ABSTRACT
The biotechnological uses of algae and cyanobacteria have been widely discussed in the context of climate change and consequent efforts to circularize economies, minimize carbon release and reuse waste streams. Their great potential in bioproduction and bioremediation has barely been exploited, particularly for the well-characterized red algae Galdieria sulphuraria and Cyanidioschyzon merolae. These and other Cyanidiales are excellent candidates for biotechnological enhancement and metabolic engineering for a broad spectrum of applications including the production of biofuels and thermostable colourants. In particular, extremophily, such as growth at thermophilic temperatures – up to 60°C – and at low pH and high salinity, make these algae unusually resistant to contamination and pathogens, and therefore potentially more commercially viable. We review existing applications of the Cyanidiales, as well as their available molecular tools. Their varied nutritional demands, from the broad heterotrophy of G. sulphuraria to strongly autotrophic C. merolae, along with their ability to grow to high densities, confer great potential as expression hosts. We also discuss the deficiencies that must be overcome to unlock further applications and ultimately to embed thermophilic red algae into a framework of circular and sustainable economic activity relying on bio-based sources.
GRAPHICAL ABSTRACT

Introduction
In recent years, the interest in photosynthetic unicellular organisms as a sustainable resource for biotechnological applications has increased significantly (Doron, Segal, & Shapira, Citation2016; Eichler-Stahlberg et al., Citation2009; Guzmán-Zapata et al., Citation2016; Imbimbo et al., Citation2019; Varshney et al., Citation2015). Research and development efforts have gradually expanded the microalgal biotechnology repertoire, but also accentuated the limitations thereof. The main drawback remains the low cost effectiveness which impedes large-scale production of a wide array of substances (Barclay, Apt, & Dong, Citation2013; Chen et al., Citation2011; Ho et al., Citation2011; Lundquist et al., Citation2010; Razzak et al., Citation2013). Several key problems will have to be addressed in order to make microalgal technologies commercially competitive: (i) the biomass and target product yields need to be increased at least by a factor of two; (ii) the costs of cultivation systems (photobioreactors, open ponds) and contamination control will have to be reduced by at least a factor of five and (iii) costs of downstream processes, such as biomass harvesting and product extraction will have to be lower (Clippinger & Davis, Citation2019; da Silva & Reis, Citation2015; Mallick et al., Citation2016; Milledge & Heaven, Citation2013). In this regard, Lundquist et al. (Citation2010) calculated a 15–20% cost reduction of microalga-derived biofuels in 100–400 ha open pond facilities if the downstream processing cost was reduced by two-thirds. Furthermore, their estimate predicts a 20–25% increase in costs of biofuels if wastewater treatment was not included in the process.
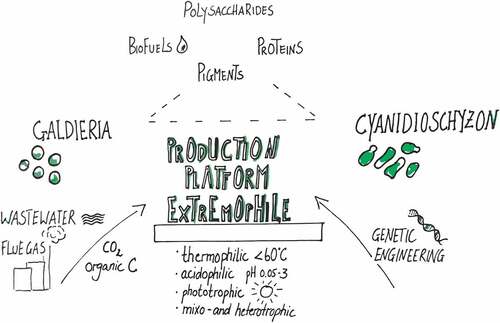
Figure 1. Graphic illustrating the biotechnological potential of Galdieria and Cyanidioschyzon. The remediation of waste streams (wastewater, flue gas, organic C) allows a valorization to products, such as pigments, lipids and polysaccharides. A toolbox for genetic engineering will render Cyanidioschyzon a heterologous expression platform.
To minimize the above-mentioned constraints, bioprospecting for suitable strains that exhibit the desired physiological properties is one way to make microalgal technologies more competitive (Barclay et al., Citation2013; Williams & Laurens, Citation2010). For example, substantial cost reductions were realized by exploitation of extremophilic algae that thrive under culture conditions that substantially inhibit the growth of predators and other algae (Patel et al., Citation2019; Varshney et al., Citation2015). These conditions could include high temperatures, high salt concentrations and extremes of pH (Varshney et al., Citation2015). Moreover, extremophiles contain unique genes and specialized, e.g., thermostable, enzymes that may prove useful for biotechnological and pharmaceutical applications (Lu et al., Citation2009; Varshney et al., Citation2015; Weber et al., Citation2007). The majority of extremophiles are bacteria and archaea; the few exceptions among eukaryotes are unique groups of green and red algae, in particular the rhodophyte lineage of Cyanidiales (Aguilera et al., Citation2007; Aguilera et al., Citation2010; Hirooka & Miyagishima, Citation2016; Weber et al., Citation2004). Despite their biotechnological potential, the number of published studies dealing with applied projects on Cyanidiales is relatively low. In a few patents, specialized production methods for cosmetics and pigments using strains of the order Cyanidiales are described (Bimonte et al., Citation2014; Cagnac, Richard, & Labro, Citation2017; Van der Maarel, Martinez-Garcia, & Sarian, Citation2016), but they are still far from commercial scale production ().
In this review, we aim to provide a comprehensive picture of the current state of the art for two species of Cyanidiales, Galdieria sulphuraria and Cyanidioschyzon merolae. We highlight the commercial and research potential of microalgae to encourage scientists towards these less-studied algal groups and move microalgal technologies towards economic viability.
Cyanidiales: an introduction
The order Cyanidiales represents a globally distributed group of unicellular rhodophytes (Archibald, Simpson, & Slamovits, Citation2017; Lee, Citation2009; Yoon et al., Citation2006), with three known genera: Cyanidium, Cyanidioschyzon and Galdieria. So far, phylogenetic analyses have identified eight different species (Guiry & Guiry, Citation2017). All are well adapted to extreme conditions such as springs with highly acidic pH (from 0.05 to 3), fumaroles, mines and temperatures up to 60°C (Ciniglia et al., Citation2004; Gross et al., Citation2001; Schonknecht et al., Citation2013; Yoon et al., Citation2006). The cells of Galdieria and Cyanidium are spherical, surrounded by a mucilage cell wall, and they propagate through the formation of four endospores. Sexual reproduction has not been described (Guiry & Guiry, Citation2017), although it has been predicted to occur in Cyanidioschyzon based on the conservation of meiosis-specific genes (Guo & Yang, Citation2015). One unique feature of Galdieria is photo-organotrophic and heterotrophic growth, resulting in substantially higher biomass yields than under phototrophic growth conditions (Graverholt & Eriksen, Citation2007; Schmidt, Wiebe, & Eriksen, Citation2005). Cyanidioschyzon is club-shaped, lacking a vacuole and cell wall, and propagates through binary fission. It contains one nucleus, mitochondrion and plastid, and is considered one of the most primitive algae (Kuroiwa, Citation1998; Matsuzaki et al., Citation2004). Previously, C. merolae was thought to be an obligate phototroph, but, in a recent study, growth on organic carbon sources was reported (Moriyama, Mori, & Sato, Citation2015). So far, C. merolae is the only member of the Cyanidiales accessible for genetic modifications (Ohnuma et al., Citation2008; ).
Table 1. Accessibility of Galdieria and Cyanidioschyzon for biotechnological applications.
Bioresources
Most micro-organisms are thought to have a worldwide distribution. Extremophilic microalgae like Cyanidiales thrive in specific and rare polyextreme environments while elsewhere they will easily be outcompeted (Castenholz & McDermott, Citation2010). This habitat specificity, along with their prominent colour, enables focused sampling of Cyanidiales, even though their typical extreme habitats like acidic geothermal environments may be hard to access physically and legally. Environmental suitability seems to be the most important factor restricting the occurrence of Cyanidiales (Barcyte et al., Citation2018), which leads to further targeted sampling of, e.g., acidic heterotrophic or metal-contaminated areas. New habitats and localities for Galdieria were found by sampling anthropogenic localities that mimic the natural environment (Barcyte et al., Citation2018; Gross et al., Citation2002).
