Abstract
Background: The benefits derived from supervised aerobic exercise in people living with human immunofeficiency virus– HIV (PLWH) have not yet been clearly identified.
Objective: To evaluate the impact of supervised aerobic exercise on immunological, cardiorespiratory, pulmonary, hemodynamic and mental parameters of PLWH.
Methods: A systematic review was carried out in accordance to PRISMA guidelines. PubMed, Physiotherapy Evidence Database (PEDro) and Cochrane Central Register of Controlled Trials (CENTRAL) were screened up to August 2021, for the identification of English written randomized trials, with participants aged 18 years and older, at any stage of the disease, with or without co-morbidities. The risk of bias assessment was conducted according to the Cochrane Collaboration’s tool for assessing risk of bias. Meta- analyses were conducted using continuous, inverse variance, random-effects model.
Results: Ten studies were suitable for meta-analysis based on inclusion criteria. Supervised aerobic exercise appeared to have beneficial effects on depressive symptoms [mean difference (MD)= −4.18 (confidence interval (CI)= (−6.55)–(−1.81), Z = 3.46, p = 0.0005, I2=0%, n = 2], forced expiratory volume in 1 sec [MD = 0.70, CI = 0.39–1.00, Z = 4.41, p < 0.0001, I2=0%, n = 2], and on the maximum oxygen uptake [MD = 1.38, CI = −0.02–2.78, Z = 1.94, p = 0.05, I2=94%, n = 4] of PLWH. No exercise effect was found for CD4 T-cell count (p = 0.16, n = 5), systolic blood pressure (p = 0.91, n = 2) and diastolic blood pressure (p = 0.72, n = 2).
Conclusions: Supervised continuous aerobic exercise may improve lung function, depressive symptomatology and aerobic capacity of PLWH, however, the small number of available studies and the high heterogeneity concerning VO2max demonstrate the need for more research in this area.
Introduction
Human immunodeficiency virus (HIV) disease and acquired immune deficiency syndrome (AIDS) epidemic remains a major global public health problem with 37.9 million people living with HIV (PLWH) in 2018.Citation1 Antiretroviral drugs significantly reduced morbidity and mortality due to HIV disease.Citation2 However, highly active antiretroviral therapy (HAART) may cause a large number of unwanted physical and mental disorders (e.g. heart failure, stroke, myocardial infarction, peripheral artery disease, lipodystrophy, dyslipidemia, insulin resistance, anxiety, depression, etc.), increasing the non-HIV- related mortality.Citation3–7
Exercise, a subset of physical activity that is planned, structured, and repetitive,Citation8 seems to provide health benefits to PLWH; it improves somatometric, metabolic and hormonal parameters, enhances cardio respiratory function and muscle strength, and promotes mental health.Citation9–14 Aerobic exercise is well established for its positive impact on human health.Citation15 In particular, it consists an important strategy for reducing the risk of cardiovascular disease that emerges in PLWH and also contributes to mitigating the symptoms of long-term HIV and long-term antiretroviral therapy (ART) use.Citation16–18 However, the participation of PLWH to exercise interventions remains below suggested levels.Citation19,Citation20 Indeed, PLWH present high levels of sedentary behavior, even after comparing with populations with other chronic diseases.Citation21,Citation22
Supervision is a variable that reinforces the participation of PLWH to exercise, by preventing a significant number of potential dropouts, an issue of great importance as regards the aerobic exercise, which is more challenging to adhere, compared to resistance training.Citation22 Ιt has also been shown that supervised exercise interventions provide more pronounced health-related gains compared to unsupervised ones in a variety of populations with or without pathological entities (i.e. hypertension, type 2 diabetes, cancer, etc.), in terms of cardiovascular, anthropometric, functional, metabolic, hemodynamic and mental improvements and improvements in quality of life.Citation23–26 Although the beneficial effects of supervised aerobic exercise have been demonstrated in literature, previous reviews of aerobic exercise in PLWH have paid minimum attention to supervision.
A substantial amount of systematic reviews, published so far, have examined both supervised and non-supervised aerobic exercise programs for PLWH, with the results of which being presented collectively.Citation13,Citation16,Citation27,Citation28 Another shortcoming yet to be addressed in previous systematic reviews/meta- analysis includes the investigation of heterogenous exercise interventions. In a recent systematic review and meta- analysis which conducted subgroup analysis to explore the impact of supervised exercise on cardiovascular parameters of PLWH, authors included both aerobic and concurrent (i.e. aerobic exercise combined with resistance training) exercise interventions.Citation17 Similarly, in another recent study, the subgroup analysis for the evaluation of supervised exercise on depression in PLWH, included only one study implementing aerobic exercise alone.Citation29 The study of Voigt et al, 2018, is the only systematic review considering supervision as the primary exercise variable on functional capacity of PLWH, however it is focused on physical activity, without any meta- analysis being made.Citation30
Therefore, to the best of the authors’ knowledge, a systematic review that focuses on the effect of supervised aerobic exercise on important clinical, physiological and mental health indices of PLWH, has not been carried out. In this light, the aim of the current systematic review and meta- analys is to examine the effects of supervised aerobic exercise on immunological, cardiorespiratory, pulmonary, hemodynamic and mental parameters of PLWH.
Materials and methods
The current systematic review and meta- analysis was registered in the International Platform of Registered Systematic Review and Meta- analysis Protocols (registration number: INPLASY202070035) and was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.Citation31
Search strategy
Using appropriate algorithms, two investigators (PK and CC) independently searched PubMed, Physiotherapy Evidence Database (PEDro) and Cochrane Library trials (CENTRAL), from the date of their inception until August 2021. Search limitations included publications written in English language. Discrepancies between the two investigators were resolved by a referee investigator (AP). The key word algorithm used in PubMed database can be found in the Appendix. In addition, a thorough search of the references lists of relevant publications was used to identify any additional eligible papers that were not appeared in the initial electronic search.
