ABSTRACT
Seaweed surface provides a suitable substratum for the settlement of microorganisms (bacteria, viruses, and plankton). These microbial partners may have either beneficial or detrimental effects on the host since surface microbiota can act either as a protective layer to the macroalgae (symbiotic beneficial relations) or, under changing environmental conditions, the microbial equilibrium may shift to a detrimental, pathogenic state thus inducing diseases in the host. In commercial aquaculture, seaweed diseases feature a growing concern. Pathogenicity of microorganisms that infect algal hosts is closely related to the release of virulence factors and the formation of biofilms, both of which are regulated by Quorum Sensing (QS). The main focus of this study is to determine the pathogenicity of the surface-associated bacteria of Halymenia floresii, a carrageenophyte that originated from distinct habitats. Twenty-five bacterial species isolated from the surface of H. floresii were individually tested for tip bleaching assay to evaluate their potential pathogenicity. Ten isolates significantly reduced the risk of tip bleaching in H. floresii and were designated as “significantly non-pathogenic”. Vibrio owensii was identified as a “significant pathogen” inducing bleaching disease in H. floresii. By using LC-MS, we here identified its HomoSerine Lactones (HSL) QS signal, as a C4-HSL (short-chain). This study thus suggests a possible involvement of QS signal (short-chain) in the disease-inducing bacterium from the aquaculture ponds (an integrated multitrophic aquaculture system). This study firstly reports on the surface-associated bacteria of a lambda-carrageenophyte. This study must contribute to the development of dedicated strategies for disease control based on HSL disruption in aquaculture.
Introduction
Macroalgae are dominant habitat-formers, primary producers, and ecosystem engineers in marine ecosystems. They play a vital role in ecosystems as foundation species by providing habitats for higher trophic levels (Fulton et al., Citation2019). Today, the main streams in macroalgal biotechnology are biofuels, agricultural biostimulants for crop plants, probiotics for aquaculture, wastewater treatment, and biomedical applications from extracted compounds (polyphenols, polysaccharides, etc.). In 2018, aquaculture produced worldwide 32.4 MT of aquatic algae for food and phycocolloid production (Barzkar, Jahromi, Poorsaheli, & Vianello, Citation2019; FAO, Citation2020). As the macroalgal aquaculture industry grew up and diversified into new species and geographical areas, new diseases have emerged and the risk of introducing non-indigenous pathogens to these new areas has increased (Cottier-Cook et al., Citation2016). In recent years, diseases such as rotting, cell wall degradation, brown points disease, and thalli bleaching, are considered to be the major contributing factors to the rapid decline in macroalgal production (Beleneva & Zhukova, Citation2006; Liu et al., Citation2019; Schroeder, Jaffer, & Coyne, Citation2003). The recent development of intensive and dense aquaculture practices has worsened the situation (Gachon, Sime-Ngando, Strittmatter, Chambouvet, & Kim, Citation2010) which ultimately results in a decline of the production yields (Ward et al., Citation2019). Seaweed diseases can be divided into non-infectious and infectious ones. Non-infectious diseases are caused by unfavourable environmental conditions, such as changes in temperature, salinity, or light exposure. Seaweeds which are stressed and weakened by such unfavourable environmental conditions are more susceptible to infectious diseases caused by pathogens (virus, bacteria, fungi) (Loureiro, Hurtado, & Critchley, Citation2017). Today, it is known that microbial partners often play a crucial part in macroalgal health, functioning, and development during the host’s various life cycle steps through mutualistic interactions (Egan et al., Citation2013; Van der Loos, Eriksson, & Falcão Salles, Citation2019; Wahl, Goecke, Labes, Dobretsov, & Weinberger, Citation2012). The macroalgal host together with its microbial partners forms a functional unit called “holobiont” (Abdul Malik et al., Citation2020a; Duarte et al., Citation2018; Egan et al., Citation2013; Hollants, Leliaert, De Clerck, & Willems, Citation2013; Wahl et al., Citation2012). Disturbance or disruption of the mutualistic association between host and microbiota can result in diseases through holobiont break-up. The holobiont approach has allowed a paradigm shift in our understanding of microbial-induced diseases (Longford et al., Citation2019). Several diseases are caused by a single pathogen (Austin & Zhang, Citation2006; Bourne et al., Citation2009; Cook et al., Citation2013), whereas other studies argue that marine diseases could also be triggered by multiple pathogens (Brown, Cornforth, & Mideo, Citation2012; Joyner et al., Citation2015; Liu et al., Citation2019; Sato, Civiello, Bell, Willis, & Bourne, Citation2016).
Epiphytic bacterial communities form a “core microbial species” of macroalgae by dominating the other microbial partners in the ratio of 640:4:1 (bacteria: diatoms: flagellates) (Abdul Malik et al., Citation2020a; Singh & Reddy, Citation2016). In that sense, either symbiotism or pathogenism must be essentially mastered by the bacterial members. The pathogenicity of such opportunists is tremendously mediated by the Quorum Sensing (QS) synchronization mechanism within the bacterial population (Zhang & Dong, Citation2004). QS, a type of bacterial communication, is ubiquitous and known to control the expression of virulence factors targeting many aquaculture pathogens and also medically important organisms (De Kievit & Iglewski, Citation2000; Donabedian, Citation2003; Natrah, Defoirdt, Sorgeloos, & Bossier, Citation2011a). Since macroalgae lack a cell-based adaptive immune response, they have evolved to some constitutive and inducible defence against bacterial diseases. As plants’ diseases are viewed as a major issue in marine ecological communities and aquaculture plants, the ability of macroalgae to counteract the bacterial QS could root the development of antibacterial solutions in regards to more friendly environmental aquacultural practices (Qian, Lau, Dahms, Dobretsov, & Harder, Citation2007; Tintillier et al., Citation2020).
