Abstract
Background
Weight gain has been well-described with integrase strand transfer inhibitors (INSTIs) and tenofovir alafenamide (TAF). Doravirine (DOR) has been identified as a relatively “weight-neutral” drug; however, there is little data describing its effect on weight change in routine clinical practice.
Methods
We conducted a retrospective chart review of weight change among people with HIV changing from an INSTI- to a non-INSTI regimen with DOR.
Results
At the time of ART switch, among 49 people with HIV, the mean age was 47 years, 24% were female, and 75% had HIV-1 viral load <200 copies/mL. Most (55%) people with HIV were taking bictegravir/TAF/emtricitabine prior to the switch. Although 84% switched due to concerns about weight gain, only 16% had a weight gain of ≥10% in the year preceding, and 49% had no substantial change in weight. 86% switched to DOR/lamivudine/tenofovir disoproxil fumarate. A weight decrease (−2.6% [95% CI: −5.1, −0.1%, p = .041] was seen over the year following the ART switch. Weight change prior to switch was greatest in the year 2021 compared to 2019, 2020, and 2022.
Conclusions
Overall, modest changes in weight were seen following ART switch from INSTI-based regimen to a DOR-based, non-INSTI regimen. Further investigations with larger people with HIV cohorts will be helpful to guide clinical practice, while the impact of the COVID-19 pandemic on weight change should also be considered.
Introduction
Modern antiretroviral therapy (ART) has improved tolerability and side effect profiles compared to older regimens. An increasing concern of modern ART is weight gain and its associated metabolic consequences, including diabetes, metabolic dysfunction-associated steatotic liver disease (MASLD), and dyslipidemia [Citation1–4]. Weight gain has been well-described with the initiation of or switch to essentially all newer ART regimens [Citation5,Citation6]. These findings are especially well described with the newer integrase strand transferase inhibitors (INSTIs) and nucleotide reverse transcriptase inhibitors (NRTIs). Numerous studies have found greater increases in weight with dolutegravir (DTG) and bictegravir (BIC) than elvitegravir or raltegravir [Citation7–9], and with tenofovir alafenamide (TAF) compared to tenofovir disoproxil fumarate (TDF) [Citation8,Citation10–12].
Weight changes with ART initiation and switch are multifactorial. These reasons may be partly due to a ‘return to health’, removal of more weight suppressive medications, genetic factors, or changes in appetite [Citation13]. Management of weight gain in people with HIV has proven difficult, and there is a paucity of data to support whether changes in ART may mitigate weight gain. Given associations between weight gain and TAF and INSTIs, some clinicians have begun switching to non-INSTI or non-TAF regimens to attenuate weight gain [Citation14,Citation15]. An ongoing clinical trial, the DO IT Trial (ACTG Study 5391), randomizes participants to remain on INSTI + TAF or switch to doravirine (DOR) with or without a concomitant switch to TDF. DOR is a non-nucleotide reverse transcriptase inhibitor (NNRTI) available as a single-tablet or combined with lamivudine (3TC) and TDF. DOR is effective in maintaining viral suppression [Citation16] and has been identified as a relatively ‘weight neutral’ option, however, few studies describe its effect on weight among people experiencing weight gain in clinical practice [Citation16,Citation17].
Here, we sought to describe changes in weight among people with HIV changing from INSTI-based regimens to DOR-based regimens in clinical practice. We hypothesized that DOR would attenuate weight gain and possibly contribute to a decrease in weight among people with HIV changing from INSTI-based to DOR-based regimens.
Methods
Study design and participants
We conducted a retrospective chart review among people with HIV at Denver Health, a county safety net health system, and the University of Colorado Hospital, a tertiary care hospital. This study was approved via the Colorado Multiple Institutional Review Board (COMIRB) Protocol #21-4286. We first identified people with HIV receiving DOR-based ART (without INSTI) using an existing clinic registry of current ART regimens (Denver Health) or Health Data Compass (University of Colorado). Individual charts were further reviewed to ensure eligibility for inclusion and collect additional clinical characteristics.