Eligibility criteria
We have included only randomized controlled trials (RCTs), which compared supervised aerobic exercise, with non-exercise (usual care) in the control condition, and examined at least one of the outcome measures under study. Studies included PLWH at all stages of the disease, with or without comorbidities, with participants 18 years and over, were considered eligible. Inclusion criteria also included the implementation of continuous aerobic exercise of moderate to vigorous intensity [i.e., 46–80% of maximum oxygen consumption (VO2max), or 40–80% of oxygen uptake reserve (VO2R), or 40–80% of heart rate reserve (HRR), or 60–85% of maximum heart rate (HRmax)], at a frequency of at least 3 times per week for a total period of at least 4 weeks. This exercise regimen is in accordance to the recommended guidelines for PLWH, in order to lessen the severity of HIV and ART related comorbidities.Citation16,Citation32–34 Moreover, it has been found that it is easier to follow compared to higher intensity levels,Citation35 especially by individuals with physical disabilities or other health conditions whose adherence to high intensity exercise may be challenging.Citation36 Only studies which reported that the exercise program, as a whole, was carried out under supervision, were accepted as eligible. Studies investigating the effects of resistance training, those including a combined program of both aerobic and resistance exercise, and those investigating the effects of aerobic exercise combined with other intervention(s) were rejected. Also, studies that used high intensity interval training (HIIT) were rejected because this type of exercise involves high intensity periods that may compromise adherence.Citation36 Furthermore, studies that they did not include a non-exercise group as control condition, or studies with only healthy individuals, were rejected. Finally, review articles, letters, theses, experts’ opinions, as well as animal studies, were also rejected.
Outcome measures
Cardiorespiratory [i.e., VO2max and 6-Minute Walk Test (6-MWT)] and pulmonary [i.e., forced vital capacity (FVC) and FEV1] parameters of physical function, as well as immunological (CD4 count cells/mm3) and hemodynamic [diastolic blood pressure (DBP) and systolic blood pressure (SBP)] variables, were assessed. Finally, depression was the variable assessed in terms of the mental health of the study population.
Study selection
Selection of the retrieved studies was performed by two independent investigators (PK and AP) using a control list, based on the above inclusion/exclusion criteria. Studies in which there was uncertainty regarding the appropriateness of their inclusion in the systematic review, were further examined by a third researcher (CC), who made the final decision. In case of unavailable data in the selected studies, the responsible author(s) was contacted via email.
Data extraction
Data extraction was performed using pre-designed data extraction form, by three independent investigators (PK, EK and AP). The extracted data were grouped as follows and include (1) publication information: author, year of publication, country of study, (2) study design: sampling method, study duration, subject participation criteria, (3) characteristics of participants: number of participants, age range, gender, disease stage, antiretroviral treatment, withdrawal of patients from the study (dropouts), (4) intervention characteristics: type of aerobic exercise, intensity, frequency, duration, data collection method/s, (5) outcome measures, difference in the values of the outcome measures between the baseline and the completion of the study and (6) limitations of the study. Disagreements in extracting or interpreting the data were resolved through discussion between the three independent investigators (PK, EK and AP). Failure to reach an agreement required the opinion of another researcher (SN).
Quality assessment
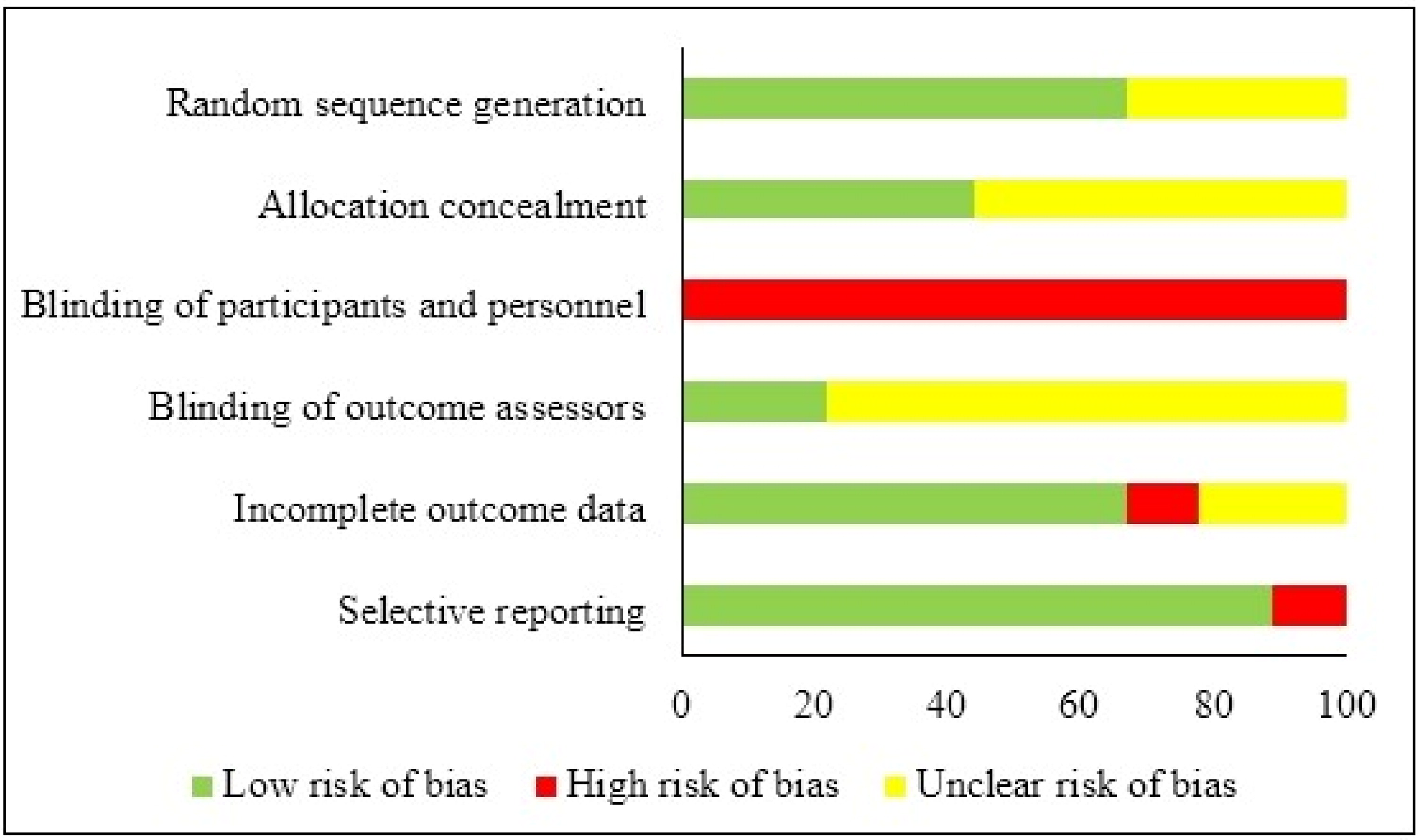
