Abstract
Objectives: To evaluate the impact of a treatment switch to dolutegravir plus lamivudine on the soluble inflammatory biomarkers of HIV-infected patients treated in a real-life setting.
Materials and methods: This was a longitudinal study that enrolled virologically-suppressed patients on stable 3-drug ART who switched at baseline to dolutegravir + lamivudine (2DR-group), based on the clinician’s decision, or maintained triple therapy (3DR-group). Subjects in the 3DR-group were matched with those in the 2DR-group for age, gender and type of anchor drug. Plasma levels of interleukin-6 (IL-6), I-FABP, D-dimer and C-reactive protein (CRP) were quantified by a microfluidic ultrasensitive ELISA assay at baseline and at 48 weeks.
Results: Overall 208 subjects were enrolled: 101 in the 2DR-group and 107 in the 3DR-group. At baseline, biomarker levels were comparable between groups. The differences in mean log10 change from baseline to 48 weeks between groups (2DR versus 3DR) were: IL-6 (pg/L) −0.051(95% CI −0.115/0.009) versus 0.004 (95% CI −0.046/0.054) (p = 0.159); I-FABP (pg/mL), −0.088 (95% CI −0.14/-0.041) versus 0.033 (95%CI −0.007/0.072) (p < 0.001); D-dimer (pg/mL), −0.011(95% CI-0.055/0.033) versus −0.021 (95% CI −0.071/0.030) (p = 0.780) and CRP (pg/mL), −0.028 (95%CI −0.118/0.063) versus 0.118 (95% CI 0.024/0.211) (p = 0.028).
Conclusions: At 1 year, switching to a dolutegravir plus lamivudine dual regimen in this setting showed a favorable trend for two biomarkers analyzed, i.e., I-FABP and CRP, as compared to continuing a triple therapy. These results add important new data in support of the safety of this approach in terms of its effect on the inflammatory milieu.
Introduction
The use of two-drug regimens is indicating a new era of significant changes in the management of antiretroviral therapy (ART) for people living with HIV (PLWH). Indeed, these therapies aim to combine virological efficacy with limited long-term toxicity associated with the use of nucleos(t)ide reverse transcriptase inhibitors (NRTIs), limited adverse effects and reduced costs when compared to standard three-drug ART.Citation1,Citation2
Within this context, the combination of dolutegravir plus lamivudine has shown excellent viroimmunological properties in both naïve and ART-experienced PLWH in clinical trials and observational studies.Citation3–11 Moreover, when compared to other virologically effective two-drug combinations, dolutegravir plus lamivudine has several advantages in terms of lower risk of drug interactions and metabolic complications.Citation1,Citation2 Thus, this strategy currently constitutes the most widely prescribed two-drug regimen. However, the impact of this simplification strategy on HIV-related so-called “residual inflammation” must still be fully determined.
Despite viral suppression, PLWH still present elevated concentrations of soluble biomarkers of systemic inflammation that decrease but do not normalize with ART.Citation12,Citation13 This condition of persistent inflammation is the result of multifactorial mechanisms, including low-level viral replication, microbial translocation or direct effects of antiretrovirals on inflammatory pathways.Citation14 Furthermore, residual inflammation can have a clinical impact, according to evidence which supports the independent association between inflammation markers and clinical outcome in treated individuals.Citation15–17 Thus, it is important to consider the relative impact of novel dual treatment strategies on these biomarkers. Dual therapy and triple therapy could affect systemic inflammation differently through several mechanisms that have been hypothesized to contribute to residual inflammation. On one hand, the use of fewer drugs could hypothetically account for the loss of control over enduring inflammation, given the reduced pharmacological pressure on residual viral replication; on the other hand, they could lead to less drug-induced oxidative stress, contributing to the containment of inflammation.Citation18
Here, we aimed to investigate changes in the levels of soluble inflammation markers following a treatment switch to dolutegravir plus lamivudine versus maintaining a triple standard combination in virologically-suppressed HIV-infected patients in a real-life setting.