Inclusion criteria included adults (aged 18 or older) with a diagnosis of HIV; current prescription of DOR without INSTI with a switch from INSTI-based regimen for any reason; no pregnancy in the year prior or following the switch; and body weight measures available at least one year before switch, at the time of switch, and ≥1 measurement within 12 months post switch. We included people with HIV regardless of viral load. All eligible patients with switch to DOR between January 1, 2016 to November 8, 2022 were included.
Data collection
Data was obtained by chart review and recorded using REDCap. Demographic data included age, birth sex, year of HIV diagnosis, CD4 at the time of ART switch, viral load at the time of ART switch, comorbidities, medications, and current tobacco or substance use. Comorbidities and tobacco or substance use information was collected via electronic medical record review of combined problem lists and provider notes. All medications were recorded via review of actively prescribed medication lists in the electronic medical record. Weights in kilograms were collected one year prior to the switch (±2 months), 0 months (at ART switch), and 3, 6, 9, and 12 (±2) months post ART switch. Any encounter for people with HIV with the exception of hospitalization with a documented weight was included. BMI calculations were performed and categorized using CDC categories of healthy (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), and obese (30.0 kg/m2 and above). Race/ethnicity categories were created from electronic medical record-derived race and ethnicity fields by first considering ethnicity information (Hispanic regardless of race vs non-Hispanic) and then race information (Black, Other, and White) in people identifying as non-Hispanic or where ethnicity was unreported.
Statistical analysis
Characteristics of eligible participants were summarized using basic descriptive statistics. Weight trajectories prior to ART switch were calculated as percent change in weight (kg) in the year prior to change. Participants were further classified as having stable or decreasing weight vs. an increase of ≥3% during the year prior to ART switch to explore subset characteristics. Differences by year of ART regimen change were examined due to concerns about disruptions caused by the COVID-19 pandemic and resulting shifts in clinical care and behavior.
Change in weight in the year after the ART switch was tested using linear mixed models with random intercept for people with HIV and random slope for time. Weight data were log-transformed due to distributional skew for these models. Factors associated with weight gain in the year prior to the ART switch were explored using Spearman correlations or Wilcox rank tests for continuous and categorical variables, respectively, due to distributional skew of % weight change prior to the ART switch. Clinically relevant sub-populations were identified a priori (sex at birth, race/ethnicity, categorical BMI at regimen switch, percent weight change in the year prior to the switch, regimen switch from TAF to TDF, tobacco use, hypertension, diabetes, age greater than 55 years and CD4 count at regimen switch). Observed means with 95% confidence intervals (CI) of subgroups were plotted by time point and used to identify comparisons for further investigation. To explore differences between subgroups, people with weight trajectories over the observed year were calculated, and subgroups were compared using t-tests or F-tests. The non-Hispanic, other race category was not included in the race/ethnicity subset analysis due to limited sample size (N = 2). Subgroup models were not further adjusted for potential confounders or precision variables due to limited sample size and should be considered of an exploratory nature.
Missing data patterns were examined to better understand the characteristics of those with fewer weights available; multiple imputation of missing data was not attempted due to the small size of data and limited number of variables collected. Three sensitivity analyses were conducted exploring the stability of estimates upon the removal of people with HIV with (1) extreme weight trajectories (>15% weight increase or decrease in the year after ART regimen switch), (2) initiation of ART switch due to resistance/virologic failure or (3) use of medications associated with significant weight loss or gain during the observation period (amphetamine/dextroamphetamine salts, dulaglutide, empagliflozin, phentermine, semaglutide, topiramate, metformin; megestrol, olanzapine, quetiapine). R version (4.3.0) was used for all analyses. All tests were two-sided and used p < 0.05 to indicate statistical significance; no adjustments for multiple comparisons are made.
Results
Baseline characteristics
A total of 49 people with HIV were identified as eligible for inclusion in the analyses. The mean age was 47.3 (SD 11.7) years, and a majority were male (76%) and White, non-Hispanic (51%). Twenty percent were Black, non-Hispanic, and 20% were Hispanic. At the time of ART switch, 76% were virally suppressed (defined as viral load < 200 copies/mL); viral load for those without suppression ranged from 384 to 112,000 copies/mL. The median CD4 at the time of the switch was 608 [IQR 421, 860] cells/mm3 ().