Halymenia floresii, a native species off the Yucatan peninsula coast of Mexico is abundant on rocky substrates. Because of its human consumption in Asia and its λ-carrageenan content, the economic interest of H. floresii has been highly considered for cultivation (Freile-Pelegrín, Azamar, & Robledo, Citation2011; Godínez-Ortega, Snoeijs, Robledo, Freile-Pelegrín, & Pedersén, Citation2008). H. floresii is also a good candidate to recycle inorganic nutrients in the Integrated Multi Trophic Aquaculture (IMTA) system, producing biomass while reducing the environmental impact of coastal ecosystems through responsible aquaculture practices (Godínez-Ortega et al., Citation2008; Robledo & Freile-Pelegrín, Citation2011). Besides, even at high concentrations of Dissolved Inorganic Nitrogen (DIN ~ 150 µM), no noticeable growth of epiphytes was observed over H. floresii thalli (Pliego-Cortés, Caamal-Fuentes, Montero-Muñoz, Freile-Pelegrín, & Robledo, Citation2017). In a previous study (Abdul Malik et al., Citation2020b) we have reported that the surface metabolites of H. floresii significantly interferes with the QS communication of its surface-associated microbial partners which was evident by a bioluminescent assay performed with Escherichia coli pSB406, as a reporter strain. In the present work, our objective is to identify the significant opportunistic pathogen(s) out of the epibacterial communities of H. floresii by their ability to induce bleaching, a loss of pigmentation in response to pathogen harm or damage and to identify their corresponding QS signals (i.e., homoserine lactones).
Materials and methods
Algal material collection
With a rigorous sampling, H. floresii beach-cast (BC) healthy individuals (n = 60) were collected from the shores near CINVESTAV Coastal Marine Station at Telchac, Yucatán, México (21.3419° N, 89.2636° W). They were thoroughly washed with ambient seawater (30 psu, from the site of collection) to remove sand and epiphytes, packed in polythene bags, and transported to the CINVESTAV in a cooler box within 1–2 h of collection. The individuals were rinsed with sterile seawater (30 psu) (autoclaved and filtered (0.5 micron) and UV-sterilized (UV-filter 0.4 micron)) and transferred to 25 l acrylic cylinders for acclimatization for 15 days under continuous aeration at a 12:12 light/dark cycle with a light intensity of 110 ± 7.2 µmol photons m–2 s–1 at 25°C. After 15 days of acclimatization under controlled culture conditions in the cultivar chamber, the healthy H. floresii individuals of similar size (n = 7) were carefully selected and healthy tips without any cut or physically damaged ends (ca. 2–3 cm) were excised. These tips (n = 6) were further used for the tip bleaching assay.
Bacterial isolates
A total of 31 epibacterial strains were previously isolated (Abdul Malik et al., Citation2020a) from the material collected from different habitats: Beach-cast (BC), Integrated MultiTrophic Aquaculture (IM), and Cultivar Chamber (CC). These strains were previously isolated and identified by 16S rRNA. To evaluate the potential ability of epibacterial strains to induce thallus bleaching in Halymenia floresii, 25 of the cryopreserved bacterial strains were revived and maintained at 25°C in Marine Agar (MA) medium in darkness until they reached an OD600 of 0.2 to 0.3. The actively growing colonies were used for the tip bleaching assay and HSL extraction.
Tip bleaching assay with single epibacterial strains
Tip bleaching assays were performed according to Saha and Weinberger (Citation2019) with minor modifications. H. floresii tips (ca. 2–3 cm long, n = 6) excised from distinct healthy individuals of similar size (n = 7) were placed into distinct 6-well plates, each well containing 3 ml of sterile seawater (SSW, 30 psu). To eliminate epibacteria, the tips were pre-treated by adding to each well two antibiotics: Vancomycin and Cefotaxim (0.1 mg ml–1 each). Then, the plates were incubated at 25°C at a photon flux density of 38.3 µmol photon m–2 s–1 for two days. Prior to the assay, the effect of the antibiotics at a concentration of 0.1 mg ml–1 each was confirmed by imprinting the treated tips on marine agar and observed for no growth of bacteria after five days of incubation. After antibiotic pre-treatment, plates were carefully emptied, and H. floresii tips were rinsed with 1.5 ml of SSW to remove antibiotics. Finally, 3 ml of SSW were added into each well, and previously revived and cultured bacteria were immediately inoculated by addition of 30 µl.
Controls consisted of the same volume of sterile culture medium. After 5 days of incubation at 20°C at a photon flux density mentioned above, wells were checked under the binocular microscope (magnification factor: 45X) and the numbers of bleached and non-bleached tips in each well were counted against a dark background. For each strain, the relative risk of thallus tip bleaching was evaluated from the comparison of the number of bleached tips in treated and non-treated samples by using the Student’s t-test. Isolates that turned out to be pathogenic after Student’s t-test (i.e., p = n.d.) were designated as “significant pathogens”, and those which were non-significant after Student’s t-test (i.e., p ≤ 0.175) were called “potential pathogens”. Isolates that reduced the risk of thallus tip bleaching were designated as “non-pathogens”. Nevertheless, after applying Student's t-test, isolates that significantly reduced the risk of thallus tip bleaching (i.e., p = n.d.) were also called “significant non-pathogens” while those that also reduced the tip bleaching (p = 0.363), but were not significant after applying Student’s t-test, are designated as ‘potential non-pathogens”.
Acylated Homoserine Lactones extraction
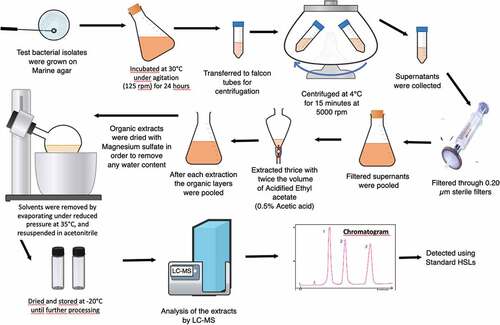
AHLs were extracted from the “significant pathogen” identified by the tip-bleaching assay (). This selected bacterium was previously revived on Marine Agar (MA) plates. A single colony was inoculated into 100 ml of Marine Broth (MB) medium and incubated for 24 hours under constant agitation (125 rpm) at 25°C. Following 24 hours of culture, liquid: liquid extraction was performed to extract the AHLs according to previously described methods (Fletcher, Diggle, Cámara, & Williams, Citation2007; Wang, Quan, Wang, Zhao, & Fan, Citation2011). Briefly, the 24 hours bacterial culture was centrifugated (4°C for 15 min at 4,000 rpm). The supernatant was collected, filtered (Sartorius 0.2 µm sterile filter) and extracted thrice by two equal volumes of acidified ethyl acetate (0.5% acetic acid). The solvent was then evaporated under vacuum at 35°C and the residue resuspended in acetonitrile. The AHL sample was further dried to remove acetonitrile using nitrogen gas and the dried sample was stored at −20°C until further analysis.