Materials and methods
Study design and participants
This longitudinal study was carried out between August 2019 and May 2021 in a cohort of PLWH who frequent the healthcare facilities of the Department of Infectious Diseases of the University Hospital “Fondazione Policlinico Universitario A. Gemelli IRCCS” in Rome. Eligible subjects were on a stable (>48 weeks) 3-drug ART regimen (anchor drug + 2 NRTI backbone) with plasma HIV-RNA <50copies/mL for ≥48 weeks and CD4 > 200 cells/mm3. The study group (2-drug regimen, 2DR) consisted of patients who switched to dolutegravir 50 mg + lamivudine 300 mg once daily based on the clinician’s decision. For the control group (3 drug regimen, 3DR), we enrolled HIV-infected outpatients who fulfilled the above-mentioned inclusion criteria and maintained their triple therapy. They were matched with the subjects of the study group according for age, gender, and type of anchor drug. Patients were followed from baseline (BL), which was defined as the date of the treatment switch for the 2DR group and the date of enrollment of the 3DR group, to 48 weeks (W48). Virological rebound was defined as any HIV-RNA determination ≥50 copies/mL that occurred during the follow-up. Changes from BL to W48 in systemic inflammation status were assessed by measuring, at the two time points, the plasma levels of two markers of inflammation, i.e., interleukine-6 (IL-6) and C-reactive protein (CRP), a marker of pro-coagulation (D-dimer) and a marker of enterocyte damage/microbial translocation (I-FABP). The clinical and viro-immunological parameters obtained for each patient in our database at the same time points were also evaluated. The study was conducted in accordance with the Good Clinical Practice and ethical principles of the Declaration of Helsinki. The protocol was reviewed and approved by our local Ethics Committees (1697/20 ID2950/2020). All participants gave their written informed consent.
Biomarker measurements
Blood samples were collected in tubes with EDTA. Plasma was aliquoted and stored at −80 °C until analysis. The levels of IL-6, CRP, D-dimer and I-FABP were measured by using an automated microfluidic ultrasensitive enzyme-linked immunosorbent assay, by the protein simple plex technology,Citation19 on the ELLA instrument (ELLA microfluidic analyzer, Protein Simple, Bio-Techne, Minneapolis, USA), according to the manufacturers’ instructions. Samples were run at 2-fold dilution for IL-6 and I-FABP, at 20-fold dilution for D-dimer and at 2000-fold dilution for CRP. The range of quantitation and lower limits of detection were, respectively: for IL-6 0.28-2652 pg/mL and 0.10 μg/mL, for I-FABP 31.1-118,560 and 5.66 pg/mL, for D-dimer 142-540,890 and 36.4 pg/mL, and for PCR 32.8-8000 and 1.24 pg/mL.
Statistical analysis
Given our sample size, and assuming a two-sided type-1 error of 5% and a medium effect size (0.5), we used post hoc analysis to calculate a power of 82%.
Baseline patient characteristics were summarized using standard descriptive statistics, with number and percentage for binary and categorical outcomes, and mean with 95% confident intervals (95%CI) or median with interquartile range (IQR) for continuous variables. Biomarker levels were log10-transformed to approximate normal distribution. Comparisons between groups were performed using Student’s t-test or the Mann-Whitney U test for independent continuous variables and comparisons within groups were analyzed using the paired-sample t test or the Wilcoxon test, as appropriate. Chi-squared or Fisher’s exact or McNemar’s test were used for categorical variables. Linear regression models were used to explore the association between the changes in inflammatory biomarker levels and patients’ demographic, clinical and therapeutic variables: variables significantly associated (p < 0.05) with biomarker changes in the univariable analysis were then included in a multivariable model.
Moreover, to further investigate if there was an effect on inflammation due to the antiretroviral drug class, we performed a subgroup analysis only on the patients with a baseline INSTI-based triple regimen, therefore excluding NNRTI- and PI-based baseline regimen.
The correlations between delta changes of the biomarkers were estimated by a Pearson correlation coefficient.
A p value lower than 0.05 was considered statistically significant. Data analysis and management was performed using the SPSS software version 22.0 (IBM, SPSS, Chicago, IL, USA).
Results
Overall, 208 subjects were enrolled: 101 in the 2DR-group and 107 in the 3DR-group. All patients completed the follow-up. The characteristics of the patients at BL are summarized in .
Table 1. Baseline characteristics of patients.
When compared, the two groups were homogeneous for all demographic and clinical characteristics, except for the CD4 cell count, which was significantly higher in the 2DR-group. The main reason for switching to dolutegravir plus lamivudine was a pro-active treatment switch (82.2%); secondary reasons included toxicity (7.0%) and drug interactions (2.0%).