Cyanidiales species typically coexist in the field. However, they can be isolated separately by their specific growth conditions. After isolation, long-term maintenance in conditions mimicking the natural situation (acidic media, high temperature and salinity) suppresses growth of contaminants under autotrophic conditions. Therefore, phototrophic conditions are traditionally used for long-term maintenance of axenic and non-axenic Cyanidiales strains, while photo-organotrophic or heterotrophic conditions are only feasible for axenic holdings.
Bioprospection for potential production strains could be based on isolation of new strains, a rather time- and resource-consuming process. A much more effective and sustainable resource lies in the public and private culture collections acting as specialized biological resource centres.
Public algal culture collections (ACCs) in particular can provide a large number of well-characterized, pre-screened strains and valuable data in a legally compliant manner (Overmann & Smith, Citation2017). As centres of competence for isolation, purification, identification, as well as characterization of newly established strains, they can underpin bioprospecting efforts. Furthermore, their expertise in cryopreservation of microbial strains eases the establishment of reliable cell banking protocols to enable long-term storage and preservation of genetic and physiological traits of production strains. Some ACCs act as International Depository Authorities (IDA) under the “Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedures and Regulations”.
Many laboratory strains of Cyanidiales have been archived since the first sustainable isolates, which were the basis for the description of the genus Galdieria by Merola (Citation1981), including the transfer of G. sulphuraria (Galdieri) Merola. Public and private culture collections maintain more than 500 Cyanidiales strains, isolated globally from different habitats (A. Rossini, Düsseldorf University, pers. comm.). The ACUF Collection (Microalgal Collection at University of Naples Federico II) specializes in thermo-acidophiles and keeps about 400 Cyanidiales strains (Del Mondo et al., Citation2019). Further strains are kept in public and private ACCs worldwide. Despite the inter- and intra-specific variability shown (Albertano et al., Citation2000; Cho et al., Citation2019; Ciniglia et al., Citation2004; Davis et al., Citation2016; Eren et al., Citation2018) only a few strains of Cyanidiales have been studied in depth for their biotechnological potential.
Cyanidiales as cell factories
In considering Cyanidiales strains for commercial scale production, it is important to evaluate their cellular properties, production parameters and the nature of the downstream processing. Thus, Cyanidium might not be the ideal candidate: it exclusively grows phototrophically, has a cell wall, and has not yet been accessible to genetic modification. Galdieria reveals its key potential in its ability to grow heterotrophically on multiple organic carbon sources including glucose, glycerol, molasses, food wastes and cherry brine (; Schmidt et al., Citation2005; Sloth, Wiebe, & Eriksen, Citation2006, Sloth et al., Citation2017). Several studies have demonstrated that heterotrophically grown G. sulphuraria achieved a 20- to 50-fold increase in biomass productivity compared to phototrophic growth conditions, reaching up to 50 g dry biomass l−1 d−1 (Graverholt & Eriksen, Citation2007; Schmidt et al., Citation2005; Sloth et al., Citation2017; Citation2006). Heterotrophic growth rates of C. merolae are substantially lower than those of Galdieria (Moriyama et al., Citation2015). However, the genomic integration of a sugar transporter gene from G. sulphuraria resulted in higher growth rates of C. merolae (Fujiwara et al., Citation2019). Furthermore, C. merolae lacks a cell wall and thus allows for a simple and effective cell extraction by osmotic shock as demonstrated by Rahman and co-workers (Rahman et al., Citation2017).
There is growing interest in using microalgae as biocatalysts for converting waste streams into valuable products. For example, wastewater is an attractive resource for algal cultivation due to its high nutrient content and low price. Researchers have attempted to grow G. sulphuraria in wastewater and demonstrated satisfactory biomass productivities and moderate removal rates of nitrogen and phosphorus species (Selvaratnam et al., Citation2014; Tchinda et al., Citation2019). Furthermore, Galdieria was shown to be a valuable biocatalyst for the recovery of rare earth elements (Čížková, Vítová, & Zachleder, Citation2019; Minoda et al., Citation2015; Vítová, Čížková, & Zachleder, Citation2019). RuBisCo of Cyanidiales strains exhibits an unusually high affinity for CO2, mediating a high CO2 tolerance of up to 100% (Kurano et al., Citation1995; Uemura, Anwaruzzaman, & Yokota, Citation1997). Coupled with the resistance to SOx and NOx species and very low pH, it makes them promising candidates for flue gas aeration in mass cultivation (Kurano et al., Citation1995; Varshney et al., Citation2015).
Natural colourants – thermostability as an advantage
Natural colourants and antioxidants have received much attention from the food, cosmetics and pharmaceutical industries in recent years (Bux & Chisti, Citation2016; Packer, Harris, & Adams, Citation2016). Here, microalgae are considered a valuable source for these compounds. The commercial production of the blue accessory pigment phycocyanin (C-PC) is achieved by mass cultivation of cyanobacteria from the genus Arthrospira (Spirulina), now in its fourth decade. The current world market size of C-PC is estimated to be 30 USD million, with Arthrospira being the sole source [https://www.lpinformationdata.com/shop/2017-2022-global-top-countries-phycocyanin-market-report-2]. Besides its role as a natural colourant in the food industry, C-PC also serves as a fluorescent probe in immunoassays, and exhibits antioxidant, anti-inflammatory and anti-cancer therapeutic properties (Bottone et al., Citation2018; Eriksen, Citation2008; Romay et al., Citation2003). With the market opening for natural blue colourants in the food sector, the demand for C-PC is expected to increase. We anticipate this development to also pave the way for alternative microalgal C-PC sources, such as Cyanidioschyzon and Galdieria.
The instability of C-PC, and its sensitivity towards light and air, is unfavourable for various biotechnological applications like thermal processing and extraction (Antelo, Costa, & Kalil, Citation2008; Chaiklahan, Chirasuwan, & Bunnag, Citation2012). The maximum temperature at which Arthrospira-derived C-PC retains its natural stability is 45°C and in a pH range of 5.5–6.0, for 30 min (Antelo et al., Citation2008; Chaiklahan et al., Citation2012; Patel et al., Citation2004). However, with preservatives, the thermostability of Arthrospira-derived C-PC for industrial applications can be increased to 60°C (Chaiklahan et al., Citation2012). There is therefore a significant market potential for a thermostable phycocyanin that does not depend on preservatives.
