2 to +4.0) kg in body weight versus +0.2 (−1.9 to +2.1) kg in the 3DR arm (p = 0.0006). This corresponded to a significant increase in BMI of +0.6 (−0.1 to +1.2) kg/m2 in the 2DR arm and +0.1 (−0.5 to +0.7) kg/m2 in the 3DR arm (p = 0.0006) ( and C).
Regarding renal function, there was a change in the MDRD eGFR by −7.8 (−17.4 to −0.3) mL/min/1.73 m2 in the 2DR arm versus −0.4 (−8.8 to +5.7) mL/min/1.73 m2 in the 3DR arm (p = 0.0002). Similar results were obtained regarding the CKD-EPI Creatinine eGFR with a decrease by −8.0 (−17.0 to −0.6) mL/min/1.73 m2 in the 2DR arm versus −0.7 (−9.4 to +4.5) mL/min/1.73 m2 in the 3DR arm (p = 0.0002). To avoid any bias due to potential creatinine-based eGFR alterations under dolutegravir, we added cystatin C-based values. Regarding the serum levels of cystatin C, there was no increase at 0.0 mg/L in both arms (2DR −0.1 to +0.1 mg/L and 3DR 0.0 to +0.1 mg/L). The median cystatin C- creatinine-based CKD-EPI Creatinine-Cystatin eGFR showed a slight decrease in both groups, by −6.7 (−14.4 to +5.3) mL/min/1.73m2 in the 2DR group (p = 0.0021) and by −2.7 (−10.0 to +4.3) mL/min/1.73 m2 in the 3DR group (p = 0.0668), with no significant differences between groups (p = 0.1572).
Discussion
A switch from 3DR to 2DR was non-inferior with regard to viral suppression in virologically suppressed PLWH in the DUALIS study, as published recently.Citation5 The findings are confirmed by data from other studies focusing on NRTI-sparing dual therapy regimes.Citation6–8 This sub-analysis now showed a non-favorable profile of 2 DR in terms of metabolic and/or renal parameters compared to standard 3DR.
In the 2DR arm, a trend towards an increase in all lipoprotein fractions, as well as in total body weight and BMI, was observed. Target values for the LDL-lipoprotein range between <116 mg/dL and <55 mg/dL according to the cardiovascular risk profile.Citation10 An increase in the LDL fraction by +13.3 mg/dL in the 2DR arm – especially with a median LDL-level of 126.8 (46.4 to 284.o) mg/dl () is thus clinically relevant in a high-risk population.
For dolutegravir, a moderate but reversible increase in serum creatinine as an adverse event, with a consecutive false negative calculated GFR, has been described due to an interaction with renal creatinine metabolism.Citation11,Citation12 This was also observed in our study. The fact that the serum cystatin C level remained stable indicated no negative impact on renal function after switching to bDRV + DTG as compared to continuing 3DR within the study period of 48 weeks. Although the CKD-EPI Creatinine-Cystatin eGFR slightly decreased in the 2DR arm this did not represent a significant difference between the 2DR and 3DR arms.
The limitations of this post-hoc analysis of renal and metabolic assessments include that this was a short study period of 48 weeks. Additional studies should be conducted with a longer follow-up period to shed more light on the metabolic and renal effects of NRTI-sparing ART.
Declaration of conflicting interests
A.B. declares no conflicts of interest.
H.B. declares no conflicts of interest.
C.B. has received grants for travel and participation in advisory boards or speaker’s honoraria from AbbVie, Gilead, Janssen-Cilag, MSD, and ViiV Healthcare.
C.C. declares no conflicts of interest.
H.H. declares no conflicts of interest.
B.J. declares no conflicts of interest.
H.J. has received personal fees from Janssen-Cilag GmbH, ViiV Helathcare GmbH, MSD GmbH, Hormosan Pharma GmbH, Ifi-Medizin GmbH, CIP Clinic GmbH and GlaxoSmithKline GmbH & Co. KG, grants and personal fees from Gilead Sciences GmbH, grants from U.S. Military HIV Research Program (MHRP) outside the submitted work.
