?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A laboratory scale prototype of a novel tube called AlgaTube™ (patent pending) was evaluated for growth of Arthrospira platensis (commonly referred to as Spirulina) in a controlled, 10-day batch trial at low light intensity. The two cultures (control and prototype) were grown in identical conditions inside a closed incubator space at 32°C with a mean light intensity of 55 µmol photons m−2 s−1 LED light, consisting mostly of red and blue wavelengths with some full spectrum background radiation. OD590 was measured daily and converted to Concentration (Cd, g l−1) using a standard curve. Four independent replicates of the trial were run. Six biomass growth metrics were evaluated and compared. Across the board, all six growth metrics showed higher rates of biomass growth in the AlgaTube™. Maximum concentration was 34% higher (p = 0.02), cumulative production was 41% higher (p = 0.02), specific growth rate was 17% higher (p = 0.02), maximum specific growth rate was 23% higher (p = 0.02), mean daily production was 41% higher (p = 0.001), and maximum daily production was 38% higher (p = 0.19). We conclude that the novel shape of the AlgaTube™ prototype increased biomass growth rates. We believe that this study constitutes successful proof-of-concept for the AlgaTube™, but further studies are needed to optimize its performance.
Introduction
Photosynthetic microorganisms have been studied as a feedstock for many commercial and industrial applications, including: CO2 sequestration, biofuels, human nutrition, fish and livestock feed, air purification, fertilizer, cosmetics, pharmaceutical manufacturing, wastewater remediation, and bioplastics (Stanley & Jones, Citation1976; Benemann, Citation1979; Kumar, Dasgupta, Nayak, Lindblad, & Das, Citation2011; Priyadarshani & Biswajit, Citation2012; Rasala & Mayfield, Citation2015; Christaki, Karatzia, & Florou-Paneri, Citation2010; Poonam & Sharma, Citation2017; Adeniyi, Azimov, & Burluka, Citation2018; Joshi, Citation2018; Liu, Pemberton, Lewis, Scales, & Martin, Citation2020; Arora, Kumar, Bose, Li, & Kulshrestha, Citation2021; Chong et al., Citation2021; Shahi et al., Citation2022). The United States Department of Energy investigated photosynthetic microorganisms as a feedstock for biofuels during the 1980s and 1990s (Sheehan, Dunahay, Benemann, Roessler, & Weissman, Citation1998). Several companies are currently marketing such products [e.g., Cyanotech, TrueAlgae, and Algeternal] (Tacon & Metian, Citation2008). During 2019, the US National Renewable Energy Laboratory published a review of the state-of-the-art for photosynthetic algae production (Clippinger & Davis, Citation2019).
Industrial applications can be divided into three steps: (1) growth and production, (2) harvest and processing, and (3) marketing and delivery. The present study is focused exclusively on the production step, which is common to all the applications listed above. Thus, the present study (and the associated product) could have a broad impact on all of the applications mentioned above. It is a platform technology.
Production technology has historically been focused on the open pond system, also known as the “raceway”, and some still use that technique (Cyanotech). More recently, closed production systems have been built using clear, cylindrical piping (Phytobloom). These are generally referred to as tubular photobioreactors (tPBRs). Closed systems are more costly to build, but they offer advantages such as protection against contamination, control of culture conditions, and increased production rates (Clippinger & Davis, Citation2019).
Different variables can impact the production rate of photobioreactors (PBRs). Temperature, light, pH, salinity, nutrients, and gas exchange can all be varied. Each variable can have a distinct impact on the culture growth rate (Hoseini, Almodares, Afsharzadeh, Shahriari, & Montazeri, Citation2014; Kendirlioglu & Cetin, Citation2017; Olaizola & Duerr, Citation1990; Soni, Sudhakar, & Rana, Citation2017, Citation2019; Srinivasan & Illanjiam, Citation2021a, Citation2021b; Tayebati, Shariati, Soltani, & Tehrani, Citation2020; Uslu et al., Citation2009; Wang, Fu, & Liu, Citation2007).
Cultures of photosynthetic algae and cyanobacteria have been observed to experience growth inhibition by the shadow effect, also referred to as the self-shading effect (Frontasyeva et al., Citation2009; Olaizola & Duerr, Citation1990; Soni, Sudhakar, & Rana, Citation2019). This occurs when the cell density is high enough that the cells shade each other, thus blocking each other’s access to needed light. At particularly high concentrations, light penetration is severely restricted. (González-Camejo et al., Citation2019; Shigesada & Okubo, Citation1981).
Other studies have successfully shown that increasing the surface-area-to-volume ratio (SA/V) improves the growth rate by exposing greater numbers of cells to direct irradiance, thus reducing the shadow effect (Converti, Lodi, Del Borghi, & Solisio, Citation2006; da Silva et al., Citation2016; Fremento et al., Citation2013). In those studies, SA/V was increased by reducing the diameter of cylindrical tubes, an effect that is inherent in the Euclidean geometry of standard cylindrical tubes. In the present study, a different approach was taken to increasing the SA Vol−1 ratio of culture vessels. The SA Vol−1 was changed, not by reducing the diameter, but by using corrugated sidewalls in lieu of conventional sidewalls.
