Abstract
Background
Globally, the rate of antiretroviral therapy coverage for pregnant women living with human immunodeficiency virus (HIV) increased by 38% between 2010 and 2015 but only by 2% between 2016 and 2020.
Objectives
We aimed to determine the prevalence of vertical transmission of HIV among infants from mothers living with HIV and associated factors in the Eastern Lake Zone and Southern Highland of Tanzania from January to December 2022.
Methods
This retrospective cross-sectional study extracted data from the Open Laboratory Data Repository database collected from January to December 2022 at 93 health facilities. A total of 1,411 infants exposed to HIV from the Mbeya (851), Songwe (304), and Mara regions (256) were enrolled.
Results
The prevalence for vertical transmission of HIV was 2.48% (35/1411). We observed a non-significant difference in the prevalence of vertical transmission in children whose first test was done below six weeks of life (1.89%) and other age groups (2.52-2.62%) (p < 0.917). Children not given antiretroviral prophylaxis had eleven times higher odds of acquiring infection (AOR 11.39, 95% CI: 3.61–35.97). Mothers who were not on ART during pregnancy had three times the odds of transmitting HIV to their infants (AOR 3.03, 95%CI: 0.91–10.15).
Conclusions
We found a low prevalence of vertical transmission of HIV compared to previous studies done in Tanzania. The use of ART prophylaxis for infants exposed to HIV is significantly associated with the low rate of HIV transmission.
Background
Globally, the rate of antiretroviral therapy (ART) coverage for pregnant women living with human immunodeficiency virus (HIV) increased by 38% between 2010 and 2015 but only by 2% between 2016 and 2020 [Citation1]. ART coverage among pregnant women increased globally from 17% in 2010 to 85% in 2020, ranging from 95% in eastern and southern Africa to 25% in the Middle East and North Africa [Citation2]. Tanzania has been implementing the prevention of vertical transmission program since 2000 to eliminate new HIV infections among children and enhance care for mothers, newborns, and children [Citation3]. The program involves the use of village/ward and council leaders to promote the prevention of mother-to-child transmission (PMTCT) services, male engagement in reproductive health, and HIV/AIDS couple testing [Citation4]. Tanzania has made significant progress in preventing vertical transmission of HIV, with an estimated 100% of pregnant women living with HIV receiving antiretroviral (ARV) in 2019, according to UNAIDS’ 2020 Country Progress Report [Citation5]. Other factors associated with HIV vertical transmission were infant feeding, infants’ ages, mother’s age, marital status, level of education, gravidity, antenatal clinic visit, and delivery method [Citation6].
ARV reduce the replication of the virus. They can prevent vertical transmission of HIV by either decreasing plasma viral loads in pregnant women or by providing post-exposure prophylaxis to newborns [Citation7,Citation8]. Infants and their families may benefit from early infant HIV diagnosis (EID) by facilitating early access to HIV treatment, enhancing newborn health, and lowering infant mortality [Citation9]. This study aimed to determine the prevalence of vertical transmission of infants exposed to HIV and the associated factors using secondary data from the Open Laboratory Data Repository (OpenLDR) database for the year 2022.
Methods
Study design and setting
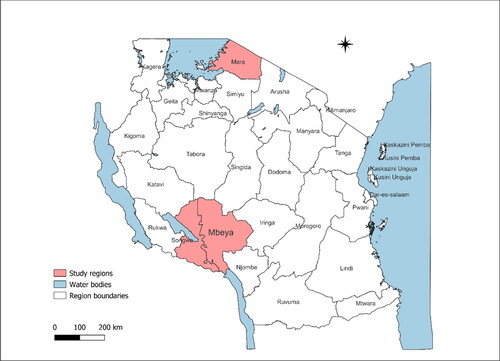
We did a retrospective cross-sectional study by analysing data from the OpenLDR for EID collected from January to December 2022. The study involved 93 healthcare facilities from three regions in the Eastern Lake and Southern Highland zones in Tanzania, including Mara (51), Mbeya (28), and Songwe region (14) (). These regions were chosen based on the high number of healthcare facilities reported in the OpenLDR database.
Study participants
The study participants included non-repeating children with dried blood spot (DBS) samples tested for first testing of HIV using Deoxyribose Nucleic Acid- Polymerase Chain Reaction (DNA-PCR). The country guideline for the prevention of vertical transmission of HIV elaborates that in infants exposed to HIV, DNA-PCR is required to confirm HIV infection. PCR tests are done at six weeks of age or any time after six weeks when a healthcare worker first sees the child.