The mean baseline levels of the biomarkers in the 2DR-group and the 3DR-group were comparable: IL-6, 3.45 (95% CI 3.39, 3.51) versus 3.47 (95% CI 3.41, 3.52) log10 pg/L (p = 0.743); I-FABP, 3.10 (95% CI 3.06, 3.15) versus 3.04 (95% CI 2.99, 3.09) log10 pg/mL (p = 0.081); D-dimer, 5.54 (95% CI 5.47, 5.60) versus 5.58 (95% CI 5.51, 5.65) log10 pg/mL (p = 0.389); and CRP 6.29 (95% CI 6.19, 6.39) versus 6.24 (95% CI 6.13, 6.35) log10 pg/mL (p = 0.533).
At W48, we observed a significant decrease in I-FABP levels in 2DR as compared to BL, whereas mean levels of CRP showed a weak increase in 3DR. We did not find significant changes for other biomarkers within each group ().
Figure 1. Evolution of inflammation markers in the 3DR-group and in the 2DR group after 48 weeks of follow-up. Dot plots represent the distribution of biomarker levels. Central horizontal bars represent the mean values and error bars represent the 95% CIs. BL, baseline; W48, 48 weeks.
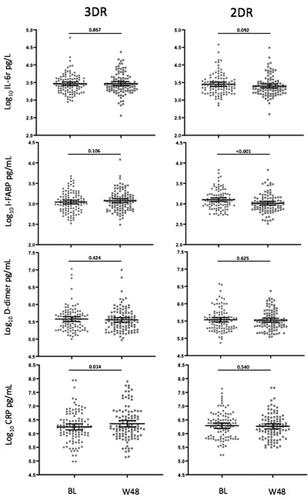
The differences in mean log10 change from BL to W48 between groups (2DR versus 3DR) were: IL-6 (pg/L) −0.051 (95% CI −0.115, 0.009) versus 0.004 (95% CI −0.046, 0.054)(p = 0.159); I-FABP (pg/mL), −0.088 (95% CI −0.14, −0.041) versus 0.033 (95%CI −0.007, 0.072)(p < 0.001); D-dimer (pg/mL), −0.011 (95% CI-0.055, 0.033) versus −0.021 (95% CI −0.071, 0.030)(p = 0.780) and CRP (pg/mL), −0.028 (95%CI −0.118, 0.063) versus 0.118 (95% CI 0.024, 0.211)(p = 0.028). shows the comparison between groups for viro-immunological and laboratory parameters at W48.
Table 2. Characteristics of patients at W48.
The virological profile was comparable to that observed at BL. Most patients maintained HIV-RNA <50 copies/mL: only two patients in 2DR exhibited a single virological rebound, HIV-RNA 65 and 54 cps/mL, respectively. Moreover, the proportion of patients with undetectable residual viremia remained similar in both groups. The CD4 cell count was still higher at W48 in 2DR as compared to 3DR; however, interestingly the CD4/CD8 ratio showed a statistically significant increase in both the 2DR (+0.035, CI −0.06, +0.12, p = 0.038) and the 3DR (+0.048, CI −0.05, +0.12, p = 0.001) group, as compared to BL. An improvement of the lipid profile was also observed at W48 in 2DR, with a decay both in the levels of total cholesterol (mg/dL) (-5.01, CI −23.7, +13,0, p = 0.046) and triglycerides (mg/dL) (-17.6, CI −50.8, +10.7, p = 0.004).
Supplemental Table S1 shows the association between the changes in I-FABP and CRP levels and patients’ demographics, clinical and therapeutic variables. By linear regression analyses, the delta change in CRP levels from W48 to BL was not correlated with any of the variables examined; however, a minor decrease in I-FABP levels was independently associated with older age in the multivariate analysis.
The subgroup analysis including only the patients with a INSTI-based regimen at baseline (70.7%) confirmed the results observed in the entire population for I-FABP, D-dimer and CRP, whereas for IL-6 a favorable trend in the 2DR group compared to 3DR group was observed (Supplemental Table S2).
Significant positively correlations were found between IL-6 and D-dimer (0.152, p = 0.003), IL-6 and CRP (0.389, p < 0.001) and CRP and D-dimer (0.187, p = 0.007).
Discussion
Current data reporting the effects of dolutegravir plus lamivudine maintenance therapy on residual inflammation include the exploratory data from the TANGO trial, in which virally-suppressed subjects were randomly assigned to receive either a dolutegravir plus lamivudine dual regimen or continue a TAF-based 3- or 4-drug regimen. These data showed uncertain effects on the soluble inflammatory biomarkers, given significant differences observed in differing directions in the two study groups.Citation9 However, it must be considered that our study evaluated the switching to dolutegravir plus lamivudine regimen compared with continuing any 3-drug ART, unlike the TANGO study included only individuals treated with TAF-based regimens.