Analysis with Liquid Chromatography-Mass Spectrometry Quadrupole Time Of Flight (LC-MS/QTOF)
Samples were resolubilized in 500 µl acetonitrile. Calibration standards were prepared at a range of 0.05–5 ng μl–1. Chromatographic analysis was performed on a liquid chromatography-mass spectrometry system (LC-MS, Ultimate 3000 Dionex – MicroTof QII, Bruker Daltonics). A total of 20 µl were injected into a Gemini C6 phenyl column (250 mm x 4.6 mm x 5 µm, Phenomenex). The column temperature was kept at 40°C and the flow rate was 0.6 ml min–1. Mobile phases A and B were water/acetonitrile (95/5) and water/acetonitrile (2/98), respectively, both with 10 mM ammonium acetate. The elution programme was performed as follows: 0 to 100% B in 15 min, held at 100% B in 5 min, 100 to 0% B in 1 min, and then returned to 0% B for the next 9 min.
Mass detector with electrospray ionization (ESI) source was performed as follows: positive mode; source temperature: 200°C; capillary voltage: 4.5 kV; nebulizer gas (N2) at 2.8 bar and dry gas (N2) at 12 l min–1. Mass spectra acquisition was set at two acquisitions from m/z 50 to 1,000.
The AHL extracts were compared with commercial standards of N-Butyryl-DL-homoserine lactone (BHL/C4 HSL), N-Hexanoyl-DL-homoserine lactone (HHL/C6 HSL), N-(β-Ketocapropyl)-L-homoserine lactone (3-O-C6-HSL), N-(3-Oxooctanoyl)-L-homoserine lactone (OOHL/3-O-C8 HSL) and N-(3-Oxododecanoyl)-L-homoserine lactone (3-O-C12 HSL) (Sigma). The AHLs were identified based on their retention time and mass spectrum.
Results
Algal material collection
At the end of the acclimatization period of 15 days there were only 20% of the H. floresii specimen which were physiologically stable and healthy. The rest of the specimen were degraded. Thus, we included only healthy H. floresii specimen (n = 7) (see section, Materials and Methods) for the tip-bleaching assay.
Tip bleaching assay
Of the 25 epibacterial strain isolates evaluated in this work, only Vibrio owensii was observed to significantly increase the risk of tip bleaching as compared to controls (no bacterial inoculation) (; ). Vibrio owensii was therefore identified as a “significant pathogen” (Student’s t-test, p = n.d.) (; ). Eight additional isolates: Pseudoalteromonas arabiensis; P. mariniglutinosa; Ruegeria sp.; Alteromonas sp. (B7CC and B12CC); Epibacterium sp.; Alteromonadaceae bacterium; Tateyamaria omphalii had the same effect but were not significantly pathogenic and were thus considered as “potential pathogens” (Student’s t-test, p ≤ 0.175) (; ). Ten out of the 16 remaining isolates were found to induce only reduced tip bleaching (Student’s t-test, p = n.d.) (; ) and were considered as “significant non-pathogens”. The last six showed no effect in inducing bleaching (Student’s t-test, p = 0.363) (; ), however they were not significantly non-pathogenic and were known as ‘potential non-pathogens. Thus, the relative risk of the bacteria analysed to induce or not bleaching in H. floresii was compared; the following pattern was observed: 40% significant non-pathogens vs 4% for significant pathogens and 24% potential non-pathogens vs 32% potential pathogens. It is to be mentioned that no bleaching occurred in control samples.
Figure 2. Risk of thallus tip bleaching in H. alymenia floresii after inoculation of 25 bacterial strains relative to control thalli without bacterial inoculation. No. of replicates for each isolate (n = 6). Error bars ± in %. Coding and colours indicates bacterial strain isolated in different habitats (yellow, BC – Beach-cast; red, IM – Integrated MultiTrophic Aquaculture; green, CC – Cultivar Chamber) species are listed in .
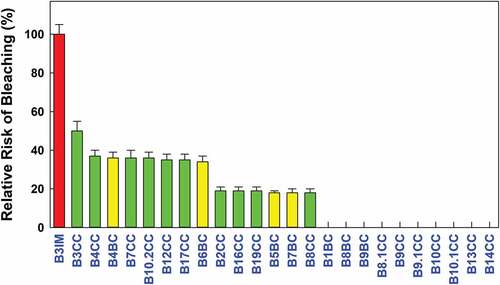
Table 1. Pathogenicity grade of epibacterial strains isolated from Halymenia floresii evaluated by the tip bleaching assay.