Consequently, extremophilic cyanobacteria and microalgae have been evaluated for their phycobiliproteins’ (PBP) thermostability and chemical stability (Edwards et al., Citation1997; Edwards, MacColl, & Eisele, Citation1996; Eisele et al., Citation2000; Kao, Edwards, & Berns, Citation1975; Leu et al., Citation2013; Liang et al., Citation2018; Rahman et al., Citation2017). Of the three common Cyanidiales species studied in the literature, C. merolae harbours the C-PC with the highest thermostability at 75°C for 30 min (Rahman et al., Citation2017). C-PC isolated from G. sulphuraria and C. caldarium showed heat resistance up to 65°C and 55°C, for at least 30 min, respectively (Eisele et al., Citation2000; Moon, Mishra, & Kim, Citation2014). In contrast to the relatively scarce data on C. merolae, C-PC production in G. sulphuraria has been studied much more extensively (Carfagna et al., Citation2018; Eisele et al., Citation2000; Lee, Citation2009; Liang et al., Citation2018; Moon et al., Citation2014; Rahman, Sarian, & van der Maarel, Citation2020; Roth, Berns, & Chen, Citation1996; Sørensen, Hantke, & Eriksen, Citation2013; Varshney et al., Citation2015). In G. sulphuraria, phototrophic growth demonstrated similar C-PC contents compared to other well-studied cyanobacteria (Wang et al., Citation2020). Pigment productivities under photo-organotrophic and heterotrophic growth conditions revealed a complex interplay between carbon and nitrogen availability, as well as light intensity (Sloth et al., Citation2006; Van der Maarel et al., Citation2016). Even though, at 1–2% of dry biomass, the cellular C-PC content was comparatively low, the high growth rate compensated for the low pigment content. The production rates were 20- to 30-fold higher than determined for A. platensis growing phototrophically in open pond systems (Graverholt & Eriksen, Citation2007; Schmidt et al., Citation2005). Contrary to many other algae, some G. sulphuraria strains maintain the photosynthetic apparatus and associated pigments even when cultivated heterotrophically (Gross & Schnarrenberger, Citation1995; Carfagna et al., Citation2018; unpublished data, University of Applied Sciences Bremerhaven).
All studies published so far have revealed the challenges of establishing a standardized process to produce high-quality thermostable C-PC with Galdieria, irrespective of the nutritional mode. This highlights the need for detailed research on Cyanidiales to further advance the technology. As outlined before, there are many Galdieria strains available in public and private collections, but only a few are discussed in the literature. Consequently, a comprehensive screen for Galdieria strains exhibiting the physiological attributes that are necessary for high C-PC yields would be required. Another option is to generate a “high C-PC” strain through mutagenesis, as in the case of Arthrospira (Takeuchi & Roberts, Citation2016).
In conclusion, both G. sulphuraria and C. merolae represent natural sources of thermostable C-PC as an advantageous alternative to the use of preservatives for Arthrospira-derived C-PC. The development of a C-PC-overexpressing C. merolae strain represents one option in addition to photo-organotrophic or heterotrophic production in an enclosed system with G. sulphuraria. For either approach, further research and development is necessary to develop standardized operations and produce high-quality pigments.
Cyanidioschyzon merolae as a model for biofuel production
In recent years, microalgae have received growing interest as a potential resource for biofuels. The advantage of microalgae over first- and second-generation biofuels is their high biomass production and ability to accumulate substantial amounts of oil (50–65% per unit dry weight) within the cells (Georgianna & Mayfield, Citation2012). Desired properties for suitable species include tolerance of changing environmental factors like light quality, temperature and water quality, as well as resistance to contamination and pathogens. C. merolae represents one such potential model organism. Its thermo-acidophilic nature enables cultivation in most kinds of culture systems including open, closed, terrestrial, marine, wastewater and cultures supplemented with industrial exhaust gas (Sato et al., Citation2017). The small nuclear genome of 16.5 Mb, genetic tractability, availability of protein expression systems and homologous recombination tools further add to the attributes of C. merolae as a potential model for biofuel production (Fujiwara et al., Citation2013; Sato et al., Citation2017). In particular, its high lipid content and precise lipid composition facilitate processing of algal biomass and its utilization as biodiesel, jet fuel or other biofuels.
Based on comparative genomics, 121 genes in total have been predicted to play a role in C. merolae lipid metabolism (Mori et al., Citation2016). In combination with protein expression data, it was possible to generate a lipid metabolic map of C. merolae. In contrast to both eukaryotic and prokaryotic pathways, lipid biosynthesis in C. merolae occurs through a novel coupled pathway, in which monogalactosyl diacyl glycerol precursors, palmitic acid and linoleic acid, are supplied by the plastid and endoplasmic reticulum, respectively (Sato & Moriyama, Citation2007). The reduced C. merolae lipid profile is unique and contains C16–C18 saturated and unsaturated fatty acids (FAs), lacks highly unsaturated lipids, and has neither cardiolipin nor phosphatidylserine (Sato & Moriyama, Citation2007; Toyoshima et al., Citation2016). This rather simple lipid profile makes C. merolae an attractive candidate for biodiesel production.
Like other microalgae, C. merolae has been shown to accumulate triacyl glyceride (TAG) up to 20% of dry weight in response to nitrogen deprivation (Takusagawa et al., Citation2016). In contrast to other oleaginous algae, lipid and starch accumulation are independent of each other, representing a potentially valuable characteristic of C. merolae since both products could be used to produce high levels of biofuels (Sato et al., Citation2017). In order to identify rate-limiting steps in TAG accumulation, overexpression of two glycerol-3-phosphate acyltransferases, CmGPAT1 and CmGPAT2, was tested. The genetically modified C. merolae strains exhibited up to 59-fold greater TAG accumulation compared to the wild-type strain under normal cell growth conditions (Fukuda et al., Citation2018). Also, in other algae, TAG accumulation has been increased by metabolic engineering and is regarded as a promising solution in strain development for algae-based biodiesel production (Ajjawi et al., Citation2017; Iwai et al., Citation2014).
All of these data together support C. merolae as a promising model organism for biofuel production. However, growth of C. merolae is still too low and further attempts with regard to strain selection and biomass productivity are necessary in order for it to become a suitable production strain.
Expression platform extremophile
Coupling photosynthesis with a homologous and/or heterologous implementation of enzymes or pathways to obtain natural products in microalgae offers great opportunities for green biotechnology. For several microalgae, genetic and metabolic engineering was demonstrated and has paved the way for the production of, e.g., bioactive compounds for pharmaceutical applications (Griesbeck, Kobl, & Heitzer, Citation2006; Hempel et al., Citation2011).
To have a maximally useful system for bioproduction, it is essential to have a broad set of molecular genetic tools. These include the means to introduce heterologous DNA into the host, genome editing tools such as CRISPR-Cas9, orthogonal inducible and repressible promoters that do not interfere with endogenous gene function, and well-characterized gene and metabolite expression pathways. Thus, a prerequisite for genetic engineering is the knowledge about the genome sequence of the respective microalga. The thermophilic red algae C. merolae and G. sulphuraria have been recognized as potentially valuable targets for green biotechnology due to their many advantageous growth properties.
The first complete algal genome sequence was that of C. merolae 10D, which became available in 2004, revealing a dramatically reduced set of 5331 genes on 20 chromosomes, and yielding unique insights into the evolution of land plants and eukaryotic cells in general (Matsuzaki et al., Citation2004). C. merolae has a genome size of around 16.5 million base pairs (bp), which is comparable to baker’s yeast or fission yeast. Only 0.5% of all C. merolae genes contain a spliceosomal intron with a strict consensus sequence (Matsuzaki et al., Citation2004). Consistent with the paucity of introns, C. merolae has a dramatically reduced set of spliceosomal factors (Stark et al., Citation2015). Apart from the nuclear genome, mitochondrial and plastid genomic sequences of C. merolae are available (Ohta, Matsuzaki, & Misumi, Citation2003; Ohta, Sato, & Kuroiwa, Citation1998). The low frequency of introns, a haploid genome and the availability of organellar DNA sequences make C. merolae an excellent target for biotechnological applications involving genetic modification.