Table 1. Baseline Characteristics stratified by weight trajectory prior to switch.
ART regimens
The most common ART regimen prior to switching was BIC/TAF/emtricitabine (FTC) (55%) followed by DTG + TAF/FTC (25%); 71% switched from an initial regimen containing TAF to a regimen containing TDF. The remaining people with HIV either remained on TAF (N = 4), switched from TAF to non-TDF (N = 2), or switched from a non-TAF regimen (N = 8). The most frequent regimen that people with HIV were prescribed with the switch was DOR/3TC/TDF (86%). The most common reason for ART switch was weight gain or weight concerns (84%), while 14% switched due to comorbidities or medical conditions and 4% because of resistance (could have multiple reasons for switch) ().
Weight characteristics prior to and at ART switch
The median weight change during the 12 months prior to the switch was +2.1% (0.0, 5.5%) or 2.0 (0.0, 5.0) kg; 16% gained 10% or more during the 12 months preceding the switch. The subset who experienced ≥10% weight gain tended to be slightly younger (mean 42, SD 8 years), have a shorter median time since HIV diagnosis (7 [5.5, 8.5] years), and had a median weight increase of 15 (13, 24) kg in the year before ART switch (Appendix ).
Greater weight gain in the year prior to ART switch was associated with greater weight at the time of switch (rho = 0.31; p = .031). Age, birth sex, Hispanic ethnicity, and years of HIV infection were not significantly associated with pre-switch weight change (p > .06; Appendix ).
Most people with HIV had an obese BMI at the time of ART switch (63.3%, mean BMI 33.9 kg/m2, SD 8.2; ).
Weight characteristics following ART switch
Seventy-one percent had an available weight measurement at the 3 and 6-month follow-up times, 69% at the 9-month, and 82% at 12 months after the ART regimen switch; see for weight change trajectories and summary statistics by time point. Forty-five (92%) had one or more available weight measurements during the last 6–12 months of the follow-up period, and the majority had three or more weight measurements available during the entire year post-ART transition (65%). People with HIV that had four weight measurements recorded during follow-up were more likely to be diabetic or pre-diabetic (p = .04), have liver disease (including MASLD p = .04), and have less weight gain prior to ART switch (0 [−1, 3] vs. 3 [0, 12] kg; p = .01) when compared to those participants with fewer than four weight measurements recorded.
Figure 1. Percent change in weight following ART switch at 3, 6, 9, and 12 months. Colored lines represent the change in weight over the one year prior to ART switch. Mean and standard deviations are illustrated by box plots included for each time period.
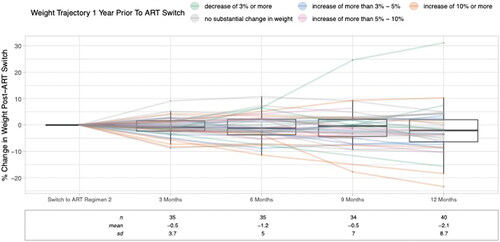
The model estimated weight change during the 1 year follow-up period was −2.6% (95% CI: −5.1, −0.1%, p = .041) relative to pre-ART switch weight, an equivalent of approximately 2.6 kg for the mean 100 kg participant in our population.
A sensitivity analysis removing three people with extreme weight trajectories after the ART switch (two that lost >15% and one that gained >15%) resulted in a similar estimate for weight change after 1 year (−2.3%, 95%CI −4.0, −0.6%); none of these extreme weight gain individuals had current prescriptions for medications associated with significant weight gain or loss. In a separate sensitivity analysis, removal of two people with HIV on medications associated with weight gain and eleven on medications associated with weight loss resulted in similar estimates, −2.5% [95%CI −5.6, 0.7%)]. A third sensitivity analysis removing two who switched ART regimens due to virologic failure also did not significantly change estimates (−2.6% [95%CI −5.1, 0.0%]) (Appendix ).