The risk of bias was assessed independently by two investigators (PK and PD) using the Cochrane Collaboration’s tool for assessing the risk of bias (CCRBT).Citation37 Disagreements between the two investigators were resolved by a referee investigator (AP). The following risk of bias components were assessed: (1) selection bias (random sequence generation and allocation sequence concealment), (2) performance bias (blinding of participants and personnel), (3) detection bias (blinding of outcome assessors), (4) attrition bias (incomplete outcome data), and (5) selective reporting bias. The above components were rated on a low, high, or unclear scale.
Meta-analyses
We conducted continuous, inverse variance, random-effect model meta-analyses via the RevMan 5.3 software. We used means and standard deviations of a) CD4 count cells, b) VO2max, c) systolic blood pressure, d) diastolic blood pressure, e) FEV1, and f) Beck’s depression inventory score to test mean differences between exercise and control conditions. The study effect sizes were synthesized to account for heterogeneity due to differences in study populations, interventions, study duration, and other factors.The statistically significant limit was set at p ≤ 0.05. We evaluated the 95% confidence interval (CI) and heterogeneity between studies using the I2 statistic. We considered a statistically significant result for heterogeneity when p < 0.10, while interpretation of I2 index was made based on previous guidelines.Citation38
Results
Searching results and selection of eligible publications
A total of 378 reports retrieved from the algorithmic search. After removing the duplicates, and due to inconsistency with the object under study or inadequacy for introduction after checking the title and/or the summary, 35 reports remained. An additional 25 reports were excluded after reading the full text. A total of seven reports derived from the algorithmic search in the electronic databases and three from the investigation of the reference lists, of the eligible publications were found to meet the eligibility criteria to be included in the systematic review ().Citation39–48
Participant characteristics
This systematic review includes a total of 370 PLWH aged 18 to 61 years (18.5% withdrawal rate). Most of the participants were in the non-AIDS stage, with the exception of Lox et al.Citation41 where patients in the AIDS stage were also included (CD4 cell count < 200 cell/mm3). Almost all studies included both genders in their research design (percentage of women at baseline: 42%). In five of the eligible studies, all participants received antiretroviral treatment,Citation42,Citation44,Citation46–48 while only in one study there was not any reference regarding the antiretroviral treatment.Citation45
Study characteristics
The duration of aerobic exercise ranged from 20 to 45 minutes, with a frequency of 3 times per week, which was observed in all studies. The duration of the intervention ranged from 4 to 15 weeks. The exercise program was supervised by specialized health professionals or other staff in four of the 10 included studies,Citation41,Citation42,Citation45,Citation48 while the rest of the studies,Citation39,Citation40,Citation43,Citation44,Citation46,Citation47 simply reported supervision without providing any further information. The status of the participants' immune system was assessed via CD4 T-lymphocytes, in fiveCitation39,Citation42,Citation43,Citation45,Citation47 of the 10 studies. Cardiorespiratory fitness assessed by VO2max was reported in four studies,Citation39,Citation41,Citation45,Citation48 while only a single study assessed it through the 6-MWT.Citation43 Two studies investigated the effect of continuous and moderate to vigorous intensity aerobic exercise on lung function,Citation39,Citation44 and two studies investigated the hemodynamic status of PLWH.Citation46,Citation48 Depression was assessed in two studies.Citation40,Citation44 Missing data of variables of interest of two studies were requested by the responsible authors via email.Citation46,Citation48 The detailed characteristics of each study can be found in the data extraction table in the Appendix.