Furthermore, recent data from the SALSA trial showed that the changes in inflammatory profile favored the switch to the dolutegravir/lamivudine regimen for one of the four biomarkers analyzed over 48 weeks, as compared to continuing an effective 3-drug therapy.Citation20
In our study, which was performed in a real-life setting, we observed that the treatment switch to dolutegravir plus lamivudine had a favorable impact on the trend for two of the four biomarkers analyzed at one year, i.e., CRP and I-FABP, when compared to continuing a standard triple therapy. Clearly, compared to clinical trials,Citation9,Citation20 the patients included in our study were those who switched to dual therapy while they were under clinical care in our center. Thus, their characteristics (i.e., long-duration of treatment, past antiretroviral treatment history or past virological failure) provided a snapshot of “real life” data that could be different from that observed in clinical trials and this should be considered when interpreting our results.
Overall, our results indicate that, despite a reduction in the number of active drugs taken after the treatment switch to dolutegravir plus lamivudine, this is unlikely to be a risk factor for the enduring inflammation compared to triple regimens. These data further extend the trials results to the real-world, supporting the effectiveness of this maintenance therapy in terms of its control over the residual inflammation. Further studies with a longer follow-up are warranted to further reveal the inflammatory consequences of switching to the dolutegravir plus lamivudine regimen in patients with sustained virological suppression, and better elucidate if it might be an advantage over triple therapy. In this context, updates to the TANGO trial at long term have confirmed minimal and similar changes in inflammatory biomarkers across dolutegravir plus lamivudine and 3/4DR groups through 144 weeks. Citation21
Rather, data from an observational study in the Spanish AIDS Research Network (CoRIS), seem to suggest a more favorable long-term anti-inflammatory profile in maintaining a 3-drug regimen than switching to 2DR. However, it should be noted that in this latter study the 2DR group was constituted by patients (n = 58) on heterogeneous regimens, including lamivudine + boosted atazanavir, lamivudine + boosted darunavir, dolutegravir + rilpivirine, and that only a very small percentage of patients was on dolutegravir plus lamivudine. Citation22
Interestingly, the significant decline of I-FABP in the 2DR group in our study is consistent with the findings of the SWORD studies, which showed a decrease in this parameter in subjects switching to a dolutegravir plus rilpivirine dual maintenance regimen when compared to those receiving standard triple ART. Citation23–24
I-FABP is a marker of enterocyte damage that has also been correlated with intestinal impairment and microbial translocation.Citation25 This could be important because microbial translocation is a driver of immune activation and systemic inflammation in HIV infection.Citation14 Consistently, SALSA trial showed a favorable effect of the switch to dolutegravir/lamivudine on the levels of sCD14Citation20, which is traditionally used as a marker of microbial translocation, even if it is not a direct and specific marker. Taken together, these data warrant further evaluation of the impact of the dolutegravir plus lamivudine simplification strategy on microbial translocation: additional markers of intestinal epithelial barrier damage should also be included, such as circulating lipopolysaccharide or EndoCAb, which were not analyzed in our study. However, in this setting we found correlation between the dynamics of the levels of PCR, IL-6 and D-dimer, which might reflect a common pathway of various inflammatory and immune-activation processes. On the opposite, the progress of the levels of I-FABP did not correlate to the progress of the other markers. This fact suggest that the variation of I-FABP observed in the 2DR group, might directly reflect a lower cytotoxic effects on the gut epithelial cells due to less toxic ART exposure. The increase in CRP levels in the 3DR group in our study was, at least in part, unexpected given previous findings reporting a plateau of this parameter after several years of effective therapy.Citation13 However, it was in line with other reports indicating that margins for an increase in CRP and/or other soluble inflammatory markers are still present after several years of suppressive ART,Citation9,Citation23 and it was likely related to the fact that persistent inflammation is the result of multifactorial mechanisms.