I.K. has received grants and/or personal fees from AbbVie, Gilead Sciences, Janssen-Cilag, ViiV Healthcare, and MSD during the study and outside the submitted work
M.M. declares no conflicts of interest.
P.K. has received grants for travel and participation in advisory boards or speaker’s honoraria from AbbVie, Gilead, Janssen-Cilag, Sanofi and ViiV Healthcare.
T. K. has received grants for travel and participation in advisory boards from Gilead Healthcare and ViiV Healthcare.
W.O. declares no conflicts of interest.
C.D.S. has received grants and/or personal fees from Gilead, Janssen-Cilag, ViiV, MSD, Healthcare/GSK during the conduct of this study, and AbbVie, Apeiron, BBraun Melsungen, Cepheid, Eli Lilly, Formycon, and Molecular partners outside this submitted work.
P.R.-S. declares no conflicts of interest.
H.J.S. has received honoraria from ViiV Healthcare, Gilead Sciences,
Janssen Cilag, MSD Sharp & Dohme, and Theratechnologies, as well as financial support for clinical trials from ViiV Healthcare, Gilead Sciences, Janssen Cilag, MSD Sharp & Dohme, Roche, AbbVie, and
Heidelberg Immunotherapeutics.
J.S. declares no conflicts of interest.
S.S. received honorarium for Study participation from ViiV, GSK, Janssen, Gilead, and Heidelberg Immunotheraputics, for lectures & talks from ViiV, Gilead, and Janssen and travel & congress participation grants from Gilead, ViiV, Janssen, and MSD.
E.W. received grants or personal fees for participation in advisory boards or speaker’s honoraria or grants for travel from AbbVie, Gilead Sciences, Janssen-Cilag, MSD, and ViiV Healthcare outside this submitted work.
Members of the DUALIS study team
Christoph D. Spinner (Technical University of Munich, School of Medicine, University Hospital Rechts der Isar, Department of Internal Medicine II, Munich, Germany), Björn Jensen (University Hospital Duesseldorf, Department of Gastroenterology, Hepatology and Infectiology, Duesseldorf, Germany), Thomas Lutz (Infektiologikum Frankfurt, Frankfurt, Germany), Petra Spornraft-Ragaller (Carl Gustav Carus University Hospital of the Technical University Dresden, Dresden, Germany), Hans Jürgen Stellbrink (ICH Study Center GmbH & Co, Hamburg, Germany), Martin Hower (Hospital Dortmund gGmbH, outpatient clinic for infectious diseases, Dortmund, Germany), Ulrich Bohr (Praxis Kaiserdamm, Berlin, Germany), Wilfried Obst (University Hospital Magdeburg, Department for Gastroenterology, Hepatology and und Infectiology, Magdeburg, Germany), Ivanka Krznaric (ZIBP Center for Infectiology Berlin Prenzlauer Berg GmbH, Berlin, Germany), Martin Sprinzl (University Hospital Mainz, Mainz, Germany), Franz Audebert (Praxiszentrum Alte Mälzerei, Köln, Germany), Tim Kuemmerle (Praxis am Ebertplatz, Köln, Germany), Stefan Scholten (Praxis Hohenstaufenring, Köln, Germany), Heribert Hillenbrand (Medizinisches Versorgungszentrum Berlin-Friedrichshain, Germany), Christiane Cordes (Praxis Cordes, Berlin, Germany), Heribert Knechten (Praxis Knechten, Aachen, Germany), Birger Kuhlmann (Praxis Kuhlmann, Hannover, Germany), Heiko Jessen (Praxis Jessen, Berlin, Germany), Patrick Beck (Gemeinschaftspraxis- Infectomed, Stuttgart, Germany), Gerd Fätkenheuer (University Hospital Köln, Department of Medicine II, Köln, Germany), Hartwig H. F. Klinker (University of Wuerzburg Medical Center, Department of Medicine II, Division of Infectious Diseases, Wuerzburg, Germany), Juergen K. Rockstroh (University Hospital Bonn, Department of Medicine I, Bonn, Germany), Stefan Esser (University Hospital Essen, Department of Dermatology, Essen, Germany), Christoph Stephan (Hospital of Johann Wolfgang Goethe University, Department of Internal Medicine, HIV-Center, Frankfurt, Germany), Olaf Degen (University Hospital Hamburg-Eppendorf Center for Infectiology, Hamburg, Germany), Andreas Bellmunt-Zsch€ape (Allgemeinmedizinische Praxis Bellmunt Zschäpe, Dortmund, Germany), Pavel Khaykin (MainFachArzt, Frankfurt/Main, Germany), Norbert Brockmeyer (Hospital BochumWIR ‘Walk In Ruhr’ at St. Elisabeth-Hospital, Bochum, Germany), Albrecht Stoehr (ifi – Institute for Interdisciplinary Medicine, Center for Infectiology at Asklepios Hospital St. Georg, Hamburg, Germany).