Quality of eligible studies
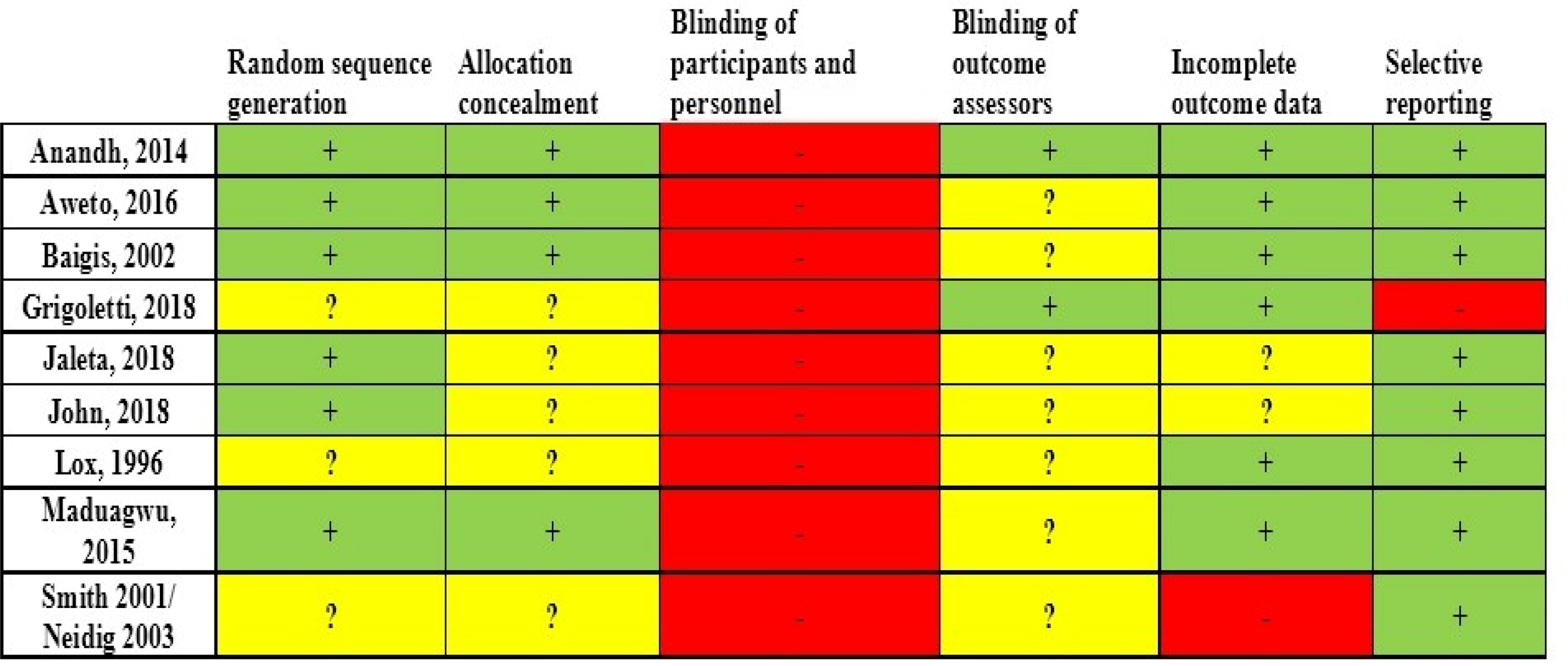
Four out of 10 studies showed low risk of bias,Citation42–45 while the remaining six studies showed high risk of bias.Citation39–41,Citation46–48 Regarding random sequence generation (selection bias), allocation concealment (selection bias) and blinding of outcome assessors (detection bias), the risk of bias was found to be low or unclear in all studies. Apart from the study by Grigoletti et al.,Citation46 all other studies showed low risk of bias for selective reporting (reporting bias). The risk of bias for blinding participants and personnel (performance bias) was found to be high in all studies, as, due to the nature of the applied intervention (exercise), blinding was impossible. The results of the assessment of study quality using CCRBT and the Cochrane risk of bias summary are reported in the Appendix.
Main outcomes
Cardiorespiratory fitness
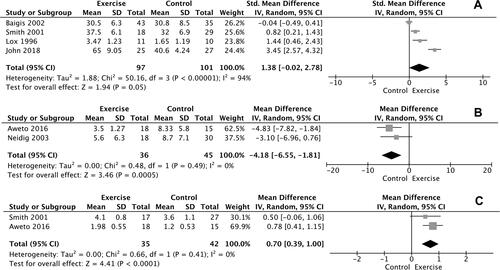
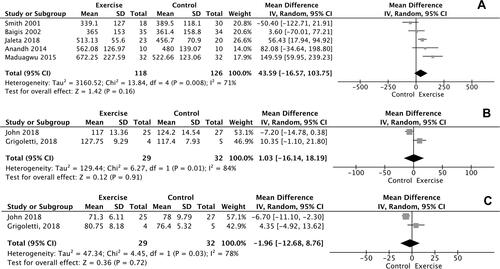
Results of meta-analysis showed a significant improvement in VO2max of 1.38 ml/kg/min, favoring participants in the intervention group compared with the non-exercisers in control group [mean difference (MD)=1.38, confidence interval (CI) = −0.02–2.78, Z = 1.94, p = 0.05, I2=94%, n = 4; ). Additionally, statistically significant improvement was found between the intervention and control group (p < 0.05) considering the performance in 6-MWT in the study of Anandh et al.Citation43 However, meta-analysis for 6-MWT performance could not be performed due to the single study investigating this particular outcome.
Depression
Meta-analysis for depression was performed for the studies of Neidig et al.Citation40 and Aweto et al.Citation44 which assessed depressive symptomatology through the same psychometric test [Beck Depression Inventory (BDI)]. The meta-analysis showed a significant difference in changes in depressive symptomatology between exercise and control groups [MD = −4.18, CI = (−6.55)–(−1.81), Z = 3.46, p = 0.0005, I2=0%, n = 2; ).
Pulmonary function
As shown in , FEV1 was increased by 0.7lt (MD = 0.70, CI = 0.39–1.00, Z = 4.41, p < 0.0001, I2 = 0%, n = 2) in the exercisers, compared to non- exercisers. FVC was examined only in the study of Aweto et al.,Citation44 where significant improvements were revealed in the exercise intervention group compared to control group (p = 0.001).