It can be hypothesized that the beneficial effect on inflammation that we observed in 2DR group could arise from the effect of ART classes, since several studies observed that integrase inhibitors may decrease inflammation and immune activation more than other antiretroviral classes.Citation26 Particularly, in switch studies changing to raltegravir-based from a PI- or NNRT-based regimen led to an improvement in inflammation and immune activation profile.Citation27–28
In our study, the subgroup analysis performed by excluding patients with NNRTI- or PI-based regimen in the baseline triple regimen confirmed the results observed in the entire population, and it even emphasized an overall amelioration in the 2DR group, including an improvement in the IL-6. Therefore, in this setting the variations observed in the biomarkers were not related to the switching from PI or NNRTI-based regimen to a INSTI-containing regimen.
Our evaluation of metabolic outcome at W48 confirms the improvement of the blood lipid profile of patients who switched from triple ART to dolutegravir plus lamivudine, which was reported in several previous studies,Citation4,Citation29 even though the high number of missing values for this parameter in our study might limit the reliability of our results.
Moreover, in our study the CD4/CD8 ratio showed a statistically significant increase in both the 2DR and the 3DR group. This finding is at variance with a previous report of an increase in the absolute number of CD8+ T cells in patients on stable triple ART who switched to NRTI-dual regimens or NRTI-sparing dual therapies.Citation30
Our study has limitations that warrant consideration. First, it was designed as a longitudinal clinical-based cohort study: as several unmeasured or uncontrolled biases can influence the results of observational non-randomized studies, this should be taken into account when interpreting our results. However, the research was strengthened by matching the study and the control group for several clinically relevant variables (i.e., age, gender, and the same anchor drug molecule), thus potentially minimizing the confounders. Moreover, the two groups at baseline were comparable for all variables except the CD4 cell count, which resulted significantly higher in the 2DR group. As a potential confounder, the CD4 cell count was explored in the regression analysis; however, no association between the CD4 cell count and changes in the inflammatory biomarkers after 48 weeks was observed in this setting.
One strength of our study is that the biomarkers quantification was performed using a microfluidic ultrasensitive enzyme-linked immunosorbent assay, i.e., the protein Simple Plex technology, a technique that has been shown to improve the accuracy and precision of the measurement as compared to traditional assays.Citation19
In summary, the present study offers a unique opportunity to assess the inflammatory consequences of switching from triple ART to dolutegravir plus lamivudine maintenance therapy in a real-world setting. We think that the reported results add important new data in support of the safety of this approach in terms of its effect on the inflammatory milieu.
Supplemental Material
Download Zip (24.2 KB)Acknowledgments
We wish to thank all the patients and clinical staff of Department of Infectious Diseases of the University Hospital “Fondazione Policlinico Universitario A. Gemelli IRCCS”, Rome, Italy.
Disclosure statement
M.F. received speakers’ honoraria and support for travel to meetings from Bristol-Myers Squibb (BMS), Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme (MSD), ViiV Healthcare, and fees for attending advisory boards from BMS, Gilead Sciences, Janssen-Cilag. A.C. received support for travel to meetings from ViiV Healthcare, A.B. received speakers’ honoraria from ViiV Healthcare, and fees for attending advisory boards from Janssen-Cilag. S.D.G. was a paid consultant or member of advisory boards for Gilead Sciences, ViiV Healthcare, Janssen-Cilag, Merck Sharp & Dohme and Bristol-Myers Squibb. All other authors: none to declare.
Data availability statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Badowski M, Pérez SE, Silva D, Lee A. Two's a company, three's a crowd: a review of initiating or switching to a two-drug antiretroviral regimen in treatment-naïve and treatment-experienced patients living with HIV-1. Infect Dis Ther. 2020;9(2):185–208.
- Rossetti B, Montagnani F, De Luca A. Current and emerging two-drug approaches for HIV-1 therapy in ART-naïve and ART-experienced, virologically suppressed patients. Expert Opin Pharmacother. 2018;19(7):713–738.
- Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143–155.
- Borghetti A, Baldin G, Lombardi F, et al. Efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multicentre cohort of patients with suppressed HIV-1 replication. HIV Med. 2018;23(7):452–454.
- Baldin G, Ciccullo A, Borghetti A, Di Giambenedetto S. Virological efficacy of dual therapy with lamivudine and dolutegravir in HIV-1-infected virologically suppressed patients: long-term data from clinical practice. J Antimicrob Chemother. 2019;74(5):1461–1463.
- Lombardi F, Belmonti S, Borghetti A, et al. Evolution of cellular HIV DNA levels in virologically suppressed patients switching to dolutegravir/lamivudine versus maintaining a triple regimen: a prospective, longitudinal, matched, controlled study. J Antimicrob Chemother. 2020;75(6):1599–1603.