Extraction and identification of HSLs from Vibrio owensii
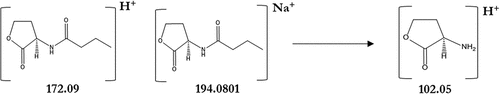
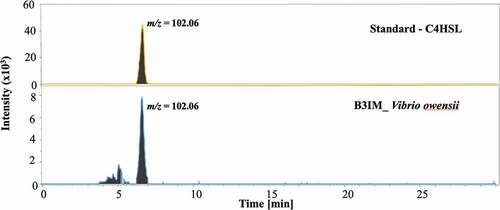
The presence of AHLs was investigated in the acidified ethyl acetate extracts of V. owensii (Accession No. MT176134) after 24 h of culture. The QS activity of V. owensii was previously detected by the bioluminescent reporter strain, Escherichia coli pSB406 (Abdul Malik et al., Citation2020a, Citation2020b). E. coli pSB406 is a lux-based biosensor containing a fusion of rhlRIP::luxCDABE on a pUC18 plasmid backbone, which enables the detection of a wide range of AHLs differing in the length of their acyl chain (Winson et al., Citation1998). To determine the chemical structure of putative HSL, V. owensii extracts were analysed by LC-MS. HSL standards with C4 to C12 acyl chain were shown to be fragmented in ESI ionization source and extraction ion chromatogram at m/z of 102.06 corresponding of lactone ring allowed to highlight the presence of putative HSL. LC-MS results confirmed the presence of N-butyryl-L-homoserine lactone (C4-HSL) in the culture medium of V. owensii grown in MB liquid media. The presence of C4-HSL in the V. owensii sample was identified from its retention time () and its fragmentation pattern () similar to the standard. The chromatograms feature the separation of the standard and the sample of C4-HSL at a retention time range of 6.2–7.0 minutes (). In , the MS spectrum of the C4-HSL in the standard and the sample are shown as at 102.06 (lactone ring); 172.09 (M + H)+ (C4-HSL) and at 194.08 (M+ Na)+ (sodium adduct). The chemical structure of C4-HSL is depicted in for a better understanding of its fundamentally based on the lactone moiety.
Figure 3. Extracted ion chromatogram at m/z 102.06 (=lactone ring) obtained by LC-MS of C4 HSL standard (upper panel) and of V. ibrio owensii (B3IM) extract (lower panel) at a retention time range from 6.2 to 7.0 minutes.
Discussion
Bacterial diseases are among the crucial issues in algal aquaculture farms where the thallus whitening or bleaching is quite common in carrageenophyte farms in which red algae are more vulnerable to bleaching symptoms: in H. floresii the risk of bleaching seems to be higher in farms as compared to beach-cast. Under cultivation, affected segments are usually cut off allowing the unaffected thallus to continue to regenerate and regrow, though with reduced overall productivity (Hurtado, Neish, & Critchley, Citation2019).
Here, opportunistic pathogens are defined as those that are present on both healthy and diseased hosts. They only become harmful following a disturbance of their host (Brown et al., Citation2012). For example, both high organic matter contents and a dense population favour the proliferation of opportunistic bacteria, this inducing in turn stress and making them even more susceptible to diseases (Bachère, Citation2003; Natrah et al., Citation2011a). As this pathogenic shift seems to play an important role in any aquacultural diseases, identifying the host-associated bacteria is a critical step in aquaculture disease management. To facilitate and develop advanced aquacultural techniques, such organized bacterial biofilms that grow at the submerged macroalgal surfaces need to be better understood for their dynamics and chemical (QS) signals that are released and/or accumulated. Identification of these signals/cues for commercial aquaculture species is obviously of interest for the aquaculture industry now and in the future (Lami, Citation2019; Qian et al., Citation2007). As the main contributor of macroalgal biofilm, bacteria assess their population density and synchronize their behaviours such as luminescence, biofilm formation, and virulence by using QS signals (Tintillier et al., Citation2020). HSLs mediated QS systems have been observed in many species of marine pathogenic bacteria which seem to play an important role in the host-microbe interactions in the marine environment (Romero, Avendaño-Herrera, Magariños, Cámara, & Otero, Citation2010) and even more in aquaculture farms.
Following the identification of V. owensii as a “significant pathogen” inducing bleaching in H. floresii (IMTA conditions) in this study, we were able to characterize one of the QS signals (C4-HSL) underlying its QS activity. In H. floresii, bleaching is characterized by a local loss of pigment at the apical ends similar to that of the red alga Gracilaria vermiculophyllum (Saha & Weinberger, Citation2019) which is often associated with the degradation of the cell wall. This bleaching is the consequence of a significant opportunist’s outgrowing (increased surface bacterial density) at the expense of least significant opportunists as well as symbionts. This outgrowing and the accompanying virulence are ruled by QS signals when the bacterial population has reached a critical threshold.
Thus, the production of C4-HSL likely mediates the virulence factors expressed by V. owensii that in turn are responsible for the observed bleaching effect. As C4-HSL was the sole QS signal observed (see ), we may safely identify it, at least in our culture conditions, as ruling the observed Vibrio behaviour. Future studies should focus to evaluate the QS signal interference of the surface extracts in inducing the bleaching disease but yet we have demonstrated that Vibrio owensii’s QS system was significantly interfered by H. floresii’s surface metabolites using a reporter strain, Escherichia coli pSB406 (Abdul Malik et al., Citation2020a). It is to be noted that, in our study, another V. owensii strain (B7BC) (MT176139), observed as a potentially non-pathogen, showed no positive response to the QS interfering activity of H. floresii’s surface metabolites (results not published). Though the strains are similar, they did behave differently, this suggesting that such behaviours depend on the environment they originated, i.e., V. owensii strain (B7BC) and V. owensii strain (B3IM) from beach-cast and IMTA ponds, respectively. It’s worth noting that Suvega & Arunkumar (Citation2019) partially characterized an N-AHL-like compound, a QS signal from the Hypnea valentiae red alga associated with the Lysinibacillus xylanilyticus HVT234 bacterium. This compound was also observed to be a quorum elicitor substance exhibiting antibacterial activity against Xanthomonas oryzae pv. It is also responsible for the growth of the red macroalga, Gracilaria edulis (Suvega & Arunkumar, Citation2019). This illustrates the diversity of the QS protective and pathogenic mechanisms which affect macroalgal growth.
Besides V. owensii, eight epibacterial isolates from H. floresii exhibited some potential to induce bleaching symptoms, although to a lesser extent (33–50% ). Since these detrimental isolates originated from healthy hosts, it suggests that opportunistic pathogens can induce bleaching symptoms when the host health status is reduced as described by several red algae (Case et al., Citation2011; Saha & Weinberger, Citation2019; Weinberger, Citation2007; Weinberger, Hoppe, & Friedlander, Citation1997). The “significant” or “potential” pathogens (9 out of 25 strains) feature 36% of the total isolates tested here, which is more than reported by Saha & Weinberger (Citation2019) who observed that only 5% of surface-associated microbes of the red alga Gracilaria vermiculophyllum induced bleaching. However, this estimation is merely a representative sample of the whole microbiota (Saha & Weinberger, Citation2019). In another red alga, Gracilaria lemaneiformis, the strongest pathogenicity to induce bleaching was observed to be associated with Aquimarina latercula (T) among eight bacterial pathogens (Liu et al., Citation2019) whereas in our study the Aquimarina sp. (B9CC) bacterium was observed as significantly non-pathogenic (showing no effect on bleaching). Possibly, the Aquimarina species were distinct, but it is still worth mentioning the play of the bacterial ecological diversity. Again, this emphasizes the diversity and specificity of pathogenic effects among distinct hosts which likely depend on the environment, including bacterial communities.