CD4 count
The meta–analysis performed for CD4 count demonstrated no difference in changes in CD4 cell counts for participants in the intervention group, compared with the non-exercise control group (p = 0.16, n = 5) ().
Hemodynamic parameters
As shown in , no significant differences in changes in SBP and DBP values were found between exercise participants and non-exercisers (p = 0.91, n = 2 and p = 0.72, n = 2, respectively).
Discussion
The aim of the current systematic review and meta-analyses was to examine the effects of supervised aerobic exercise on important clinical (CD4 T-cell counts, blood pressure, FEV1, and FVC), physiological (maximum oxygen uptake, 6MWT), and mental health (depression) parameters in PLWH. Ten studies met the eligibility criteria for inclusion in our systematic review and were included in meta-analysis for CD4 T-cell counts, SBP, DBP, FEV1, VO2 max and depression.Citation39–48 The main results of these meta-analyses suggest that a supervised aerobic exercise progmam has beneficial effects on cardiorespiratory capacity, pulmonary function and mental health of PLWH.
Scientific data show that cardiorespiratory capacity is a strong and independent predictor of mortality, and several biological mechanisms suggest that by increasing it, cardiometabolic risk factors such as insulin resistance, lipid profile, body composition, blood pressure and inflammation are also improved.Citation49 These benefits are particularly important for PLWH, as they experience an increased incidence of cardio-metabolic disorders.Citation50 The clinical significance of cardiorespiratory capacity improvement, as assessed by VO2 max, has not yet been established in PLWH. Myers et al.Citation51 reported that 1-MET (3.5 ml/kg/min) increase in exercise capacity was associated with a 12% improvement in survival of both healthy subjects and those with cardiovascular disease. For PLWH, O’ Brien et all.Citation16 considered that an improvement of 2 ml/kg/min in VO2 max could indicate an important clinical change. Our meta-analysis showed a significant improvement of 1.38 ml/kg/min in VO2 max, which does not reach the afore mentioned clinically beneficial levels. However, this might not be an established conclusion as much lower changes are possibly associated with improvements in clinically important outcomes. Notably, Swank et al.Citation52 reported that an improvement of 0.4 ml/kg/min in VO2 max over a period of three months resulted in improved primary time endpoint to all-cause mortality or all-cause hospitalization by 5% in patients with chronic heart failure. Considering cardiovascular adaptations, our findings support those of previous, though heterogeneous in terms of supervision and exercise intensity, systematic reviews, regarding the beneficial effect of aerobic exercise on cardiorespiratory fitness of PLWH.Citation16,Citation17,Citation53–55
Moreover, chronic pulmonary disabilities and respiratory symptoms are common findings among PLWH.Citation56–58 HIV disease is associated with reduced diffusion capacity and the development of asthma, cardiopulmonary dysfunction and obstructive ventilatory disorders.Citation56,Citation58 Indeed, the association between chronic obstructive pulmonary disease (COPD) and HIV is becoming increasingly recognized,Citation57,Citation58 as HIV disease is considered as an independent risk factor for COPD.Citation59,Citation60 Despite that, however, only 2 RCTsCitation39,Citation44, investigating the effect of aerobic exercise on the lung function of PLWH, were detected and analyzed in this review, the results of which showed a significant improvement of FEV1 in the exercised participants. This finding is of particular interest since no related therapies have been tested, specifically in PLWH, and the optimal therapeutic strategy for chronic lung diseases in PLWH is largely unknown.Citation56
In addition, hypertension is a traditional risk factor for cardiovascular disease and a significant cause of death in people under antiretroviral therapy.Citation61 Specifically, elevated blood pressure (BP) is one of the strongest modifiable risk factor, with exercise playing a key role in its prevention and treatment.Citation62 Based on the inclusion and exclusion criteria set in this systematic review, only two studiesCitation46,Citation48 have emerged that investigated the effect of supervised aerobic exercise on the SBP and DBP of PLWH. The analysis of these studies revealed no significant differences in the changes of SBP and DBP values between the exercise and control groups. These findings are in line with those of a recent systematic review and meta-analysis,Citation17 which found no significant association between aerobic exercise and a decrease of BP in PLWH. That review included three studies investigating the effect of aerobic exercise and eight studies investigating the effect of aerobic combined with resistance exercise on PLWH, showing that these forms of exercise did not affect SBP and DBP, or heart rate in these people.
Our systematic review and meta-analysis also revealed no significant exercise-induced changes in CD4 count outcomes, suggesting that continuous supervised aerobic exercise had no impact on the progression of HIV. This finding is in agreement with the results of previous systematic reviews and/or meta-analyses that investigated the effect of both aerobic exercise alone or the combination of aerobic and resistance exercise, showing that CD4 T-lymphocyte values did not change after the completion of the exercise intervention.Citation16,Citation63,Citation64 Nevertheless, the lack of deterioration of CD4 T-lymphocyte population post-exercise suggests that supervised aerobic exercise did not impair the immune status of PLWH.
The prevalence of mental disorders in PLWH is high and is associated with poor treatment outcomes. More specifically, depression rates range from 22 to 50% among PLWH.Citation65 The results of our meta-analysis conducted for depressive symptomatology, are consistent with findings from previous studies concluding that aerobic exercise is beneficial for the mental health of PLWH.Citation16,Citation29
To our knowledge, this is the first study investigating the effects of continuous supervised aerobic exercise on clinical, physiological and mental health variables of PLWH. It is also the first study that performed meta-analysis on the effect of aerobic exercise on pulmonary parameters in this specific population. We used strict inclusion criteria in order to achieve homogeneity between the studies. Only studies with usual care in the control condition and exclusively those that implemented supervised aerobic exercise, without the combination of another intervention, were introduced so that the results can be attributed exclusively to the exercise regimen under study while, further, homogeneity regarding the frequency of exercise sessions was high in the studies included in this systematic review.