Mother to child prevention of HIV in Tanzania
As per National guidelines of 2019, pregnant or breast-feeding women start a lifelong ART at the time of diagnosis. The recommended first-line regimen is a fixed-dose regimen of Tenofovir (TDF)+Lamivudine (3TC)+Dolutegravir (DTG). The combination of TDF + 3TC + Efavirenz (EFV) may be an option during the pre-conception period through the first eight weeks of pregnancy to avoid the potential risk of neural tube defects and among those who do not tolerate DTG. Infants exposed to HIV receive NVP syrup prophylaxis after birth (within 6 –12 h) for six weeks. Infants at high risk receive enhanced postnatal prophylaxis (ePNP) for 12 wk. Infants exposed to HIV should exclusively breastfeed for six months and continue breastfeeding until one year. At six months, infants should begin complementary foods. Infants who are HIV-positive continue breastfeeding for at least two years [Citation10].
Variables
The independent variables for HIV vertical transmission included demographic and clinical factors. The infants’ demographic factors included age, sex, and residence. The clinical characteristics included the child’s prophylaxis given since birth and the maternal ART taken during pregnancy. We included types of breastfeeding (exclusive breastfeeding, complementary feeding, and replacement feeding). We defined exclusive breastfeeding as feeding with breast milk only below six months. Feeding with formula milk below six months is termed replacement feeding, while complementary foods are given with breast milk from 6 months and above until cessation of breastfeeding. The data were collected during the antenatal care visits, one before 16 wk, at 20-24 wk, at 28-32 wk, and 36 wk of gestation.
Data source
Socio-demographic data were collected at the time of sample collection. Clinical characteristics, including child ART regimen and feeding methods, referred from childbirth to sample collection. Mothers’ ART regimen was considered during pregnancy to sample collection. The data were extracted from OpenLDR and exported to a Microsoft Excel sheet. HIV DNA-PCR results were also extracted from the database. OpenLDR is the national database that collects and integrates testing information from laboratory information systems for data repository and dashboard visualisation.
Study sample size
The sample size used was 1411 infants exposed to HIV from pregnant women living with HIV whose data were completed in the database and enrolled in the study.
Laboratory procedures
Samples collection
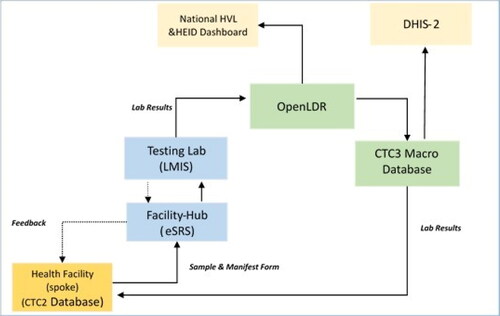
Samples from infants exposed to HIV were collected using DBS kits. Three to five blood spots, 75 μL each, were collected at care and treatment centres (spokes). DBS samples were then sent to Hub, where the quality of samples was checked and taken to laboratories for testing. Results were transmitted via Hub to the care and treatment centres (spokes) where samples were collected. The national turnaround time from sample collection to results back to the care and treatment centres is fourteen days. The open-LDR dataset collects information on the date and time of the sample collection, the sample received at the testing laboratory, and the results approved and sent to the facility. Data is then sent to OpenLDR for EID surveillance visualisation (). Samples collected from the three regions were tested at Mbeya Zonal Referral Hospital and Bugando Medical Centre, where a conventional laboratory is centralised. The HIV DNA-PCR positive results were repeated for the same sample before being reported. After an initial positive DNA-PCR, another sample is collected for a confirmatory test. If the second (confirmatory) DNA-PCR is negative, a third DNA-PCR is performed before considering ART interruption to a child. The indeterminate and error results are repeated during the testing, and the final results are reported accordingly.
Figure 2. Illustrates the electronic laboratory data flow.
HVL: HIV Viral Load; HEID: HIV Early Infant Diagnosis; OpenLDR: Open Laboratory Data Repository; DHIS2: District Health Information System; LMIS: Laboratory Management Information System; eSRS: Electronic Sample Referral System; CTC: Centre for Treatment and Care.