Saha & Weinberger (Citation2019) categorized 36 out of 58 isolates (62%) as neutral ones but in this study we excluded the “neutral phenotype” as we didn’t observe any bleaching in the control treatment (only with media). We assumed that our control conditions were not enough drastic to reveal potential neutral phenotypes. So, we considered them as significant non-pathogens, this to say inducing no bleaching at all.
Fernandes et al. (Citation2011) elucidated the ability of Nautella sp. R11 to induce bleaching in the Rhodophyta Dilsea pulchra and they observed that a combination of virulence factors and QS-dependent regulatory mechanisms enabled a shift from symbiotic to a pathogenic lifestyle of the epibacterial community, especially under environmental conditions unfavourable for the host. As the most abundant culturable microbe, the mechanisms related to Vibrio’s pathogenicity are critically needed (Rizzo, Fraschetti, Alifano, Tredici, & Stabili, Citation2016). As reviewed by Natrah et al. (Citation2011b), several different virulence factors of Vibrio causing diseases in aquaculture systems were tightly related to the presence of HSLs, such as N-hexanoyl-L-homoserine lactone (HHL), N-(3-oxohexanoyl)-L-homoserine lactone (OHHL), N-butanoyl-L-homoserine lactone (BHL), N-3-octanoyl homoserine lactone (OHL), N-(3-oxodecanolyl)-L-homoserine Lactone (ODHL). Moreover, V. owensii has been identified as a causative agent of AHPND (Acute Hepato Pancreatic Necrosis Disease) in Litopenaeus vannamei ponds (Liu et al., Citation2018). Interestingly, the V. owensii strain (B3IM) exerts a pathogenic effect on H. floresii cultivated in a land-based IMTA system whereas the B7BC strain isolated from the beach-cast samples failed to induce any bleaching. This stresses that the specific effect of a particular strain depends on the culture conditions and, in turn, on the actual bacterial community.
H. floresii surface compounds at their 1-fold natural surface concentration, significantly interfered with the QS signals of V. owensii by reducing the luminescence of the reporter strain, E. coli pSB406. However, when we tested a higher concentration of the extract it increased the luminescence that may result from an antagonistic effect of the surface-extract on to the Vibrio’s QS system (Abdul Malik et al., Citation2020b). The quorum sensing interference of H. floresii is likely due to the presence of two halogenated secondary metabolites putatively identified on the surface: (4-bromophenyl)[4-({(2E)-4-[cyclopropyl(methyl)amino]but-2-enyl}oxy)phenyl] methanone (Compound 7) and (2E)-N-allyl-4-{[3-(4-bromophenyl)-5-fluoro-1-methyl-1 H-indazol-6-yl]oxy}-N-methyl-2-buten-1-amine (Compound 8) (Abdul Malik et al., Citation2020b). Interestingly, these two brominated compounds are widely observed in red algae such as Bonnemaisonia asparagoides, D. pulchra, and Asparagopsis taxiformis to exhibit antimicrobial activities (De Nys, Dworjanyn, & Steinberg, Citation1998; Greff et al., Citation2014; Nylund et al., Citation2010).
Elucidating the interplay between QS systems and host surface secondary metabolites in maintaining microbial balance in holobionts will promote important applications in aquaculture and/or antifouling industries (Lami,) since bacteria-induced infectious diseases like those triggered by Vibrio are the main cause of economic losses in aquaculture. The expression of virulence genes in some Vibrio species is controlled by signal molecules such as HSLs. One of the aquaculture-related pathogenic Vibrio strains, V. owensii VibC-Oc-106, isolated from the seawater, was reported to produce N-(3-hydroxy – hexanoyl)-homoserine lactone (3-OH-C6-HSL), N-(3-hydroxy-heptanoyl)-homoserine lactone (3-OH-C7-HSL), and N-tridecanoyl-homoserine lactone (C13-HSL) (Torres et al., Citation2018). In our work, it was observed that the macroalgae surface-associated V. owensii produced the extracellular C4-HSL. However, Girard et al. (Citation2017) did not detect any AHLs from strains of V. owensii from geographically distinct marine environments even after using three different biosensors (Pseudomonas putida [pKR-C12], E. coli [pJBA-132], and Chromobacterium violaceum [CV026]) which covered a large spectrum of AHLs. These findings suggest that many factors originate the diversity of QS signals expressed in a particular ecosystem like the Vibrio strain, the local microbiont community and the host that should influence the molecular handshake and/or fighting within the holobiont. We may further hypothesize that the QS signal of the pathogenic vibrio is tuned in order to not be interfered by those of other strains, making it thereby a critical factor for bacterial populations dynamic balances.
In conclusion the world production of carrageenophytes is around 160 MT of macroalgae (dry weight), from which 28KT of carrageenan are extracted for a value of USD270 million (Freile-Pelegrín & Robledo, Citation2016). Preventing the disease outbreak in aquaculture with a more environmentally friendly approach as compared to treating or removing the diseased segments after the disease outbreak is critical. Indeed, QS interference is cost-effective for preventing and/or inhibiting the virulence of pathogens and hence reducing diseases while increasing productivity in aquaculture systems (Zhao, Chen, Quan, & Fan, Citation2015). As the surface extracts of H. floresii interfere with the QS activity of V. owensii, this alga appears as a potential candidate to produce compounds for disease control in aquaculture. Moreover, Pliego-Cortés et al. (Citation2019) described H. floresii as an efficient cultivar to recycle inorganic nutrients in a land-based IMTA reducing thereby the environmental impact on coastal ecosystems through responsible aquaculture practices. Besides its use as a carrageenophyte, H. floresii could play a significant role among green approaches, an eco-friendly way to improve aquaculture productivity through sustainable environmental practices and thus preventing the environmental pollution, for instance, reducing the use of antibiotics in aquaculture system.