Our study has several limitations; the strict inclusion/exclusion criteria set, only allowed the introduction and analysis of a small number of studies. In addition, participants involved in the included studies may exhibit differences in certain characteristics such as race/ethinicity, age-related exclusions, administration and type of antiretroviral therapy, the stage of the disease, and the presence of co-morbidities. Also, the duration of the exercise intervention that lasted up to 15 weeks, did not allow examining long-term benefits of exercise. However, we have used a random-effect model meta-analysis to allow a statistical analysis of these data. A random effect model meta-analysis assumes a variation among the included studies, due to differences in interventions and other factors.Citation66 Also, thresholds for heterogeneity can be avoided, given the small number of studies included in our meta-analyses.Citation66 Therefore, the outcomes of our meta-analyses can be considered with the limitation of the small number of participants (small sample size) due to small number of the included studies. In this light, a sensitivity analysis was not optimal, that would further decrease the sample size of the analysis. Finally, to determine publication bias via funnel plots was not possible, due to the small number of studies included in each meta-analysis (<10).Citation66
Further research is needed to identify PLWH who would be expected to benefit most from performing a supervised aerobic exercise program depending on the stage of their disease, as well as to determine the optimum dosage of exercise required to reduce disease-related symptoms, aiming at maximizing the clinical benefits. In addition, more RCTs are needed to explore the impact of supervised aerobic exercise across the spectrum of clinical, psysiological, and mental health variables, emphasizing in important understudied measures such as pulmonary and hemodynamic indices, the improvement of which may benefit this clinical population. RCTs with higher numbers of participants and longer duration than those conducted so far are warranted, in order to draw safe conclusions regarding the effects of supervised aerobic exercise on clinical, physiological and mental health variables in PLWH. Moreover, future studies should focus on the comparison between supervised and non-supervised exercise in this population, the elucidation of which, may lead to the prescription of more effective exercise regimens.
In conclusion, performing supervised, continuous aerobic exercise, appears to lead in pulmonary, cardiorespiratory and mental health improvements in PLWH, while the effects of this exercise regimen on immunological and hemodynamic variables are less clear. Physicians should consider supervised exercise as an intervention for improving quality of life in PLWH. The results of this systematic review and meta-analyses should, though, be treated with caution, given the small number of related studies identified.
Disclosure of interest
The authors report no conflict of interest. No specific funding was received for this work.
Ethical approval
Ethical approval was not required for this study.
Appendix
Search query in Pub Med database
Search: (((((((((((((((((((((((((((((((((("Cardiorespiratory Fitness"[Mesh]) OR ("Walk Test"[Mesh])) OR ("Pulmonary Ventilation"[Mesh])) OR ("Pulmonary Gas Exchange"[Mesh])) OR ("Hemodynamics"[Mesh])) OR ("Blood Pressure"[Mesh])) OR ("Arterial Pressure"[Mesh])) OR ("CD4 Lymphocyte Count"[Mesh])) OR ("Depression"[Mesh])) OR ("cardiorespiratory fitness"[Title/Abstract])) OR ("cardiovascular fitness"[Title/Abstract])) OR ("cardiovascular parameters"[Title/Abstract])) OR ("VO2 max"[Title/Abstract])) OR ("maximal oxygen consumption"[Title/Abstract])) OR ("maximal oxygen uptake"[Title/Abstract])) OR ("peak oxygen uptake"[Title/Abstract])) OR ("maximal aerobic capacity"[Title/Abstract])) OR ("6 minute walk test"[Title/Abstract])) OR ("6MWT"[Title/Abstract])) OR ("pulmonary ventilation"[Title/Abstract])) OR ("pulmonary gas exchange"[Title/Abstract])) OR ("FVC"[Title/Abstract])) OR ("forced vital capacity"[Title/Abstract])) OR ("forced expiratory volume in one second"[Title/Abstract])) OR ("FEV1"[Title/Abstract])) OR ("hemodynamics"[Title/Abstract])) OR ("haemodynamics"[Title/Abstract])) OR ("arterial pressure"[Title/Abstract])) OR ("systolic blood pressure"[Title/Abstract])) OR ("diastolic blood pressure"[Title/Abstract])) OR ("CD4 lymphocyte count"[Title/Abstract])) OR ("CD4 cell count"[Title/Abstract])) OR ("depression"[Title/Abstract])) AND (((((((((HIV[MeSH Terms]) OR (hiv infections[MeSH Terms])) OR (acquired immunodeficiency syndrome[MeSH Terms])) OR ("HIV"[Title/Abstract])) OR ("HIV infection"[Title/Abstract])) OR ("acquired immunodeficiency syndrome"[Title/Abstract])) OR ("HIV seropositivity"[Title/Abstract])) OR ("human immunodeficiency virus"[Title/Abstract])) OR ("AIDS"[Title/Abstract]))) AND ((((((((((exercise[MeSH Terms]) OR (endurance training[MeSH Terms])) OR ("exercise"[Title/Abstract])) OR ("endurance training"[Title/Abstract])) OR ("physical exercise"[Title/Abstract])) OR ("exercise training"[Title/Abstract])) OR ("cardiovascular exercise"[Title/Abstract])) OR ("aerobic training"[Title/Abstract])) OR ("cardio exercise"[Title/Abstract])) OR ("aerobic exercise"[Title/Abstract])) Filters: Randomized Controlled Trial, English
Data extraction table
Risk of bias assessment within studies
Risk of bias assessment across studies
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Communities at the Centre. Defending rights breaking barriers reaching people with HIV services. Global AIDS Update 2019. Geneva: UNAIDS. https://www.unaids.org/en/resources/documents/2019/2019-global-AIDS-update. Published 2019 (Accessed: 11 Aug 2020).
- Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533.
- Hawkins T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res. 2010;85(1):201–209.
- Alonso A, Barnes AE, Guest JL, et al. HIV infection and incidence of cardiovascular diseases: an analysis of a large healthcare database. J Am Heart Assoc. 2019;8(14):e012241.
- Mikhail N. Insulin resistance in HIV-related lipodystrophy. Curr Hypertens Rep. 2003;5(2):117–121.
- Nanni MG, Caruso R, Mitchell AJ, et al. Depression in HIV infected patients: a review. Curr Psychiatry Rep. 2015;17(1):530.
- Clucas C, Sibley E, Harding R, et al. A systematic review of interventions for anxiety in people with HIV. Psychol Health Med. 2011;16(5):528–547.
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131.
- Jaggers JR, Hand GA. Health benefits of exercise for people living with HIV: a review of the literature. Am J Lifestyle Med. 2016;10(3):184–192.
- Ortiz A. Exercise for adults living with human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Int J Phys Med Rehabil. 2014; 2:4.
- Kamitani E, Sipe TA, Higa DH, et al. Evaluating the effectiveness of physical exercise interventions in persons living with HIV: overview of systematic reviews. AIDS Educ Prev. 2017;29(4):347–363.
- Pérez Chaparro CGA, Zech P, Schuch F, et al. Effects of aerobic and resistance exercise alone or combined on strength and hormone outcomes for people living with HIV. A meta-analysis. PLoS One. 2018;13(9):e0203384.
- Fillipas S, Cherry CL, Cicuttini F, et al. The effects of exercise training on metabolic and morphological outcomes for people living with HIV: a systematic review of randomised controlled trials. HIV Clin Trials. 2010;11(5):270–282.
- Webel AR, Perazzo J, Longenecker CT, et al. The influence of exercise on cardiovascular health in sedentary adults with human immunodeficiency virus. J Cardiovasc Nurs. 2018;33(3):239–247.
- Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25_suppl_2):76–99.
- O'Brien KK, Tynan AM, Nixon SA, et al. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis. 2016;16:182.
- Zech P, Pérez-Chaparro C, Schuch F, Wolfarth B, Rapp M, Heissel A. Effects of aerobic and resistance exercise on cardiovascular parameters for people living with HIV: a meta-analysis. J Assoc Nurses AIDS Care. 2019;30(2):186–205.
- Ezema CI, Onwunali AA, Lamina S, et al. Effect of aerobic exercise training on cardiovascular parameters and CD4 cell count of people living with human immunodeficiency virus/acquired immune deficiency syndrome: a randomized controlled trial. Niger J Clin Pract. 2014;17(5):543–548.
- Vancampfort D, Mugisha J, De Hert M, et al. Global physical activity levels among people living with HIV: a systematic review and meta-analysis. Disabil Rehabil. 2018;40(4):388–397.
- Simonik A, Vader K, Ellis D, et al. Are you ready? Exploring readiness to engage in exercise among people living with HIV and multimorbidity in Toronto, Canada: a qualitative study. BMJ Open. 2016;6(3):e010029.
- Vancampfort D, Mugisha J, De Hert M, et al. Sedentary behavior in people living with HIV: a systematic review and meta-analysis. J Phys Act Health. 2017;14(7):571–577.
- Vancampfort D, Mugisha J, Richards J, et al. Dropout from physical activity interventions in people living with HIV: a systematic review and meta-analysis. AIDS Care. 2017;29(5):636–643.
- Albayrak İ, Kaya İ, Gulec A, et al. Effects of supervised versus unsupervised aerobic exercise training on weight loss, functional capacity, quality of life and depression level in patients with essential hypertension. Authorea. 2021:1–10.
- Beaudry R, Kruger C, Liang Y, et al. Effect of supervised exercise on aerobic capacity in cancer survivors: adherence and workload predict variance in effect. WJMA. 2015; 3(1):43–53.
- Slivovskaja I, Buzinskaitė J, Ryliškytė L, et al. Positive impact of a 4-week duration supervised aerobic training on anthropometric, metabolic, hemodynamic and arterial wall parameters in metabolic syndrome subjects. Semin Cardiovasc Med. 2017;23(1):11–16..
- Maduabuchi JN, Goddy CO, Afamefuna VE, et al. The effect of twelve weeks supervised aerobic exercise intervention on lower extremities oxygenation and wound healing among diabetic ulcer subjects. Int J Diabetes Res 2017;6(3):47–53.
- Gomes Neto M, Ogalha C, Andrade AM, et al. A systematic review of effects of concurrent strength and endurance training on the health-related quality of life and cardiopulmonary status in patients with HIV/AIDS. Biomed Res Int. 2013;2013:319524.
- Gomes-Neto M, Conceição CS, Oliveira Carvalho V, et al. A systematic review of the effects of different types of therapeutic exercise on physiologic and functional measurements in patients with HIV/AIDS. Clinics (Sao Paulo). 2013;68(8):1157–1167.
- Heissel A, Zech P, Rapp MA, et al. Effects of exercise on depression and anxiety in persons living with HIV: a meta-analysis. J Psychosom Res. 2019;126:109823.
- Voigt N, Cho H, Schnall R. Supervised physical activity and improved functional capacity among adults living with HIV: a systematic review. J Assoc Nurses AIDS Care. 2018;29(5):667–680.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
- Yahiaoui A, McGough EL, Voss JG. Development of evidence-based exercise recommendations for older HIV-infected patients. J Assoc Nurses AIDS Care. 2012;23(3):204–219.