Data analysis
We performed data analysis using Stata software version 15.1. Categorical variables are presented using frequencies and proportions and the median (Interquartile range) for continuous variables. Pearson’s chi-square test was used to compare categorical variables. We performed a logistic regression analysis to determine the factors associated with the vertical transmission of HIV. Variables with a p-value of < 0.20 in the bivariate analysis and those reported to have an association with vertical transmission were included in the multivariable model using forward selection. Variables in the multivariable model were the age of infants, Sex, child feeding methods, Child prophylaxis and maternal regimen. Crude and adjusted prevalence ratios with their corresponding 95% confidence intervals (CI) were presented, and a significance level of p ≤ 0.05 was used.
Patient and public involvement
Patients and the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Results
Participants’ characteristics
A total of 1411 HIV-exposed children were included in the study. For most children, 762 (54.00%) were between seven weeks to nine months, with a median of 1.9 months (IQR: 1.5-9.2), while half of the participants, 709 (50.25%), were female. A high proportion of children, 1029 (72.93%), were exclusively breastfed only at a median age of 1.6 months, and most children, 1359 (96.31%), were given ARV prophylaxis up to six weeks of age. Most mothers, 1358 (96.24%), were on ART during pregnancy ().
Table 1. Participants’ characteristics and prevalence of vertical transmission of HIV among infants exposed in Mbeya, songwe, and Mara regions (N = 1411).
Vertical transmission among HIV-exposed infants
The overall prevalence of HIV infection in infants exposed was 2.48% (35/1411) at diagnosis. The proportion of vertical transmission of HIV was significantly higher among mothers who did not receive ART, 12/53(22.64%) (p < 0.001). Children not given ARV prophylaxis up to six weeks of age had a significantly higher proportion of acquiring the HIV infection, 14/52 (26.92%) (p ≤ 0.001). We observed a non-significant difference in the prevalence of vertical transmission in children whose first test was done below six weeks of life (1.89%) and other age groups (2.52-2.62%) (p = 0.917) ().
Factors associated with vertical transmission of HIV
The use of ART prophylaxis among infants exposed to HIV was independently associated with vertical transmission of HIV infection. Children who were not given ARV prophylaxis up to six weeks of age had eleven times higher odds of acquiring HIV infection (AOR 11.39, 95% CI: 3.61 − 35.97). Mothers who were not on ART during pregnancy had three times the odds of transmitting HIV infection to their infants (AOR 3.03, 95%CI: 0.91–10.15) ().
Table 2. Bivariate and multivariate logistic regression analysis for the factors associated with vertical transmission among infants exposed to HIV (N = 1411).
Discussion
The current study found that the overall prevalence of vertical transmission of HIV infection was 2.48% at the time of first testing. The majority of infants exposed to HIV and their mothers were using ART. We found minimal risk of vertical transmission for both infants exposed to HIV and for mothers who used ART during pregnancy and after delivery, which reflects the benefit of ART.
This study found that the prevalence of vertical transmission of infants exposed to HIV was lower compared to the study done at Kilimanjaro, Tanzania, which revealed a prevalence of 9.6% [Citation11]. The time difference could explain the variation, as the study at Kilimanjaro was conducted nine years ago when PMTCT implementation was minimal compared to recent years. However, vertical transmission rates similar to our study have been reported in a study conducted in Yaoundé, Cameroon (2.8%) and in a European collaborative study (2.87%) [Citation12,Citation13]. Our findings reflect the impact of the country’s effort to reach 90-90-90% HIV targets [Citation14]. However, developed countries like Italy and France have reported a low prevalence of 1.3% [Citation15,Citation16]. A stable and sustainable health system with more healthcare resources in developed countries could contribute to the variation of HIV vertical transmission in our country.
We found a high prevalence (27%) of HIV transmission among infants who were not using ARV prophylaxis up to six weeks of age; this was lower than the studies conducted in Nigeria, which revealed a 52.1% prevalence [Citation17]. This shows that failure to provide postpartum ARV treatment for babies who are HIV-exposed increases the risk of perinatal HIV transmission. Furthermore, a higher transmission rate (22.64%) was also noted in a group of mothers who were not on ART treatment during pregnancy, which was higher compared to the prevalence of 9.3% reported in Nigeria [Citation17]. Higher rates of transmission call for more emphasis on the importance of ART use during pregnancy and post-delivery to prevent HIV transmission.