Weight change by year of switch with consideration of COVID-19 pandemic
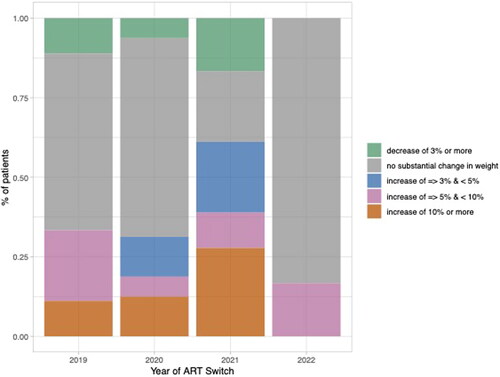
As the majority of data was collected from 2019 to 2022, we wanted to explore the impact of the COVID-19 pandemic on weight changes. Among years 2019 to 2022, the median percent weight change by year prior to the ART switch was greatest among those who switched ART in 2021 (3.7%[−1.0, 10.7%] or 3 kg [−1.0, 11.3 kg]). The median percent weight change in the year prior to the ART switch was 1.2% ([0, 3.7%] or 1.5 kg [0.0, 3.3 kg]) among those who switched ART in 2020. Additionally, more than 25% experienced a weight change of 10% or more if they switched ART in 2021 (Appendix ).
Subgroup analyses
Timepoint means with 95% CI by subgroups were plotted, and race/ethnicity, regimen switch group, BMI category at regimen switch, CD4 count at regimen switch, weight trajectory prior to ART switch, sex at birth and year of regimen switch were chosen for additional investigation (Appendix and ).
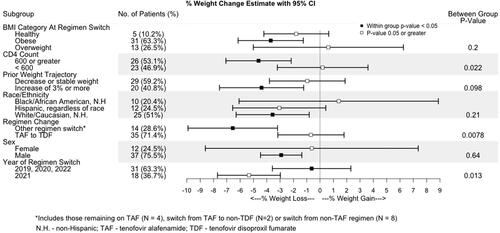
shows the results of within- and between-group comparisons after switch. People with HIV who did not switch from TAF to TDF had an estimated change of −6.6% (−9.9, −3.2%; p: 0.001) over the observed year post ART switch, while those who switched from TAF to TDF did not have a significant change in weight post ART switch; the between-group difference in change was significant (p = .008; see for patient characteristics by regimen change). People with HIV with a CD4 count of 600 or greater had an estimated weight change of −4.6% (−7.1%, −2.2%; p: <.001) in the year following the ART switch, which was significantly different from those with a CD4 count <600 at time of ART regimen change (p = .022). People with HIV switching ART in 2021 had a −5.3% weight change post ART switch (−7.7%, −3.0%), p < .001, significantly different from those not switching ART in 2021.
Figure 2. Differences in weight change estimates by different subgroups are shown in Forest plots. Significant (p < .05) changes from pre to post switch are indicated by the black box, and p values ≥.05 or greater are indicated by the white box on the Forest plot. Statistical significance for comparisons across categories are indicated in the right column. BMI (body mass index), NH (non-Hispanic), TAF (tenofovir alafenamide fumarate), TDF (tenofovir disoproxil fumarate).
Discussion
In this small retrospective description of weight change observed within routine clinical practice, we found a modest 2.6% average decrease in weight following ART switch from INSTI-based to non-INSTI, DOR-based regimens, most of which contained TDF. With a mean weight at the time of switch of approximately 100 kg in our study population, this represents a 2.6 kg decrease. Weight gain of about 1 kg per year is typically observed with increasing age, thus any decrease in weight may have some clinical relevance. However, a weight loss of 5% (i.e. a 5 kg weight loss for the average 100 kg individual in our population) is typically recommended for more clinically relevant metabolic improvements [Citation18–20]. Furthermore, we found that one of the strongest predictors of weight gain before switch was a switch in the year 2021, likely reflective of changes in diet, physical activity, and stress during the initial year of the COVID-19 pandemic ().
Figure 3. Pre-ART switch weight trajectories categorized by year of ART switch. Percentage of patients categorized into percent weight change categories or no substantial change in weight based on ART switch in years 2019, 2020, 2021 or 2022.