- Grace JM, Semple SJ, Combrink S. Exercise therapy for human immunodeficiency virus/AIDS patients: guidelines for clinical exercise therapists. J Exerc Sci Fit. 2015;13(1):49–56.
- Riebe D, Ehrman JK, Liguori G, et al. ACSM's Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia: Wolters Kluwer; 2018.
- Perri MG, Anton SD, Durning PE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002;21(5):452–458..
- Prout EC, Mansfield A, McIlroy WE, Brooks D. Patients' perspectives on aerobic exercise early after stroke. Disabil Rehabil. 2017;39(7):684–690.
- Higgins JT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London, UK: The Cochrane Collab; 2011.
- Smith BA, Neidig JL, Nickel JT, et al. Aerobic exercise: effects on parameters related to fatigue, dyspnea, weight and body composition in HIV-infected adults. AIDS. 2001;15(6):693–701.
- Neidig JL, Smith BA, Brashers DE. Aerobic exercise training for depressive symptom management in adults living with HIV infection. J Assoc Nurses AIDS Care. 2003;14(2):30–40.
- Lox CL, McAuley E, Tucker RS. Aerobic and resistance exercise training effects on body composition, muscular strength, and cardiovascular fitness in an HIV-1 population. Int J Behav Med. 1996;3(1):55–69.
- Maduagwu SM, Kaidal A, Gashau W. Effect of aerobic exercise on CD4 cell count and lipid profile of HIV infected persons in North Eastern Nigeria. J AIDS Clin Res. 2015;06 (10):508.
- Anandh V, D’Sa IP, Alagesan J. Effect of aerobic and progressive resistance training on functional capacity, quality of life and CD4 count in people with HIV/AIDS. GJRA. 2014;3(7):226–231.
- Aweto HA, Aiyegbusi AI, Ugonabo AJ, et al. Effects of aerobic exercise on the pulmonary functions, respiratory symptoms and psychological status of people living with HIV. J Res Health Sci. 2016;16(1):17–21.
- Baigis J, Korniewicz DM, Chase G, et al. Effectiveness of a home-based exercise intervention for HIV-infected adults: a randomized trial. J Assoc Nurses AIDS Care. 2002;13(2):33–45.
- Grigoletti SS, Ribeiro JP, Sprinz E, et al. Short-term folinic acid supplementation and aerobic exercise improve vascular reactivity in HIV-infected individuals. HIV Clin Trials. 2018;19(4):148–151.
- Jaleta GΤ, Mondal S, Abdulkedar M, et al. Effects of aerobic exercise on CD4 count among people living with HIV/AIDS in Nekemte, Ethiopia. Int. J. Immunol. 2018;6(3):43–47.
- John DO, Tella BA, Olawale OA, et al. Effects of a 6-week aerobic exercise program on the cardiovascular parameters, body composition, and quality of life of people living with human immune virus. J Exerc Rehabil. 2018;14(5):891–898.
- Lee DC, Artero EG, Sui X, et al. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010;24(4 Suppl):27–35.
- Serrão R, Piñero C, Velez J, et al. Non-AIDS-related comorbidities in people living with HIV-1 aged 50 years and older: the AGING POSITIVE study. Int J Infect Dis. 2019;79:94–100..
- Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801.
- Swank AM, Horton J, Fleg JL, et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail. 2012;5(5):579–585.
- Poton R, Polito MD. The effects of aerobic training on the CD4 cells, VO2 max, and metabolic parameters in HIV-infected patients: a meta-analysis of randomized controlled trials. J Sports Med Phys Fitness. 2020;60(4):634–642.
- Ibeneme SC, Omeje C, Myezwa H, et al. Effects of physical exercises on inflammatory biomarkers and cardiopulmonary function in patients living with HIV: a systematic review with meta-analysis. BMC Infect Dis. 2019;19(1):359.
- Gomes-Neto M, Saquetto MB, Alves IG, et al. Effects of exercise interventions on aerobic capacity and health-related quality of life in people living with HIV/AIDS: systematic review and network meta-analysis. Phys Ther. 2021;101(7):pzab092.
- Fitzpatrick ME, Kunisaki KM, Morris A. Pulmonary disease in HIV-infected adults in the era of antiretroviral therapy. AIDS. 2018;32(3):277–292.
- Hull MW, Phillips P, Montaner JSG. Changing global epidemiology of pulmonary manifestations of HIV/AIDS. Chest 2008;134(6):1287–1298.
- Calligaro GL, Gray DM. Lung function abnormalities in HIV-infected adults and children. Respirology. 2015;20(1):24–32.
- Crothers K, Butt AA, Gibert CL, et al. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006;130(5):1326–1333.
- Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–395.
- Nsagha DS, Assob JC, Njunda AL, et al. Risk factors of cardiovascular diseases in HIV/AIDS patients on HAART. TOAIDJ 2015;9(1):51–59.
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289(19):2560–2571.
- Ibeneme SC, Irem FO, Iloanusi NI, et al. Impact of physical exercises on immune function, bone mineral density, and quality of life in people living with HIV/AIDS: a systematic review with meta-analysis. BMC Infect Dis. 2019;19(1):340.
- Pedro RE, Guariglia DA, Peres SB, et al. Effects of physical training for people with HIV-associated lipodystrophy syndrome: a systematic review. J Sports Med Phys Fitness. 2017;57(5):685–694.
- Nedelcovych MT, Manning AA, Semenova S, et al. The psychiatric impact of HIV. ACS Chem Neurosci. 2017;8(7):1432–1434.
- Higgins JPT, Thomas J, Chandler J, eds., et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane. www.training.cochrane.org/handbook. Published 2021 (Accessed: 17 Aug 2021).