Our study revealed that infants on exclusive breastfeeding had a lower prevalence of HIV transmission (2.24%), while infants who were given complementary foods had a higher prevalence of HIV transmission (3.23%). This indicates that the antigens found in non-breast milk may enhance intestinal wall permeability, resulting in inflammation and increasing the risk of HIV transmission [Citation18]. Our study showed a slightly lower prevalence for exclusive breastfeeding than Nigeria’s, 2.7% at six weeks and 11.8% at six months, respectively [Citation19]. Our findings suggest that the increase in prevalence among infants on complementary foods may be due to a longer duration of exposure to HIV via breastmilk, possible missed diagnosis at a younger age because they were not tested early; their HIV status was unknown. However, the difference was not statistically significant.
Children tested above six weeks of life had a high prevalence of HIV transmission (2.5-2.6%). Our finding in the proportion of HIV positive at the age above six weeks was lower than the report in the Ethiopian study of 15.8% [Citation20]. Our study findings suggest that despite a low HIV prevalence noted in infants exposed to HIV, the slight difference at the time of test may suggest some infection after six weeks of age. The national standard time of testing for infants exposed to HIV is below six weeks of age; however, our findings suggest that if all children were tested below six weeks, they could have a low HIV prevalence. Our study found that almost 69% of children were tested less or equal to nine months. Therefore, DNA PCR testing was achieved by 69% to confirm HIV infection in children at six weeks or at any time ≤ 9 (months) as a first test [Citation10]. The findings indicate a need to strengthen the application of the guidelines.
We found that the use of ART prophylaxis to exposed infants was significantly associated with the low vertical transmission rate. The vertical transmission of HIV from mothers not on ART during pregnancy was an almost threefold increase. This was lower than a study in Ethiopia, which found that vertical transmission for mothers not on ART had a fivefold increased odds [Citation21]. The finding was still lower than other studies in Ethiopia, which revealed a fifteen-fold likelihood of transmission [Citation22]. Our study revealed that infants without ARV prophylaxis had eleven times of the odds of contracting HIV; our findings were lower than that of late transmission in the Yaoundé, Cameroon study [Citation23]. Thus, our study showed a trend that ART treatment reduces the chance of vertical transmission of HIV to infants.
Strengths and limitations of this study
Our study has some limitations because of the use of secondary data, including missing important variables, maternal demographics and clinical characteristics like age, Viral load, and ART adherence rate, making it difficult to interpret the findings. The findings may be associated with biases from non-representative participants because data were collected from the facilities with data completeness. We were faced with challenges in controlling for inconsistencies and missing variables, which are significant in assessing the risk of vertical transmission of HIV. The missing follow-up testing outcomes for infants exposed to HIV who tested negative from the first test, and we were unable to conclude the final status of the HIV DNA-PCR test for those who tested negative after the first test. It was difficult to establish when the HIV infection occurred after the child missed a first test at the age below six weeks and the contribution factors of infections. We were unable to identify the infants who were given mixed food from the complementary feeding group. We were unable to differentiate between utero and perinatal HIV transmission. However, the study provides preliminary insights into the PMTCT program’s effectiveness. The large sample size we used in the study from OpenLDR, the national laboratory data repository, increased the accuracy of the findings.
Conclusions
Overall, we found a low prevalence of vertical transmission of HIV in infants exposed when compared to previous studies done in Tanzania. The use of ART prophylaxis for infants exposed to HIV is significantly associated with the low rate of HIV transmission. We recommend further studies to be done to validate the findings of this study on a larger scale.
Ethical approval
We obtained permission to use routine data from the Ministry of Health, Tanzania, Ref. No. BA.1/107/01/27B dated 30 August 2023. We anonymised the data before it was analysed to ensure patient confidentiality.
Authors’ contributors
PRT, LU, MVM, and AJ participated in the conception and study design. PRT, JNA, DJO, FMS, JM, LM, and TPM were involved in data collection and cleaning. PRT, LU, MVM, AJ were involved in data analysis, interpretation and drafting of the manuscript. MR, MAN, MM, MC, HC, GDM reviewed and aligned the manuscript to national guidelines requirements. AM, GE, ASM, RJ, AK reviewed the final manuscript. All authors read and approved the final manuscript.
Acknowledgments
We are very grateful to the Muhimbili University of Health and Allied Sciences, Ministry of Health, PMTCT department, Tanzania Field Epidemiology and laboratory management program.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data supporting this study’s findings are available from the National AIDS Control Program under the Ministry of Health.
Additional information
Funding
References
- UNICEF. HIV and AIDS global snapshot: pregnant women, children and adolescents. Bmi. 2021;2021:1–9.