Author contributions
M.M. drafted the manuscript. T.K., J.S., C.C., H.H., H.J.S., I.K., S.S., B.J., H.J., C.S., P.S.R., and P.K. supervised patient care, assisted in data interpretation, and edited the manuscript. E.W. and A.N.B. performed the statistical analysis, data interpretation, and assisted with editing of the manuscript. C.D.S. and C.B. coordinated the entire study, supervised patient care, and edited the manuscript.
Acknowledgments
We would like to thank the team of Munich Study Center, CRO, Technical University of Munich and the staff of MUC Research for supporting this study. We also thank the study centers and participants of the DUALIS study.
Data sharing statement
For the time being, data sharing is not planned. On request, we will decide on sharing data on a by-case basis.
Additional information
Funding
References
- Study Group IS, Lundgren JD, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807.
- Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. HPTN 052-ACTG Study Team. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–290.
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505.
- Achhra AC, Mwasakifwa G, Amin J, et al. Efficacy and safety of contemporary dual-drug antiretroviral regimens as first-line treatment or as a simplification strategy: a systematic review and meta-analysis. Lancet Hiv. 2016;3(8):e351–e360.
- Spinner CD, Kümmerle T, Schneider J, et al. DUALIS Study Group. Efficacy and safety of switching to dolutegravir with boosted darunavir in virologically suppressed adults with HIV-1: A randomized, open-label, multicenter, phase 3, noninferiority trial: The DUALIS Study. Open Forum Infect Dis. 2020;7:ofaa356.
- Young J, Scherrer AU, Calmy A, Swiss HIV Cohort Study, et al. The comparative effectiveness of NRTI-sparing dual regimens in emulated trials using observational data from the Swiss HIV Cohort Study. Antivir Ther. 2019;24(5):343–353. PMID: 30985290.
- Gay CL, Neo DT, Devanathan AS, et al. Efficacy, pharmacokinetics and neurocognitive performance of dual, NRTI-sparing antiretroviral therapy in acute HIV-infection. AIDS. 2020;34(13):1923–1931. PMID: 32773474; PMCID: PMC7541775.
- Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018;391(10123):839–849.
- Schneider E, Whitmore S, Glynn KM, et al. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years–United States. 2008. MMWR Recomm Rep. 2008;57(RR-10):1–12.
- Visseren FLJ, Mach F, Smulders YM, ESC Scientific Document Group, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337.
- Ribera E, Podzamczer D. Mecanismo de acción, farmacología e interacciones de dolutegravir [Mechanisms of action, pharmacology and interactions of dolutegravir. Enferm Infecc Microbiol Clin. 2015;33 (Suppl 1):2–8. Spanish. PMID: 25858605.
- DeJesus E, Rockstroh JK, Henry K, GS-236-0103 Study Team, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–2438.