Initially, comparative genomics were conducted based on the C. merolae genome sequence and an EST set of G. sulphuraria (Barbier et al., Citation2005; Weber et al., Citation2004). Finally, the 13.7 million bp genome sequence of G. sulphuraria was published in 2013 (Schonknecht et al., Citation2013). To date, full genomes of 13 Cyanidiales strains have been reported, isolated from Italy, the Czech Republic, Spain, Azores, the USA, Taiwan and Indonesia, and including authentic strains derived from the type material of G. sulphuraria, G. phlegrea and C. merolae (Rossoni et al., Citation2019a). Notably, the authors found a low frequency (1%) of horizontal gene transfer among the sequenced species and their finding may have implications for the entire eukaryotic lineage (Rossoni et al., Citation2019a). However, G. sulphuraria was shown to contain 5% of horizontally transferred genes, and with RNA-seq analyses these genes were identified as conferring polyextremophilic traits like cold tolerance and a concomitantly altered carbon metabolism, even affecting photosynthesis (Rossoni, Schönknecht, & Lee, Citation2019b). Obviously, the horizontal gene transfer to G. sulphuraria is responsible for the adaption of this alga to extreme environments (Rossoni et al., Citation2019a).
Despite the available G. sulphuraria nuclear genome sequences, progress has only been made in genetic and metabolic engineering with C. merolae (Matsuzaki et al., Citation2004; Minoda et al., Citation2004; Fujiwara et al., Citation2013; Zienkiewicz et al., Citation2017a, Citation2017b) and these initial experiments offer great opportunities for homologous or heterologous protein expression.
The entire genomic and organellar DNA sequences of C. merolae are available and thus, the codon usage of an expressed heterologous target gene can be optimized, and sites of gene integration by homologous recombination are easily defined (Nozaki, Citation2007). Harbouring a haploid genome, C. merolae is ideal for gene modification by homologous recombination – to replace a wild-type gene by a mutated version, alter a promoter, introduce heterologous genes or delete endogenous ones. Nuclear gene modification is well established (see below), and organellar gene modification has also been reported (Zienkiewicz et al., Citation2017a).
For C. merolae, a reliable PEG transformation protocol has been published (Ohnuma et al., Citation2009; Citation2008), optimized (Ohnuma et al., Citation2011) and is now used as the standard in the field. Several studies have documented successful PEG transformations, e.g., with B tubulin or tubulin (Ohnuma et al., Citation2009; Citation2008). One potentially useful method for rapidly selecting transformants is cell sorting. Flow cytometry has been reported in C. merolae to assess cell density and viability (Nikolova et al., Citation2017), but there have not yet been any demonstrations that C. merolae cells can be grown after cell sorting.
For a stable transformation, selectable markers are required. One potential drawback to growing microbes at acidic pH is the possibility that antibiotics or other small molecule drugs could be degraded in the medium. This has recently been reported for CDK inhibitors (Kobayashi & Tanaka, Citation2018) and may explain some of the apparent antibiotic resistance of C. merolae. Therefore, initially auxotrophic strains were characterized for their suitability for transformation protocols.
The endogenous URA5.3 gene has been used as a selectable marker for homologous recombination in the uracil auxotrophic strain M4 (Fujiwara et al., Citation2013), but integration at the target locus is competed by integration at the URA5.3 locus. When the Galdieria URA5.3 gene was used as the marker, this competition was markedly reduced (Imamura et al., Citation2010). More recently, a uracil auxotroph with a complete URA5.3 deletion has been reported that circumvents problems with integration at the URA5.3 locus (Taki et al., Citation2015). Finally, a second selection marker for C. merolae has been introduced – chloramphenicol acetyl transferase, or CAT, which can be stably integrated into the nuclear and also chloroplast genomes (Fujiwara et al., Citation2017; Zienkiewicz et al., Citation2017a). Using the CAT marker, the authors were able to determine that 200 bp of flanking homology is the minimum required for homologous recombination of DNA into the genome, but 500 bp substantially increases the recombination efficiency (Fujiwara et al., Citation2017). However, a stable vector for plasmid-based expression for C. merolae is not yet available and would greatly advance this red alga as an expression host.
With methods available to introduce and select for heterologous DNA, the next question is how to regulate expression of the transgene(s) by suitable promoters. Several target promoters have been identified. Watanabe et al. (Citation2011) report successful implementation of the phycocyanin-associated rod linker protein promoter, APCCp. This promoter is strongly induced under illumination, and was used in their study to drive GFP expression and a CENH3-GFP fusion protein. Furthermore, Imamura et al. (Citation2010) identified a nitrogen-responsive transcription factor, CmMyb1, that up-regulates nitrogen assimilation genes in response to nitrogen depletion (Imamura et al., Citation2010). CmMyb1 acts by binding several promoters, including NRp, NRTp and NiRp, which were subsequently shown to be effective regulators of heterologous expression (Fujiwara et al., Citation2015). Similarly, a 200 bp region containing the promoter from the CmHsp20 gene was found to reliably induce expression of two different genes in response to a shift to temperatures above 50°C (Sumiya et al., Citation2014).
Two methods for down-regulation of genes are to either express anti-sense RNA, complementary to the gene of interest, or simply to delete the gene by homologous integration of a selectable marker. As C. merolae lacks the RNAi machinery (Casas-Mollano et al., Citation2008), gene expression can be down-regulated by expression of an anti-sense RNA, as reported for the catalase gene (Ohnuma et al., Citation2009). Regarding homologous recombination, Imamura et al. (Citation2010) report knocking out the nitrite reductase gene by targeting a selectable marker to the appropriate locus. Both methods appear to be reliable for reducing gene expression, with transient expression of anti-sense RNA being substantially faster as transformed cells can be selected within 24 h.
For cells growing at elevated temperatures, an elaborate protein folding machinery, e.g., chaperones are required. Since C. merolae grows up to temperatures of 60°C, we expect the presence of specialized chaperones that aid protein solubility and stability and thus compensate for the elevated temperatures in the hot sulphur springs (Kobayashi et al., Citation2010). Nevertheless, stable expression of GFP required the use of the superfolder variant (Sumiya et al., Citation2014), and to date there are only a few reports of other heterologously expressed genes in C. merolae.
In summary, the basic tools for heterologous gene expression in C. merolae are available, but have so far only been used in the context of homologous recombination. There is still no suitable self-replicating – and thus stable – plasmid for C. merolae which would facilitate fast and reliable expression trials.
The future for exploitation of Cyanidiales
Both G. sulphuraria and C. merolae have unique advantages for biotechnological applications – on one hand an efficient generation of biomass for Galdieria and on the other an advanced understanding of the genome and options for genetic manipulation in C. merolae (). It is evident that knowledge of these organisms could be combined, as shown by generating modified C. merolae strains with metabolite transporters from G. sulphuraria (Fujiwara et al., Citation2019). Lately, the potential of Cyanidiales as a biotechnological resource has increasingly been recognized. Cultivation procedures, extraction protocols and the validation of biomass extracts for applications in human health and food have been developed, however, only for a handful of strains. Public and private culture collections maintain more than 500 Cyanidiales strains, isolated globally from different habitats. These precious bioresources harbour great potential to further advance the selection for suitable strains for various applications. Activities related to cell banking and strain development have recently been initiated and are indispensable for the establishment of biotechnological processes.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aguilera, A., Souza-Egipsy, V., Gómez, F., & Amils, R. (2007). Development and structure of eukaryotic biofilms in an extreme acidic environment, Río Tinto (SW, Spain). Microbial Ecology, 53, 294–305.
- Aguilera, A., Souza-Egipsy, V., González-Toril, E., Rendueles, O., & Amils, R. (2010). Eukaryotic microbial diversity of phototrophic microbial mats in two Icelandic geothermal hot springs. International Microbiology : The Official Journal of the Spanish Society for Microbiology, 13, 21–32.
- Ajjawi, I., Verruto, J., Aqui, M., Soriaga, L. B., Coppersmith, J., Kwok, K., & Moellering, E. R. (2017). Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nature Biotechnology, 35, 647–652.