- Joly V, Burdet C, Landman R, et al. Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: Results of the ANRS 167 trial (LAMIDOL). J Antimicrob Chemother. 2019;74(3):739–745.
- Taiwo BO, Marconi VC, Berzins B, et al. Dolutegravir plus lamivudine maintains human immunodeficiency virus-1 suppression through week 48 in a pilot randomized trial. Clin Infect Dis. 2018;66(11):1794–1797.
- van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide–based 3-or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis. 2020;71(8):1920–1929.
- Cento V, Perno CF. Dolutegravir plus lamivudine two-drug regimen: safety, efficacy and diagnostic considerations for its use in real-life clinical practice-a refined approach in the COVID-19 era. Diagnostics. 2021;11(5):809.
- Fabbiani M, Rossetti B, Ciccullo A, et al. Efficacy and durability of two- vs. three-drug integrase inhibitor-based regimens in virologically suppressed HIV-infected patients: data from real-life ODOACRE cohort. HIV Med. 2021;22(9)Jul:843–853. Epub ahead of print. PMID: 34318591.
- Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795.
- Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29(4):463–471.
- Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9(2):139–147.
- Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–1259.
- Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203(11):1637–1646.
- Freiberg MS, Bebu I, Tracy R, et al. D-dimer levels before HIV seroconversion remain elevated even after viral suppression and are associated with an increased risk of non-AIDS events. PLoS One. 2016;11(4):e0152588.
- Tincati C, Mondatore D, Bai F, d'Arminio Monforte A, Marchetti G. Do combination antiretroviral therapy regimens for HIV infection feature diverse T-cell phenotypes and inflammatory profiles? Open Forum Infect Dis. 2020;7(9):ofaa340.
- Aldo P, Marusov G, Svancara D, David J, Mor G. Simple Plex(™): a novel multi-analyte, automated microfluidic immunoassay platform for the detection of human and mouse cytokines and chemokines. Am J Reprod Immunol. 2016;75(6):678–693.
- Llibre JM, Brites CA Cheng CY Osiyemi O, Galera C, et al. Switching to the 2-drug regimen of dolutegravir/lamivudine (dtg/3tc) fixed-dose combination is non-inferior to continuing a 3-drug regimen through 48 weeks in a randomized clinical trial (salsa). In: 11th International AIDS Society Conference on HIV Science (IAS2021); July 18–21, 2021, Berlin, Germany, (OALB030).
- van Wyk J, Ait-Khaled M, Santos J, et al. Metabolic health outcomes at week 144 in the TANGO study, comparing a switch to DTG/3TC versus maintenance of TAF-based regimen. In: 11th International AIDS Society Conference on HIV Science (IAS2021); July 18–21, 2021, Berlin, Germany, (OPEB164).
- Serrano-Villar S, López-Huertas MR, Jiménez D, et al. Effects of switch from 3dr to 2dr on inflammatory biomarkers. In: Conference on Retroviruses and Opportunistic Infectious, March 6–11, 2021 | Virtual (Abstract n 527.)
- Aboud M, Orkin C, Podzamczer D, et al. Efficacy and safety of dolutegravir–rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1and SWORD-2 studies. Lancet HIV. 2019;6:576–587.
- María Llibre J, Cortés L, Aylott LF, et al. Inflammatory and atherogenesis markers 148 weeks postswitch to dtg + rpv in sword-1/-2. In: Conference on Retroviruses and Opportunistic Infectious, March 6–11, 2021 | Virtual (Abstract n 489).
- Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26(1):2–18.
- Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep. 2017;14(3):93–100.
- Martínez E, D’Albuquerque PM, Llibre JM, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS. 2012;26(18):2315–2326.
- Lake JE, McComsey GA, Hulgan T, et al. Switch to raltegravir decreases soluble CD14 in virologically suppressed overweight women: the Women, Integrase and Fat Accumulation Trial. HIV Med. 2014;15(7):431–441.
- Ciccullo A, Baldin G, Capetti A, et al. A comparison between two dolutegravir-based two-drug regimens as switch strategies in a multicentre cohort of HIV-1-infected patients. Antivir Ther. 2019;24(1):63–67.
- Mussini C, Lorenzini P, Cozzi-Lepri A, et al. Switching to dual/monotherapy determines an increase in CD8+ in HIV-infected individuals: an observational cohort study. BMC Med. 2018;16(1):79.