HSL production by Vibrio owensii is also significant as this bacterium is a well-known pathogen in shrimp aquaculture. In addition to the HSLs regulation of virulence mechanisms in other Vibrio species so far described (Frans et al., Citation2011; Girard et al., Citation2017; Lilley & Bassler, Citation2000; Zhu et al., Citation2002), the present data improve our understandings of the role of HSLs in the physiology and pathogenicity of these microorganisms (Girard et al., Citation2017) since its versatility – either pathogenic or synergic- regarding both the host and the culture conditions is emphasized. From our knowledge, this work is the first about identification of an HSL secreted by a pathogen strain of H. floresii. Future works should highlight the link between this signal molecule and the pathogenicity of this strain. Addition of a quorum quencher such as a lactonase could decrease the bleaching of the algae and comfort this possible link. This will contribute to a better understanding of the molecular mechanisms behind algal diseases.
Supplemental Material
Download MS Excel (11.2 KB)Acknowledgments
The authors thank V. Avila-Velazquez for H. floresii cultivation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/26388081.2022.2086483
Additional information
Funding
References
- Abdul Malik, S. A., Bazire, A., Gamboa-Muñoz, A., Bedoux, G., Robledo, D., García-Maldonado, J. Q., & Bourgougnon, N. (2020a). Screening of surface-associated bacteria from the Mexican red alga Halymenia floresii for quorum sensing activity. Microbiology, 89, 778–788.
- Abdul Malik, S. A., Bedoux, G., Robledo, D., García-Maldonado, J. Q., Freile-Pelegrín, Y., & Bourgougnon, N. (2020b). Chemical defense against microfouling by allelopathic active metabolites of Halymenia floresii (Rhodophyta). Journal of Applied Phycology, 32, 2673–2687. doi:10.1007/s10811-020-02094-4
- Austin, B., & Zhang, X.-H. (2006). Vibrio harveyi: A significant pathogen of marine vertebrates and invertebrates. Letters in Applied Microbiology, 43, 119–124.
- Bachère, E. (2003). Anti-infectious immune effectors in marine invertebrates: Potential tools for disease control in larviculture. Aquaculture, 227, 427–438.
- Barzkar, N., Jahromi, S. T., Poorsaheli, H. B., & Vianello, F. (2019). Metabolites from marine microorganisms, micro, and macroalgae: Immense scope for pharmacology. Marine Drugs, 17, 464.
- Beleneva, I. A., & Zhukova, N. V. (2006). Bacterial communities of some brown and red algae from peter the Great Bay, the sea of Japan. Microbiology, 75, 348–357.
- Bourne, D. G., Garren, M., Work, T. M., Rosenberg, E., Smith, G. W., & Harvell, C. D. (2009). Microbial disease and the coral holobiont. Trends in Microbiology, 17, 554–562.
- Brown, S. P., Cornforth, D. M., & Mideo, N. (2012). Evolution of virulence in opportunistic pathogens: Generalism, plasticity, and control. Trends in Microbiology, 20, 336–342.
- Case, R. J., Longford, S. R., Campbell, A. H., Low, A., Tujula, N., Steinberg, P. D., & Kjelleberg, S. (2011). Temperature-induced bacterial virulence and bleaching disease in a chemically defended marine macroalga. Environmental Microbiology, 13, 529–537.
- Cook, G. M., Rothenberger, J. P., Sikaroodi, M., Gillevet, P. M., Peters, E. C., & Jonas, R. B. (2013). A comparison of culture-dependent and culture-independent techniques used to characterize bacterial communities on healthy and white plague-diseased corals of the Montastraea annularis species complex. Coral Reefs, 32, 375–388.
- De Kievit, T. R., & Iglewski, B. H. (2000). Bacterial quorum sensing in pathogenic relationships. Infection and Immunity, 68, 4839–4849.
- De Nys, R., Dworjanyn, S. A., & Steinberg, P. D. (1998). A new method for determining surface concentrations of marine natural products on seaweeds. Marine Ecology Progress Series, 162, 79–87.
- Donabedian, H. (2003). Quorum sensing and its relevance to infectious diseases. Journal of Infection, 46, 207–214.
- Duarte, C., Navarro, J. M., Quij, P. A., Loncon, D., Torres, R., Manríquez, P. H., & Lagos, N. A. (2018). The energetic physiology of juvenile mussels, (Hupe): The prevalent role of salinity under current and predicted pCO2 scenarios. Environmental Pollution, 242, 156–163.
- Egan, S., Harder, T., Burke, C., Steinberg, P., Kjelleberg, S., & Thomas, T. (2013). The seaweed holobiont: Understanding seaweed–bacteria interactions. FEMS Microbiology Reviews, 37, 462–476.
- FAO. (2020). The state of world fisheries and aquaculture 2020. Sustainability in action. https://doi.org/10.4060/ca9229en
- Fernandes, N., Case, R. J., Longford, S. R., Seyedsayamdost, M. R., Steinberg, P. D., Kjelleberg, S., & Thomas, T. (2011). Genomes and virulence factors of novel bacterial pathogens causing bleaching disease in the marine red alga Delisea pulchra. PLoS One, 6, e27387.
- Fletcher, M. P., Diggle, S. P., Cámara, M., & Williams, P. (2007). Biosensor-based assays for PQS, HHQ and related 2-alkyl-4-quinolone quorum sensing signal molecules. Nature Protocols, 2, 1254–1262.
- Frans, I., Michiels, C. W., Bossier, P., Willems, K. A., Lievens, B., & Rediers, H. (2011). Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. Journal of Fish Diseases, 34, 643–661.
- Freile-Pelegrín, Y., Azamar, J. A., & Robledo, D. (2011). Preliminary characterization of carrageenan from the red seaweed Halymenia floresii. Journal of Aquatic Food Product Technology, 20, 73–81.