DOR has been described as relatively ‘weight neutral’, and may have weight suppressive effects, especially when combined with TDF [Citation21]. In a post hoc analysis of three randomized controlled trials evaluating weight change in ART-naïve people with HIV initiating treatment, similar weight gain was observed among participants randomized to TDF/FTC with either DOR, DRV/r, or EFV groups (2.4 (1.9, 2.8), 1.8 (0.8, 2.7), and 1.6 (0.9, 2.3) kg, respectively), and a majority had <5% weight gain by week 96 [Citation17]. Similar findings were observed in the DRIVE-SHIFT trial, showing modest changes in weight among ART-experienced, virologically suppressed people with HIV switching from a regimen of 2 NRTIs + a boosted PI, COBI/EVG, or an NNRTI to DOR/3TC/TDF [Citation16]. Adjusted mean weight gain was 1.4 kg at 2.8 years after the immediate switch to DOR/3TC/TDF and 1.2 kg at 2.3 years after the delayed switch.
In our subgroups, we observed a greater percent weight decrease in the year following the ART switch among those with higher CD4 T-cell count (>600 cell/µLs compared to <600). While some studies have attributed weight gain to a ‘return to health’, this was clearly not the case in our study population with a mean CD4 count of over 600 cells/µL [Citation22–24]. Additionally, we found those with more profound weight gain (>10%) in the year before ART switch had fewer years of HIV infection, which may have reflected a population who had only been exposed to INSTI-based therapy and initiated therapy at higher CD4 counts [Citation6].
Weight gain with TAF has been described in many studies [Citation6,Citation12,Citation25,Citation26], especially in the setting of a switch from TDF to TAF [Citation5,Citation12,Citation27,Citation28]. Indeed, weight gain attributed to TAF may reflect TDF's ‘weight suppressive’ effects [Citation29]. In contrast to other studies describing weight change with a switch from TDF to TAF, we found that people with HIV who switched from TAF to TDF had significantly less weight decrease than those who remained on TAF or changed to a non-TDF regimen. However, this may have represented a biased population with more older adults or persons with renal or bone disease.
Given that most people with HIV switched ART in our study period, which spanned the years 2020 to 2021, we focused on possible influences from the COVID-19 pandemic. A majority (37%) of people with HIV switched ART regimens in 2021, and weight change was greatest in the year prior to the ART switch among these individuals, compared to other years. Multiple studies have shown weight gain among the general population during the COVID-19 pandemic [Citation30–32], with stress, anxiety, boredom, and depression cited as the most frequent reasons. Those who gained ≥5% body weight tended to start with a higher BMI and were more likely to eat take-out meals and comfort foods, overeat, or binge-eat [Citation33]. Specific to people with HIV, in a study amongst Latinx and non-Latinx Black young adults with HIV in the United States, greater anxiety, stress, and weight gain were reported as a result of the COVID-19 pandemic [Citation34]. These indirect effects of the COVID-19 pandemic may have influenced weight gain in our population as well.
Lastly, we note that the overall BMI of this population was approximately 34 kg/m2. With this degree of obesity, the 1–3 kg weight changes associated with ART switch are unlikely to have significant metabolic impact, or alter obesity-related disease processes. While diet and exercise are essential components for overall health, obesity needs to be managed similar to other medical conditions, which often involves prescription of weight loss medications. Numerous barriers to prescription of these medications are present both in the HIV clinic and in general practice, including under or lack of insurance, provider comfort, polypharmacy, side effects, and potential drug-drug interactions [Citation35–37]. Our findings highlight that removal of barriers to weight loss medication prescription may be more effective for long-term weight management than ART switch.
There were many limitations of this study. Our cohort was small and included relatively few women. As women appear to experience the more weight gain with ART initiation or switch, our findings may have differed with a larger proportion of women. With the retrospective nature, we cannot rule out that those who switched to ART may have also been more motivated to make other lifestyle changes that contributed to weight change. Additionally, given many encounters occurred during the years 2020 to 2021 of the COVID-19 pandemic, there were fewer in-person clinic visits and fewer weight measurements. Weight was measured and recorded as part of routine clinical practice at each site, thus subject to variability. The strength of this study is that it is one of the first to show real-world clinical data describing weight change with a switch from an INSTI-based regimen to a DOR-based regimen.