- Albertano, P., Ciniglia, C., Pinto, G., & Pollio, A. (2000). The taxonomic position of Cyanidium, Cyanidioschyzon and Galdieria: An update. Hydrobiologia, 433, 137–143.
- Antelo, F. S., Costa, J. A. V., & Kalil, S. J. (2008). Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochemical Engineering Journal, 41, 43–47.
- Archibald, J. M., Simpson, A. G. B., & Slamovits, C. H. (2017). Handbook of the protists. Cham: Springer International Publishing.
- Barbier, G., Oesterhelt, C., Larson, M. D., Halgren, R. G., Wilkerson, C., Garavito, R. M., … Weber, A. P. M. (2005). Comparative genomics of two closely related unicellular thermo-acidophilic red algae, Galdieria sulphuraria and Cyanidioschyzon merolae, reveals the molecular basis of the metabolic flexibility of Galdieria sulphuraria and significant differences in carbohydrate metabolism of both algae. Plant Physiology, 137, 460–474.
- Barclay, W., Apt, K., & Dong, X. D. (2013). Commercial production of microalgae via fermentation. In John Wiley & Sons, Ltd, Handbook of microalgal culture (pp. 134–145). Oxford, UK: John Wiley & Sons, Ltd.
- Barcyte, D., Nedbalova, L., Culka, A., Kosek, F., & Jehlicka, J. (2018). Burning coal spoil heaps as a new habitat for the extremophilic red alga Galdieria sulphuraria. Fottea, 18, 19–29.
- Bimonte, M., de Lucia, A., Tito, A., et al. (2014). Cosmetic compositions based on extracts derived from the microalga Galdieria sulphuraria, particularly indicated to reduce the harmful effects caused by acne. Patent: MI2014A 000186.
- Bottone, C., Camerlingo, R., Miceli, R., Salbitani, G., Sessa, G., Pirozzi, G., & Carfagna, S. (2018). Antioxidant and anti-proliferative properties of extracts from heterotrophic cultures of Galdieria sulphuraria. Natural Product Research, 1–5.
- Bux, F., & Chisti, Y. (2016). Algae biotechnology. Products and processes. Green Energy Technology, 344.
- Cagnac, O., Richard, L., & Labro, J. (2017). (Nouveau procede de culture d’algues rouges unicellulaires). Patent Application PCT/EP2016/072582.
- Carfagna, S., Landi, V., Coraggio, F., Salbitani, G., Vona, V., Pinto, G., … Ciniglia, C. (2018). Different characteristics of C-phycocyanin (C-PC) in two strains of the extremophilic Galdieria phlegrea. Algal Research, 31, 406–412.
- Carfagna, S., Napolitano, G., Barone, D., Pinto, G., Pollio, A., & Venditti, P. (2015). Dietary supplementation with the microalga Galdieria sulphuraria (rhodophyta) reduces prolonged exercise-induced oxidative stress in rat tissues. Oxidative Medicine and Cellular Longevity, 2015, 732090.
- Casas-Mollano, J. A., Rohr, J., Kim, E.-J., Balassa, E., van Dijk, K., & Cerutti, H. (2008). Diversification of the core RNA interference machinery in Chlamydomonas reinhardtii and the role of DCL1 in transposon silencing. Genetics, 179, 69–81.
- Castenholz, R. W., & McDermott, T. R. (2010). The Cyanidiales: Ecology, biodiversity, and biogeography. In J. Seckbach, D. Chapman (Eds.), Red Algae Genomic Age (pp. 357–371). Springer International Publishing.
- Chaiklahan, R., Chirasuwan, N., & Bunnag, B. (2012). Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochemistry, 47, 659–664.
- Chen, C.-Y., Liu, C.-H., Lo, Y.-C., & Chang, J.-S. (2011). Perspectives on cultivation strategies and photobioreactor designs for photo-fermentative hydrogen production. Bioresource Technology, 102, 8484–8492.
- Cho, C. H., Park, S. I., Ciniglia, C. Y. H., Cho, C., Park, S. H., Imke. (2019). Keynote and oral papers. European Journal of Phycology, 54, 31–117.
- Ciniglia, C., Yoon, H. S., Pollio, A., Pinto, G., & Bhattacharya, D. (2004). Hidden biodiversity of the extremophilic Cyanidiales red algae. Molecular Ecology, 13, 1827–1838.
- Čížková, M., Vítová, M., & Zachleder, V. (2019). The red microalga Galdieria as a promising organism for applications in biotechnology. In Microalgae - From physiology to application [Working Title]. IntechOpen.
- Clippinger, J., & Davis, R. (2019). Techno-economic analysis for the production of algal biomass via closed photobioreactors: Future cost potential evaluated across a range of cultivation system designs. Golden: CO Natl Renew Energy Lab.
- da Silva, T. L., & Reis, A. (2015). Scale-up problems for the large scale production of algae. In Algal biorefinery: An integrated approach (pp. 125–149). Cham: Springer International Publishing.
- Davis, A. M., Iovinella, M., James, S., et al. (2016). Using MinION nanopore sequencing to generate a de novo eukaryotic draft genome: Preliminary physiological and genomic description of the extremophilic red alga Galdieria sulphuraria strain SAG 107.79. BioRxiv, 076208.
- Del Mondo, A., Langellotti, A. L., Petraretti, M., Ciniglia, C., Pinto, G., Pollio, A. (2019). Seventh European phycological congress. European Journal of Phycology, 54, 108.
- Doron, L., Segal, N., & Shapira, M. (2016). Transgene expression in microalgae—from tools to applications. Frontiers in Plant Science, 7, 505.
- Edwards, M. R., Hauer, C., Stack, R. F., Eisele, L. E., & MacColl, R. (1997). Thermophilic C-phycocyanin: Effect of temperature, monomer stability, and structure. Biochimica Et Biophysica Acta (BBA) - Bioenergetics, 1321, 157–164.
- Edwards, M. R., MacColl, R., & Eisele, L. E. (1996). Some physical properties of an unusual C-phycocyanin isolated from a photosynthetic thermophile. Biochimica Et Biophysica Acta (BBA) - Bioenergetics, 1276, 64–70.
- Eichler-Stahlberg, A., Weisheit, W., Ruecker, O., & Heitzer, M. (2009). Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta, 229, 873–883.
- Eisele, L. E., Bakhru, S. H., Liu, X., MacColl, R., & Edwards, M. R. (2000). Studies on C-phycocyanin from Cyanidium caldarium, a eukaryote at the extremes of habitat. Biochimica Et Biophysica Acta (BBA) - Bioenergetics, 1456, 99–107.
- Eren, A., Iovinella, M., Yoon, H. S., Cennamo, P., de Stefano, M., de Castro, O., & Ciniglia, C. (2018). Genetic structure of Galdieria populations from Iceland. Polar Biology, 41, 1681–1691.
- Eriksen, N. T. (2008). Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Applied Microbiology and Biotechnology, 80, 1–14.
- Fujiwara, T., Hirooka, S., Mukai, M., Ohbayashi, R., kanesaki, Y., Watanabe, S., & Miyagishima, S.-Y. (2019). Integration of a Galdieria plasma membrane sugar transporter enables heterotrophic growth of the obligate photoautotrophic red alga Cynanidioschyzon merolae. Plant Direct, 3, e00134.
- Fujiwara, T., Kanesaki, Y., Hirooka, S., Era, A., Sumiya, N., Yoshikawa, H., & Miyagishima, S.-Y. (2015). A nitrogen source-dependent inducible and repressible gene expression system in the red alga Cyanidioschyzon merolae. Frontiers in Plant Science, 6, 657.