- Freile-Pelegrín, Y., & Robledo, D. (2016). Prospects for carrageenan production in tropical waters of Yucatan peninsula. In L. Pereira (Ed.), Carrageenans (pp. 87–100). NewYork: Nova Publishers.
- Fulton, C. J., Abesamis, R. A., Berkström, C., Depczynski, M., Graham, N. A. J., Holmes, T. H., & Wilson, S. K. (2019). Form and function of tropical macroalgal reefs in the Anthropocene. Functional Ecology, 33, 989–999.
- Gachon, C. M. M., Sime-Ngando, T., Strittmatter, M., Chambouvet, A., & Kim, G. H. (2010). Algal diseases: Spotlight on a black box. Trends in Plant Science, 15, 633–640.
- Girard, L., Blanchet, É., Intertaglia, L., Baudart, J., Stien, D., Suzuki, M., & Lami, R. (2017). Characterization of N-Acyl homoserine lactones in Vibrio tasmaniensis LGP32 by a biosensor-based UHPLC-HRMS/MS method. Sensors (Switzerland), 17, 906.
- Godínez-Ortega, J. L., Snoeijs, P., Robledo, D., Freile-Pelegrín, Y., & Pedersén, M. (2008). Growth and pigment composition in the red alga Halymenia floresii cultured under different light qualities. Journal of Applied Phycology, 20, 253–260.
- Greff, S., Zubia, M., Genta-Jouve, G., Massi, L., Perez, T., & Thomas, O. P. (2014). Mahorones, highly brominated Cyclopentenones from the red alga Asparagopsis taxiformis. Journal of Natural Products, 77, 1150–1155.
- Hollants, J., Leliaert, F., De Clerck, O., & Willems, A. (2013). What we can learn from sushi: A review on seaweed-bacterial associations. FEMS Microbiology Ecology, 83, 1–16.
- Hurtado, A. Q., Neish, I. C., & Critchley, A. T. (2019). Phyconomy: The extensive cultivation of seaweeds, their sustainability and economic value, with particular reference to important lessons to be learned and transferred from the practice of eucheumatoid farming. Phycologia, 58, 472–483.
- Joyner, J. L., Sutherland, K. P., Kemp, D. W., Berry, B., Griffin, A., Porter, J. W., & Lipp, E. K. (2015). Systematic analysis of white pox disease in Acropora palmata of the Florida keys and role of Serratia marcescens. Applied and Environmental Microbiology, 81, 4451–4457.
- Lami, R. (2019). Quorum sensing in marine biofilms and environments. In G. Tommonaro (Ed.), Quorum sensing: Molecular mechanism and biotechnological application (pp. 55–96). Academic Press. doi:10.1016/B978-0-12-814905-8.00003-4
- Lilley, B. N., & Bassler, B. L. (2000). Regulation of quorum sensing in Vibrio harveyi by LuxO and Sigma-54. Molecular Microbiology, 36, 940–954.
- Liu, L., Xiao, J., Zhang, M., Zhu, W., Xia, X., Dai, X., & Wang, Y. (2018). A Vibrio owensii strain as the causative agent of AHPND in cultured shrimp, Litopenaeus vannamei. Journal of Invertebrate Pathology, 153, 156–164.
- Liu, X., Chen, Y., Zhong, M., Chen, W., Lin, Q., & Du, H. (2019). Isolation and pathogenicity identification of bacterial pathogens in bleached disease and their physiological effects on the red macroalga Gracilaria lemaneiformis. Aquatic Botany, 153, 1–7.
- Longford, S. R., Campbell, A. H., Nielsen, S., Case, R. J., Kjelleberg, S., & Steinberg, P. D. (2019). Interactions within the microbiome alter microbial interactions with host chemical defences and affect disease in a marine holobiont. Scientific Reports, 9, 1363.
- Loureiro, R. R., Hurtado, A. Q., & Critchley, A. T. (2017). Impacts of AMPEP on Epiphytes and Diseases in Kappaphycus and Eucheuma Cultivation. In A. Hurtado, A. Critchley, I. Neish (Eds.), Tropical Seaweed Farming Trends, Problems and Opportunities. Developments in Applied Phycology (Vol. 9.). Cham: Springer. doi:10.1007/978-3-319-63498-2_6
- Natrah, F. M. I., Defoirdt, T., Sorgeloos, P., & Bossier, P. (2011a). Disruption of bacterial cell-to-cell communication by marine organisms and its relevance to aquaculture. Marine Biotechnology, 13, 109–126.
- Natrah, F. M. I., Ruwandeepika, H. A. D., Pawar, S., Karunasagar, I., Sorgeloos, P., Bossier, P., & Defoirdt, T. (2011b). Regulation of virulence factors by quorum sensing in Vibrio harveyi. Veterinary Microbiology, 154, 124–129.
- Nylund, G. M., Persson, F., Lindegarth, M., Cervin, G., Hermansson, M., & Pavia, H. (2010). The red alga Bonnemaisonia asparagoidesa regulates epiphytic bacterial abundance and community composition by chemical defence. FEMS Microbiology Ecology, 71, 84–93.
- Pliego-Cortés, H., Caamal-Fuentes, E., Montero-Muñoz, J., Freile-Pelegrín, Y., & Robledo, D. (2017). Growth, biochemical and antioxidant content of Rhodymenia pseudopalmata (Rhodymeniales, Rhodophyta) cultivated under salinity and irradiance treatments. Journal of Applied Phycology, 29, 2595–2603.
- Pliego-Cortés, H., Bedoux, G., Boulho, R., Taupin, L., Freile-Pelegrín, Y., Bourgougnon, N., & Robledo, D. (2019). Stress tolerance and photoadaptation to solar radiation in Rhodymenia pseudopalmata (Rhodophyta) through mycosporine-like amino acids, phenolic compounds, and pigments in an integrated multi-trophic aquaculture system. Algal Research, 41, 101542.
- Qian, P.-Y., Lau, S. C. K., Dahms, H.-U., Dobretsov, S., & Harder, T. (2007). Marine biofilms as mediators of colonization by marine macroorganisms: Implications for antifouling and aquaculture. Marine Biotechnology, 9, 399–410.