In summary, we observed small weight decreases of 2.6% over the year following a switch from INSTI-based to DOR-based ART. Ongoing clinical trials, such as the ACTG A5391, randomizing people with HIV with suppressed HIV viral load and an obese BMI on INSTI + TAF/FTC to switch to a DOR-based regimen with or without a switch to TDF or remain on the current regimen will provide additional data. Ultimately, the most important factor in ART management is long-term adherence. If people with HIV are considering stopping therapy due to frustration with weight gain, DOR-based regimens may offer a possible weight suppressive option among those without known resistance to ensure satisfaction and viral suppression. Larger scale studies will provide greater insight into attributable weight changes with switch and provide the informed guidance needed for clinical practice and treatment guidelines.
Geolocation information
Denver, Colorado. Aurora, Colorado.
Disclosure statement
KME has received grant funding (to the University of Colorado) from Gilead Sciences, and consulting payments (to the University of Colorado) from Gilead Sciences, ViiV, and Merck.
Additional information
Funding
References
- Riebensahm C, Berzigotti A, Surial B, et al. Factors associated with liver steatosis in people with human immunodeficiency virus on contemporary antiretroviral therapy. Open Forum Infect Dis. 2022;9(11):ofac538.
- Bailin SS, Gabriel CL, Wanjalla CN, et al. Obesity and weight gain in persons with HIV. Curr HIV/AIDS Rep. 2020;17(2):138–150.
- Kuo PH, Sun HY, Chuang YC, et al. Weight gain and dyslipidemia among virally suppressed HIV-positive patients switching to co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Int J Infect Dis. 2020;92:71–77.
- Rebeiro PF, Jenkins CA, Bian A, et al. Risk of incident diabetes mellitus, weight gain, and their relationships with integrase inhibitor-based initial antiretroviral therapy among persons with human immunodeficiency virus in the United States and Canada. Clin Infect Dis. 2021;73(7):e2234–e2242.
- Erlandson KM, Carter CC, Melbourne K, et al. Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin Infect Dis. 2021;73(8):1440–1451.
- Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71(6):1379–1389.
- Bai R, Lv S, Wu H, et al. Effects of different integrase strand transfer inhibitors on body weight in patients with HIV/AIDS: a network meta-analysis. BMC Infect Dis. 2022;22(1):118.
- Sjaarda A, Bernstein A, Sparks A, et al. Comparison of weight gain after antiretroviral switch to integrase strand transfer inhibitor or tenofovir alafenamide-based therapy. Infection. 2022;50(2):407–412.
- Lake JE, Wu K, Bares SH, et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis. 2020;71(9):e471–e477.
- Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267–1274.
- Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815.
- Surial B, Mugglin C, Calmy A, et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med. 2021;174(6):758–767.
- Chandiwana NC, Siedner MJ, Marconi VC, et al. Weight gain after HIV therapy initiation: pathophysiology and implications. J Clin Endocrinol Metab. 2023;109(2):e478–e487.
- Garcia M, Martellosio JP, Giraud V, et al. Efficacy and safety of Doravirine-Based regimens in real life: a prospective monocentric French study. AIDS Res Hum Retroviruses. 2022;38(10):779–781.
- Mazzitelli M, Antoni MD, Castelli F, et al. Real-life use of doravirine in treatment-experienced people living with HIV: a multicenter Italian study. Medicine. 2022;101(30):e29855.
- Johnson M, Kumar P, Molina J-M, et al. Switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) maintains HIV-1 virologic suppression through 48 weeks: Results of the DRIVE-SHIFT trial. J Acquir Immune Defic Syndr. 2019;81(4):463–472.
- Orkin C, Elion R, Thompson M, et al. Changes in weight and BMI with first-line doravirine-based therapy. AIDS. 2021;35(1):91–99.
- Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404.
- Douketis JD, Macie C, Thabane L, et al. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond). 2005;29(10):1153–1167.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation. 2014;129(25 Suppl 2):S102–S138.
- Erlandson KM, Mohaweche R, Morrow M, et al. Energy balance and body composition after switch between integrase strand transfer inhibitors and doravirine among people with HIV. J Antimicrob Chemother. 2023;79(1):179–185.
- Yuh B, Tate J, Butt AA, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60(12):1852–1859.