- Fujiwara, T., Ohnuma, M., Kuroiwa, T., Ohbayashi, R., Hirooka, S., & Miyagishima, S.-Y. (2017). Development of a double nuclear gene-targeting method by two-step transformation based on a newly established chloramphenicol-selection system in the red alga Cyanidioschyzon merolae. Frontiers in Plant Science, 8, 343. PM - 28352279 M4 - Citavi.
- Fujiwara, T., Ohnuma, M., Yoshida, M., Kuroiwa, T., & Hirano, T. (2013). Gene targeting in the red alga Cyanidioschyzon merolae: Single- and multi-copy insertion using authentic and chimeric selection markers. PloS One, 8, e73608.
- Fukuda, S., Hirasawa, E., Takemura, T., Takahashi, S., Chokshi, K., Pancha, I., … Imamura, S. (2018). Accelerated triacylglycerol production without growth inhibition by overexpression of a glycerol-3-phosphate acyltransferase in the unicellular red alga Cyanidioschyzon merolae. Scientific Reports, 8, 12410.
- Georgianna, D. R., & Mayfield, S. P. (2012). Exploiting diversity and synthetic biology for the production of algal biofuels. Nature, 488, 329–335.
- Graverholt, O. S., & Eriksen, N. T. (2007). Heterotrophic high-cell-density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin. Applied Microbiology and Biotechnology, 77, 69–75.
- Graziani, G., Schiavo, S., Nicolai, M. A., Buono, S., Fogliano, V., Pinto, G., & Pollio, A. (2013). Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Function, 4, 144–152.
- Griesbeck, C., Kobl, I., & Heitzer, M. (2006). Chlamydomonas reinhardtii: A protein expression system for pharmaceutical and biotechnological proteins. Molecular Biotechnology, 34, 213–224.
- Gross, W., Heilmann, I., Lenze, D., & Schnarrenberger, C. (2001). Biogeography of the Cyanidiaceae (Rhodophyta) based on 18S ribosomal RNA sequence data. European Journal of Phycology, 36, 275–280.
- Gross, W., Oesterhelt, C., Tischendorf, G., & Lederer, F. (2002). Characterization of a non-thermophilic strain of the red algal genus Galdieria isolated from Soos (Czech Republic). European Journal of Phycology, 37, 477–482.
- Gross, W., & Schnarrenberger, C. (1995). Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant & Cell Physiology, 36, 633–638.
- Guiry, M. D., & Guiry, G. M. (2017). AlgaeBase. Galway: World-wide electronic publication, National University of Ireland. Retrieved from https://www.algaebase.org
- Guo, L., & Yang, G. (2015). Predicting the reproduction strategies of several microalgae through their genome sequences. Journal of Ocean University of China, 14, 491–502.
- Guzmán-Zapata, D., Macedo-Osorio, K. S., Almaraz-Delgado, A. L., Durán-Figueroa, A., Badillo-Corona, J. A. (2016). Production of recombinant proteins in the chloroplast of the green alga Chlamydomonas reinhardtii. In Recombinant proteins from plants: Methods and protocols (pp. 69–85).
- Hempel, F., Lau, J., Klingl, A., & Maier, U. G. (2011). Algae as protein factories: Expression of a human antibody and the respective antigen in the diatom Phaeodactylum tricornutum. PloS One, 6, e28424.
- Hirooka, S., & Miyagishima, S. Y. (2016). Cultivation of acidophilic algae Galdieria sulphuraria and Pseudochlorella sp. YKT1 in media derived from acidic hot springs. Frontiers in Microbiology, 7, 2022.
- Ho, S.-H., Chen, C.-Y., Lee, D.-J., & Chang, J.-S. (2011). Perspectives on microalgal CO2-emission mitigation systems — A review. Biotechnology Advances, 29, 189–198.
- Imamura, S., Terashita, M., Ohnuma, M., Maruyama, S., Minoda, A., Weber, A. P. M., … Tanaka, K. (2010). Nitrate assimilatory genes and their transcriptional regulation in a unicellular red alga Cyanidioschyzon merolae: Genetic evidence for nitrite reduction by a sulfite reductase-like enzyme. Plant and Cell Physiology, 51, 707–717.
- Imbimbo, P., Romanucci, V., Pollio, A., Fontanarosa, C., Amoresano, A., Zarrelli, A., & Monti, D. M. (2019). A cascade extraction of active phycocyanin and fatty acids from Galdieria phlegrea. Applied Microbiology and Biotechnology, 103, 9455–9464.
- Iwai, M., Ikeda, K., Shimojima, M., & Ohta, H. (2014). Enhancement of extraplastidic oil synthesis in Chlamydomonas reinhardtii using a type-2 diacylglycerol acyltransferase with a phosphorus starvation-inducible promoter. Plant Biotechnology Journal, 12, 808–819.
- Kao, O. H., Edwards, M. R., & Berns, D. S. (1975). Physical-chemical properties of C-phycocyanin isolated from an acido-thermophilic eukaryote, Cyanidium caldarium. Biochemical Journal, 147, 63–70.
- Kobayashi, Y., Ohnuma, M., Tanaka, K., & Ha-, M. (2010). The basics of cultivation and molecular genetic analysis of the unicellular red alga Cyanidioschyzon merolae. Cell, 20, 53–61.
- Kobayashi, Y., & Tanaka, K. (2018). Lability in sulfur acidic cultivation medium explains unstable effects of CDK inhibitors on Cyanidioschyzon merolae cell proliferation. The Journal of General and Applied Microbiology, 64, 299–302.
- Kurano, N., Ikemoto, H., Miyashita, H., Hasegawa, T., Hata, H., & Miyachi, S. (1995). Fixation and utilization of carbon dioxide by microalgal photosynthesis. Energy Conversion and Management, 36, 689–692.
- Kuroiwa, T. (1998). The primitive red algae Cyanidium caldarium and Cyanidioschyzon merolae as model system for investigating the dividing apparatus of mitochondria and plastids. BioEssays, 20, 344–354.
- Lee, R. E. (2009). Phycology (4th ed.). Cambridge: Cambridge University Press.
- Leu, J.-Y., Lin, T.-H., Selvamani, M. J. P., Chen, H.-C., Liang, J.-Z., & Pan, K.-M. (2013). Characterization of a novel thermophilic cyanobacterial strain from Taian hot springs in Taiwan for high CO2 mitigation and C-phycocyanin extraction. Process Biochemistry, 48, 41–48.
- Liang, Y., Kaczmarek, M. B., Kasprzak, A. K., Tang, J., Shah, M. M. R., Jin, P., … Daroch, M. (2018). Thermosynechococcaceae as a source of thermostable C-phycocyanins: Properties and molecular insights. Algal Research, 35, 223–235.
- Lu, Y., Chi, X., Yang, Q., Li, Z., Liu, S., Gan, Q., & Qin, S. (2009). Molecular cloning and stress-dependent expression of a gene encoding Δ12-fatty acid desaturase in the Antarctic microalga Chlorella vulgaris NJ-7. Extremophiles, 13, 875–884.
- Lundquist, T., Woertz, I., Quinn, N., & Benemann, J. (2010). A realistic technology and engineering assessment of algae biofuel production. Energy Biosci Inst. Retrieved January 23, 2018 from http://digitalcommons.calpoly.edu/cenv_fac/188
- Mallick, N., Bagchi, S. K., Koley, S., & Singh, A. K. (2016). Progress and challenges in microalgal biodiesel production. Frontiers in Microbiology, 7.