- Rizzo, L., Fraschetti, S., Alifano, P., Tredici, M. S., & Stabili, L. (2016). Association of Vibrio community with the Atlantic Mediterranean invasive alga Caulerpa cylindracea. Journal of Experimental Marine Biology and Ecology, 475, 129–136.
- Robledo, D., & Freile-Pelegrín, Y. (2011). Prospects for the cultivation of economically important carrageenophytes in Southeast Mexico. Journal of Applied Phycology, 23, 415–416.
- Romero, M., Avendaño-Herrera, R., Magariños, B., Cámara, M., & Otero, A. (2010). Acylhomoserine lactone production and degradation by the fish pathogen Tenacibaculum maritimum, a member of the Cytophaga-Flavobacterium-Bacteroides (CFB) group. FEMS Microbiology Letters, 304, 131–139.
- Saha, M., & Weinberger, F. (2019). Microbial “gardening” by a seaweed holobiont: Surface metabolites attract protective and deter pathogenic epibacterial settlement. Journal of Ecology, 1365–2745. doi:10.1111/1365-2745.13193
- Sato, Y., Civiello, M., Bell, S. C., Willis, B. L., & Bourne, D. G. (2016). Integrated approach to understanding the onset and pathogenesis of black band disease in corals. Environmental Microbiology, 18, 752–765.
- Schroeder, D. C., Jaffer, M. A., & Coyne, V. E. (2003). Investigation of the role of a β(1 - 4) agarase produced by Pseudoalteromonas gracilis B9 in eliciting disease symptoms in the red alga Gracilaria gracilis. Microbiology, 149, 2919–2929.
- Singh, R. P., & Reddy, C. R. K. (2016). Unraveling the functions of the macroalgal microbiome. Frontiers in Microbiology, 6, 1488.
- Sun, X., He, Y., Xu, N., Xia, Y., & Liu, Z. (2012a). Isolation and identification of two strains of pathogenic bacteria and their effects on the volatile metabolites of Gracilariopsis lemaneiformis (Rhodophyta). Journal of Applied Phycology, 24, 277–284.
- Sun, Y., Zhang, J., Xu, S., Li, W., & Wang, C. (2012b). Growth inhibition of Karenia mikimitoi by extracts from Gracilaria lemaneiformis using five solvents. In E., Z., S., S (Ed.), Information technology and agricultural engineering. advances in intelligent and soft computing (pp. 199–210). Berlin, Heidelberg: Springer. doi:10.1007/978-3-642-27537-1_26.
- Suvega, T., & Arunkumar, K. (2019). Probiotic bacteria promote the growth of associating host (red seaweed, Gracilaria edulis) also synthesize antibacterial protein. Biocatalysis and Agricultural Biotechnology, 19, 101136.
- Tintillier, F., Moriou, C., Petek, S., Fauchon, M., Hellio, C., Saulnier, D., & Debitus, C. (2020). Quorum sensing inhibitory and antifouling activities of new bromotyrosine metabolites from the polynesian sponge pseudoceratina n. sp. Marine Drugs, 18, 272.
- Torres, M., Reina, J. C., Fuentes-Monteverde, J. C., Fernández, G., Rodríguez, J., Jiménez, C., & Llamas, I. (2018). AHL-lactonase expression in three marine emerging pathogenic Vibrio spp. reduces virulence and mortality in brine shrimp (Artemia salina) and Manila clam (Venerupis philippinarum). PLoS One, 13, e019517.
- Van der Loos, L. M., Eriksson, B. K., & Falcão Salles, J. (2019). The macroalgal holobiont in a changing sea. Trends in Microbiology, 27, 635–650.
- Wahl, M., Goecke, F., Labes, A., Dobretsov, S., & Weinberger, F. (2012). The second skin: Ecological role of epibiotic biofilms on marine organisms. Frontiers in Microbiology, 3, 292.
- Wang, J., Quan, C., Wang, X., Zhao, P., & Fan, S. (2011). Extraction, purification and identification of bacterial signal molecules based on N-acyl homoserine lactones. Microbial Biotechnology, 4, 479–490.
- Ward, G. M., Faisan, J. P., Cottier-Cook, E. J., Gachon, C., Hurtado, A. Q., Lim, P. E., & Brodie, J. (2019). A review of reported seaweed diseases and pests in aquaculture in Asia. Journal of the World Aquaculture Society (JWAS), 12649, 1–14.
- Weinberger, F., Hoppe, H. G., & Friedlander, M. (1997). Bacterial induction and inhibition of a fast necrotic response in Gracilaria conferta (Rhodophyta). Journal of Applied Phycology, 9, 277–285.
- Weinberger, F. (2007). Pathogen-induced defense and innate immunity in macroalgae. The Biological Bulletin, 213, 290–302.
- Winson, M. K., Swift, S., Fish, L., Throup, J. P., Jã¸rgensen, F., Chhabra, S. R., & Stewart, G. S. A. (1998). Construction and analysis of luxCDABE -based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiology Letters, 163, 185–192.
- Zhang, L. H., & Dong, Y. H. (2004). Quorum sensing and signal interference: Diverse implications. Molecular Microbiology, 53, 1563–1571.
- Zhao, J., Chen, M., Quan, C. S., & Fan, S. D. (2015). Mechanisms of quorum sensing and strategies for quorum sensing disruption in aquaculture pathogens. Journal of Fish Diseases, 38, 771–786.
- Zhu, J., Miller, M. B., Vance, R. E., Dziejman, M., Bassler, B. L., & Mekalanos, J. J. (2002). Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proceedings of the National Academy of Sciences of the United States of America, 99, 3129–3134.

![Figure 4. MS spectra of C4-HSL standard and V. ibrio owensii extract. Marked m/z correspond to lactone ring (m/z 102.06), [M + H] + proton adduct of C4-HSL (m/z 172.09) and [M+ Na] + sodium adduct of C4-HSL (m/z 194.08).](/cms/asset/e98d5ce8-8baa-4f17-8a43-bdf30c5ca219/tapy_a_2086483_f0004_oc.jpg)