- UNICEF. Elimination of mother-to-child transmission | Portal. 2023. [cited 2023 Jun 12]. Available from: https://open.unaids.org/priority/strategy-result-areas/elimination-mother-child-transmission
- MoH. Ministry of Health. 2023 [cited 2023 Oct 31]. Available from: https://www.moh.go.tz/services/19
- MoHSW. Tanzania elimination of mother to child transmission of HIV plan, 2012-2015. MoHSW. 2012; (October 2017):17–103. Available from: http://www.nbs.go.tz/nbs/takwimu/this2016-17/Tanzania_SummarySheet_English.pdf%0Ahttp://www.emtct-iatt.org/wp-content/uploads/2012/11/Costed-eMTCT-Plan-Final-Nov-20121.pdf
- Global AIDS Monitoring. Progress reports submitted by countries - United republic of Tanzania. Ctry Prog Rep. 2020;5–48. Available from: https://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2020countries
- Tarimo CS, Wu J. The first confirmed case of COVID-19 in Tanzania: Recommendations based on lesson learned from China. Trop Med Health. 2020;48(1):25.
- Volmink J, Siegfried NL, van der Merwe L, et al. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2007;(1):CD003510.pub2
- Siegfried N, et al. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane database Syst Rev]. 2011;(7). Available from: https://pubmed.ncbi.nlm.nih.gov/21735394/
- Naiwatanakul T, et al. Uptake of early infant diagnosis in thailand’s national program for preventing mother-to-child HIV transmission and linkage to care, 2008-2011. J Int AIDS Soc. 2016;19(1). Available from: https://pubmed.ncbi.nlm.nih.gov/26968214/PMCID: PMC4788772
- MoHSW. National guidelines for the management of HIV and AIDS. 7th Edition, 2019;1–303.
- Mwendo EM, et al. Effectiveness of prevention of mother-to-child HIV transmission programmes in kilimanjaro region, Northern Tanzania. Trop Med Int Health. 2014;19(3):267–274.
- Njom E, et al. Preventing HIV-1 transmission in breastfed infants in low resource settings : Early HIV infection and late postnatal transmission in a routine prevention of mother-to-child transmission program in yaounde. Cameroon. 2013;59(5):387–392.
- Thorne C, et al. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(3):458–465.
- Global, United. The Path That Ends. 2023. Available from: https://www.unaids.org/en/resources/documents/2023/global-aids-update-2023
- Galli L, Puliti D, Chiappini E, et al. Is the interruption of antiretroviral treatment during pregnancy an additional major risk factor for mother-to-child transmission of HIV type 1. Clin Infect Dis. 2009;48(9):1310–1317.
- Warszawski J, Tubiana R, Le Chenadec J, et al. Mother-to-child HIV transmission despite antiretroviral therapy in the ANRS french perinatal cohort. AIDS. 2008;22(2):289–299.
- Ugochukwu EF, Onubogu CU. A review and analysis of outcomes from prevention of mother-to- child transmission of HIV infant follow-up services at a pediatric infectious diseases unit of a major tertiary hospital in Nigeria : 2007-2020. Int J MCH AIDS. 2021;10(2):269–279.
- Smith MM, Kuhn L. Exclusive breast-feeding: Does it have the potential to reduce breast-feeding transmission of HIV-1? Nutr Rev. 2000;58(11):333–340.
- Anoje C, Aiyenigba B, Suzuki C, et al. Reducing mother-to-child transmission of HIV: findings from an early infant diagnosis program in South-South region of Nigeria. BMC Public Health. 2012;12(1):184. http://www.biomedcentral.com/1471-2458/12/184
- Yitayew YA, Bekele DM, Wondimeneh B, et al. Mother to child transmission of HIV and associated factors among HIV exposed infants at public health facilities, dessie town, Ethiopia. HIV AIDS (Auckl). 2019;11:343–350.
- Koye DN, Zeleke BM. Mother-to-child transmission of HIV and its predictors among HIV-exposed infants at a PMTCT clinic in northwest Ethiopia. BMC Public Health. 2013;13(1):398.
- Moges NA, Kassa GM, Boneya DJ. Rate of HIV transmission and associated factors among HIV-exposed infants in selected health facilities of east and west gojjam zones, northwest Ethiopia; retrospective cohort study. BMC Infect Dis. 2017;17(1):475.
- Nlend AEN, et al. Preventing hiv-1 transmission in breastfed infants in low resource settings: Early hiv infection and late postnatal transmission in a routine prevention of mother-to-child transmission program in yaounde, Cameroon. J Trop Pediatr. 2013;59(5):387–392.