- Martinez-Garcia, M., & van der Maarel, M. J. E. C. (2016). Floridoside production by the red microalga Galdieria sulphuraria under different conditions of growth and osmotic stress. AMB Express, 6, 71.
- Massa, M., Buono, S., Langellotti, A. L., Martello, A., Russo, G. L., Troise, D. A., Sacchi, R., Vitaglione, P., & Fogliano, V. (2019). Biochemical composition and in vitro digestibility of Galdieria sulphuraria grown on spent cherry-brine liquid. New Biotechnol ogy, 53, 9–15. (2019).
- Matsuzaki, M., Misumi, O., Shin-I, T., Maruyama, S., Takahara, M., Miyagishima, S.-Y., … Kuroiwa, T. (2004). Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature, 428, 653–657.
- Merola, A., Castaldo, R., De Luca, P., Gambardella, R., Musacchio, A., & Taddei, R. (1981). Revision of Cyanidium caldarium. three species of acidophilic algae. Giornale Botanico Italiano, 115, 189–195.
- Milledge, J. J., & Heaven, S. (2013). A review of the harvesting of micro-algae for biofuel production. Reviews in Environmental Science and Bio/Technology, 12, 165–178.
- Minoda, A., Sakagami, R., Yagisawa, F., Kuroiwa, T., & Tanaka, K. (2004). Improvement of culture conditions and evidence for nuclear transformation by homologous recombination in a red alga, Cyanidioschyzon merolae 10D. Plant and Cell Physiology, 45, 667–671.
- Minoda, A., Sawada, H., Suzuki, S., Miyashita, S.-I., Inagaki, K., Yamamoto, T., & Tsuzuki, M. (2015). Recovery of rare earth elements from the sulfothermophilic red alga Galdieria sulphuraria using aqueous acid. Applied Microbiology and Biotechnology, 99, 1513–1519.
- Modeste, V., Brient, A., Thirion-Delalande, C., Forster, R., Aguenou, C., Griffiths, H., & Cagnac, O. (2019). Safety evaluation of Galdieria high-protein microalgal biomass. Toxicology Research and Application, 3.
- Moon, M., Mishra, S. K., & Kim, C. W. (2014). Isolation and characterization of thermostable phycocyanin from Galdieria sulphuraria. Korean Journal of Chemical Engineering, 29, 490–495.
- Mori, N., Moriyama, T., Toyoshima, M., & Sato, N. (2016). Construction of global acyl lipid metabolic map by comparative genomics and subcellular localization analysis in the red alga Cyanidioschyzon merolae. Frontiers in Plant Science, 7. doi:10.3389/fpls.2016.00958.
- Moriyama, T., Mori, N., & Sato, N. (2015). Activation of oxidative carbon metabolism by nutritional enrichment by photosynthesis and exogenous organic compounds in the red alga Cyanidioschyzon merolae: Evidence for heterotrophic growth. Springerplus, 4, 559.
- Nikolova, D., Weber, D., Scholz, M., Bald, T., Scharsack, J. P., & Hippler, M. (2017). Temperature-induced remodeling of the photosynthetic machinery tunes photosynthesis in the thermophilic alga Cyanidioschyzon merolae. Plant Physiology, 174, 35–46.
- Nozaki, H., Takano, H., Misumi, O., Terasawa, K., Matsuzaki, M., Maruyama, S., Nishida, K., Yagisawa, F., Yoshida, Y., Fujiwara, T., Takio, S., Tamura, K., Chung, S. J., Nakamura, S., Kuroiwa, H., Tanaka, K., Sato, N., & Kuroiwa, T. (2007). A 100%-complete sequence reveals unusually simple genomic features in the hot-spring red alga Cyanidioschyzon merolae. BMC Biology, 5. doi:10.1186/1741-7007-5-28
- Oesterhelt, C., Schnarrenberger, C., & Gross, W. (1999). Characterization of a sugar/polyol uptake system in the red alga Galdieria sulphuraria. European Journal of Phycology, 34, 271–277.
- Ohnuma, M., Misumi, O., Fujiwara, T., Watanabe, S., Tanaka, K., & Kuroiwa, T. (2009). Transient gene suppression in a red alga, Cyanidioschyzon merolae 10D. Protoplasma, 236, 107–112.
- Ohnuma, M., Yokoyama, T., Inouye, T., Sekine, Y., Kuroiwa, T., & Tanaka, K. (2011). Optimization of polyethylene glycol (PEG)-mediated DNA introduction conditions for transient gene expression in the unicellular red alga Cyanidioschyzon merolae. The Journal of General and Applied Microbiology, 60, 156–159.
- Ohnuma, M., Yokoyama, T., Inouye, T., Sekine, Y., & Tanaka, K. (2008). Polyethylene glycol (PEG)-mediated transient gene expression in a red alga, Cyanidioschyzon merolae 10D. Plant and Cell Physiology, 49, 117–120.
- Ohta, N., Matsuzaki, M., & Misumi, O. (2003). Complete sequence and analysis of the plastid genome of the unicellular red alga Cyanidioschyzon merolae. DNA Research, 10, 67–77.
- Ohta, N., Sato, N., & Kuroiwa, T. (1998). Structure and organization of the mitochondrial genome of the unicellular red alga Cyanidioschyzon merolae deduced from the complete nucleotide sequence. Nucleic Acids Research, 26, 5190–5198.
- Overmann, J., & Smith, D. (2017). Microbial resource centers contribute to bioprospecting of bacteria and filamentous microfungi. In Bioprospecting (pp. 51–79). Cham: Springer.
- Packer, M. A., Harris, G. C., & Adams, S. L. (2016). Food and feed applications of algae (pp. 217–247). Cham: Springer.
- Patel, A., Matsakas, L., Rova, U., & Christakopoulos, P. (2019). A perspective on biotechnological applications of thermophilic microalgae and cyanobacteria. Bioresource Technology, 278, 424–434.
- Patel, A., Pawar, R., Mishra, S., Sonawane, S., & Ghosh, P. K. (2004). Kinetic studies on thermal denaturation of C-phycocyanin. Indian journal of Biochemistry & Biophysics, 41, 254–257.
- Rahman, D. Y., Sarian, F. D., & van der Maarel, M. J. E. C. (2020). Biomass and phycocyanin content of heterotrophic Galdieria sulphuraria 074G under maltodextrin and granular starches–feeding conditions. Journal of Applied Phycology, 32, 51–57.
- Rahman, D. Y., Sarian, F. D., van Wijk, A., Martinez-Garcia, M., & van der Maarel, M. J. E. C. (2017). Thermostable phycocyanin from the red microalga Cyanidioschyzon merolae, a new natural blue food colorant. Journal of Applied Phycology, 29, 1233–1239.
- Razzak, S. A., Hossain, M. M., Lucky, R. A., Bassi, A. S., & de Lasa, H. (2013). Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renewable and Sustainable Energy Reviews, 27, 622–653.
- Romay, C., González, R., Ledón, N., Remirez, D., & Rimbau, V. (2003). C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Current Protein & Peptide Science, 4, 207–216.
- Rossoni, A. W., Price, D. C., Seger, M., et al. (2019a). The genomes of polyextremophilic cyanidiales contain 1% horizontally transferred genes with diverse adaptive functions. Elife, 8.
- Rossoni, A. W., Schönknecht, G., & Lee, H. J. (2019b). Cold acclimation of the thermoacidophilic red alga Galdieria sulphuraria: Changes in gene expression and involvement of horizontally acquired genes. Plant and Cell Physiology, 60, 702–712.
- Roth, L. G., Berns, D. S., & Chen, C.-H. (1996). Comparative thermodynamic elucidation of the structural stability of thermophilic proteins. Biophysical Chemistry, 60, 89–97.