- Chang E, Sekhar R, Patel S, et al. Dysregulated energy expenditure in HIV-infected patients: a mechanistic review. Clin Infect Dis. 2007;44(11):1509–1517.
- Mangili A, Murman DH, Zampini AM, et al. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006;42(6):836–842.
- Martínez-Sanz J, Serrano-Villar S, Muriel A, et al. Metabolic-Related outcomes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in adults with human immunodeficiency virus (HIV): a multicenter prospective cohort study. Clin Infect Dis. 2023;76(3):e652–e660.
- Venter WDF, Sokhela S, Simmons B, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7(10):e666–e676.
- Mallon PW, Brunet L, Hsu RK, et al. Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J Int AIDS Soc. 2021;24(4):e25702.
- Plum P-E, Maes N, Sauvage A-S, et al. Impact of switch from tenofovir disoproxil fumarate-based regimens to tenofovir alafenamide-based regimens on lipid profile, weight gain and cardiovascular risk score in people living with HIV. BMC Infect Dis. 2021;21(1):910.
- Cahn P, Sierra Madero J, Arribas JR, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy – naive adults with HIV-1 infection. AIDS. 2022;36(1):39–48.
- Bhutani S, vanDellen MR, Cooper JA. Longitudinal weight gain and related risk behaviors during the COVID-19 pandemic in adults in the US. Nutrients. 2021;13(2):671.
- Goitia J, Chen A, Patel S, et al. Factors associated with weight gain during the COVID-19 pandemic. Obes Res Clin Pract. 2022;16(2):174–176.
- Mulugeta W, Desalegn H, Solomon S. Impact of the COVID-19 pandemic lockdown on weight status and factors associated with weight gain among adults in Massachusetts. Clin Obes. 2021;11(4):e12453.
- Almandoz JP, Xie L, Schellinger JN, et al. Changes in body weight, health behaviors, and mental health in adults with obesity during the COVID-19 pandemic. Obesity. 2022;30(9):1875–1886.
- Sauceda JA, Dubé K, Harris O, et al. Brief report: the influence of the COVID-19 pandemic on the physical, social, and mental health of black and latinx young people with HIV in the United States. J Acquir Immune Defic Syndr. 2023;93(3):187–190.
- Fujioka K, Harris SR. Barriers and solutions for prescribing obesity pharmacotherapy. Endocrinol Metab Clin North Am. 2020;49(2):303–314.
- Hung A, Wong ES, Dennis PA, et al. Real world use of anti-obesity medications and weight change in veterans. J Gen Intern Med. 2023;39(4):519–528.
- Granara B, Laurent J. Provider attitudes and practice patterns of obesity management with pharmacotherapy. J Am Assoc Nurse Pract. 2017;29(9):543–550.
Appendix
Figure A1. Percent weight change from ART switch. Trends show percent weight change from 12 months prior to ART switch to 12 months post ART switch. Individual graphs show subgroups and trends in percent weight change between subgroups. ART: Antiretroviral therapy; BMI: Body Mass Index; N.H.: non-Hispanic; TAF: Tenofovir Alafenamide Fumarate; TDF: Tenofovir Disoproxil Fumarate; HIV: Human Immunodeficiency Virus; T2DM: Type 2 Diabetes Mellitus; HTN: Hypertension.
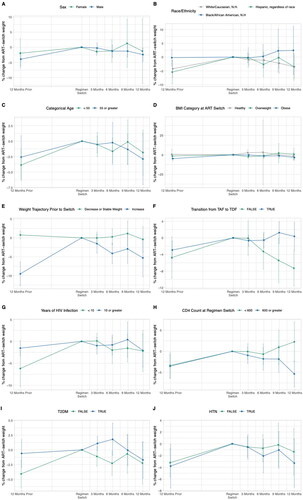
Table A1. PWH characteristics by more nuanced weight trajectory prior to ART switch.
Table A2. Pre-ART weight change characteristic testing.
Table A3. Results of sensitivity analyses.
Table A4. PWH characteristics stratified by 2021 year of switch.
Table A5. Subgroup model results.
Table A6. PWH characteristics by TDF to TAF or non-TDF to TAF Switch.