- Sato, N., & Moriyama, T. (2007). Genomic and biochemical analysis of lipid biosynthesis in the unicellular rhodophyte Cyanidioschyzon merolae: Lack of a plastidic desaturation pathway results in the coupled pathway of galactolipid synthesis. Eukaryotic Cell, 6, 1006–1017.
- Sato, N., Moriyama, T., Mori, N., & Toyoshima, M. (2017). Lipid metabolism and potentials of biofuel and high added-value oil production in red algae. World Journal of Microbiology and Biotechnology, 33, 74.
- Schmidt, R. A., Wiebe, M. G., & Eriksen, N. T. (2005). Heterotrophic high cell-density fed-batch cultures of the phycocyanin-producing red alga Galdieria sulphuraria. Biotechnology and Bioengineering, 90, 77–84.
- Schonknecht, G., Chen, W.-H., Ternes, C. M., Barbier, G. G., Shrestha, R. P., Stanke, M., … Weber, A. P. M. (2013). Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science (80-), 339, 1207–1210.
- Selvaratnam, T., Pegallapati, A. K., Montelya, F., Rodriguez, G., Nirmalakhandan, N., Van Voorhies, W., & Lammers, P. J. (2014). Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresource Technology, 156, 395–399.
- Sloth, J. K., Jensen, H. C., Pleer, D., & Eriksen, N. T. (2017). Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresource Technology, 238, 296–305.
- Sloth, J. K., Wiebe, M. G., & Eriksen, N. T. (2006). Accumulation of phycocyanin in heterotrophic and mixotrophic cultures of the acidophilic red alga Galdieria sulphuraria. Enzyme and Microbial Technology, 38, 168–175.
- Sørensen, L., Hantke, A., & Eriksen, N. T. (2013). Purification of the photosynthetic pigment C-phycocyanin from heterotrophic Galdieria sulphuraria. Journal of the Science of Food and Agriculture, 93, 2933–2938.
- Stark, M. R., Dunn, E. A., Dunn, W. S. C., Grisdale, C. J., Daniele, A. R., Halstead, M. R. G., & Rader, S. D. (2015). Dramatically reduced spliceosome in Cyanidioschyzon merolae. Proceedings of the National Academy of Sciences, 112, E1191–E1200.
- Sumiya, N., Fujiwara, T., Kobayashi, Y., Misumi, O., & Miyagishima, S.-Y. (2014). Development of a heat-shock inducible gene expression system in the red alga Cyanidioschyzon merolae. PloS One, 9, e111261.
- Takeuchi, R., & Roberts, J. (2016). Targeted mutagenesis in spirulina. Patent US20170298319A1.
- Taki, K., Sone, T., Kobayashi, Y., Watanabe, S., Imamura, S., & Tanaka, K. (2015). Construction of a URA5.3 deletion strain of the unicellular red alga Cyanidioschyzon merolae: A backgroundless host strain for transformation experiments. The Journal of General and Applied Microbiology, 61, 211–214.
- Takusagawa, M., Nakajima, Y., Saito, T., & Misumi, O. (2016). Primitive red alga Cyanidioschyzon merolae accumulates storage glucan and triacylglycerol under nitrogen depletion. The Journal of General and Applied Microbiology, 62, 111–117.
- Tchinda, D., Henkanatte-Gedera, S. M., Abeysiriwardana-Arachchige, I. S. A., Delanka-Pedige, H. M. K., Munasinghe-Arachchige, S. P., Zhang, Y., & Nirmalakhandan, N. (2019). Single-step treatment of primary effluent by Galdieria sulphuraria: Removal of biochemical oxygen demand, nutrients, and pathogens. Algal Research, 42, 101578.
- Toyoshima, M., Mori, N., Moriyama, T., Misumi, O., & Sato, N. (2016). Analysis of triacylglycerol accumulation under nitrogen deprivation in the red alga Cyanidioschyzon merolae. Microbiology, 162, 803–812.
- Uemura, K., Anwaruzzaman, M. S., & Yokota, A. (1997). Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase from thermophilic red algae with a strong specificity for CO2Fixation. Biochemical and Biophysical Research Communications, 233, 568–571.
- Van der Maarel, M. J. E. C., Martinez-Garcia, M., & Sarian, F. D. (2016). (Patent: PCT/NL20 15/050867 Natural blue photopigments, methods for producing them and to uses thereof as colorant).
- Varshney, P., Mikulic, P., Vonshak, A., Beardall, J., & Wangikar, P. P. (2015). Extremophilic micro-algae and their potential contribution in biotechnology. Bioresource Technology, 184, 363–372.
- Vítová, M., Čížková, M., & Zachleder, V. (2019). Lanthanides and algae. In Lanthanides. IntechOpen.
- Wan, M., Wang, Z., Zhang, Z., Wang, J., Li, S., Yu, A., & Li, Y. (2016). A novel paradigm for the high-efficient production of phycocyanin from Galdieria sulphuraria. Bioresource Technology, 218, 272–278.
- Wang, H., Zhang, Z., Wan, M., Wang, R., Huang, J., Zhang, K., Guo, J., Bai, W., & Li, Y. (2019).Comparative study on light attenuation models of Galdieria sulphuraria for efficient production of phycocyanin. Journal of Applied Phycology, 37, 165-174.
- Wang, H., Zhang, Z., Wan, M., Wang, R., Huang, J., Zhang, K., … Li, Y. (2020). Comparative study on light attenuation models of Galdieria sulphuraria for efficient production of phycocyanin. Journal of Applied Phycology, 32, 165–174.
- Watanabe, S., Ohnuma, M., Sato, J., Yoshikawa, H., & Tanaka, K. (2011). Utility of a GFP reporter system in the red alga Cyanidioschyzon merolae. The Journal of General and Applied Microbiology, 57, 69–72.
- Weber, A., Oesterhelt, C., Gross, W., Brutigam, A., Imboden, L., Krassovskaya, I., … Benning, C. (2004). EST-analysis of the thermo-acidophilic red microalga Galdieria sulphuraria reveals potential for lipid A biosynthesis and unveils the pathway of carbon export from rhodoplasts. Plant Molecular Biology, 55, 17–32.
- Weber, A. P. M., Horst, R. J., Barbier, G. G., & Oesterhelt, C. (2007). Metabolism and metabolomics of eukaryotes living under extreme conditions. International Review of Cytology, 256, 1–34. PM - 17241903 M4 - Citavi.
- Williams, P. J. L. B., & Laurens, L. M. L. (2010). Microalgae as biodiesel & biomass feedstocks: Review and analysis of the biochemistry, energetics and economics. Energy & Environmental Science, 3, 554.
- Yoon, H. S., Ciniglia, C., Wu, M., Comeron, J. M., Pinto, G., Pollio, A., & Bhattacharya, D. (2006). Establishment of endolithic populations of extremophilic Cyanidiales (Rhodophyta). BMC Evolutionary Biology, 6, 78. PM - 17022817 M4 - Citavi.
- Zienkiewicz, M., Krupnik, T., Drożak, A., Golke, A., & Romanowska, E. (2017a). Transformation of the Cyanidioschyzon merolae chloroplast genome: Prospects for understanding chloroplast function in extreme environments. Plant Molecular Biology, 93, 171–183. T4.
- Zienkiewicz, M., Krupnik, T., Drożak, A., Golke, A., & Romanowska, E. (2017b). Chloramphenicol acetyltransferase—a new selectable marker in stable nuclear transformation of the red alga Cyanidioschyzon merolae. Protoplasma, 254, 587–596.