The purpose of the present study was to evaluate a prototypic tube with enhanced, corrugated surface area, the AlgaTube™, for its ability to reduce the shadow effect and, thereby, improve the rate of photosynthetic production, when compared to the standard, cylindrical tubes that are widely used as state-of-the-art in tubular photobioreactors. The novel shaped culture vessel tested here was shown to significantly improve the growth rate. The increase was most likely due to changing the shape of the sidewalls of the cylindrical culture vessel significantly reducing the self-shading effect by increasing the surface area of the sidewalls of the culture vessel. This new configuration may help to improve the economics of any industrial process that utilizes photosynthetic microorganisms such as cyanobacteria and algae.
Materials and methods
Culture vessels
The surface area of the sidewalls of the AlgaTube™ was the primary variable that we isolated and evaluated in this experiment. Here, we use the term Photon Surface Area (PSA) to label this key variable and to differentiate it from the cross-sectional surface area (CSSA), which is a different measure altogether. In the present study, PSA Vol−1 is equivalent to the “SA/V” metric used by Converti, da Silva and other referenced authors. The control flask was a standard, cylinder-shaped vessel. Both vessels were custom made by 3-D printing, using the same resin and the same manufacturing technology (DMS Somos® Watershed XC 11,122 resin and Stereolithography (SLA)). The other dimensions of the two flasks were chosen such that the remaining flasks were chosen such that the remaining functional dimensions of the two flasks were identical ( and ).
Figure 1. Representation of the cross sections of the two flasks. The control flask (a) on the left, and the prototype flask (b) on the right have identical cross-sectional areas, but the sidewall configuration of the two flasks is different. These diagrams are intended for illustrative purposes only. They are not presented to scale, nor are they intended for manufacturing or engineering purposes. The key geometric measures of the respective flasks are presented in .
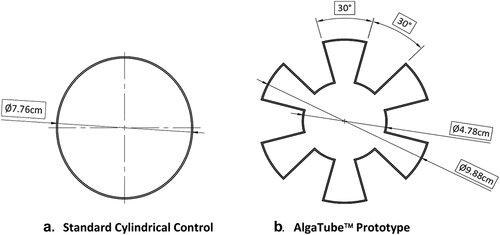
Table 1. Key Geometric Measures of the Flasks.
Culture species and growth conditions
Seed culture was purchased from Algae Research Supply in Carlsbad, CA, USA, who identify the strain only as Arthrospira platensis (A. platensis). Modified Schlosser Medium (da Silva et al., Citation2016) was prepared using deionized water from the Brigham Young University (BYU) Life Science Building (LSB). Two YOHAYOH LED Light Panels (45W, 50-60 Hz, 300 mA, 110 V, 30.99 cm x 30.99 cm x 4.06 cm) were fixed to the inside of the incubator. They emitted narrow spectral peaks at 460 nm (blue) and 630 nm (red) and a substantially lower level of full visible spectrum background radiation. The ratio of red to blue in the present study is calculated from the manufacturer’s data as approximately 60:40. A 15-Watt incandescent bulb (General Electric) was also included. The mean intensity applied to each culture was 55 µmol photons m−2 s−1 and was identical between the two cultures. The lights were maintained 24 hours a day with no period of darkness.
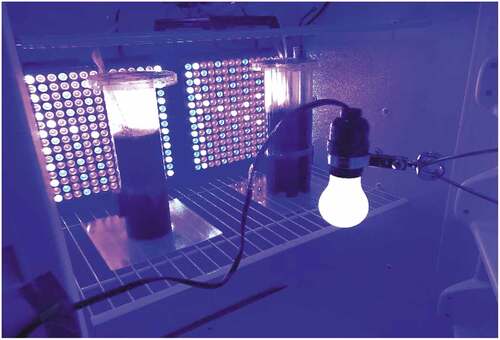
The two cultures were exposed to a continuous magnetic field of approximately 300mT (milliTesla) each (). The magnets (K&J Magnetics) were Neodymium Grade N52 with a Brmax rating of 14 800 Gauss and a BHmax rating of 52MGOe. Pure gaseous CO2 was supplied from a pressurized tank through a regulator (FZone Model#FZ-2020PRO), a pH controller (Milwaukee MC122Pro), and a sparger stone (CR Brewbeer, 0.5um Diffusion Stones). The CO2 was dosed as needed using the automated pH controller set for 9.0 ± 0.2. Ambient air was continuously bubbled through both cultures using a HITOP Aquarium Air Pump (Haisen). Dissolved oxygen levels were consistently lower than 150% of ambient levels. The temperature was maintained at 32°C. The Relative Humidity fluctuated mostly within a range of 10–15%. Periodically, due to the passage of storm systems, the Relative Humidity fluctuated outside that range. The setup is shown in .
Figure 2. Configuration of the growth chamber. The control flask was on the left, and the prototype flask was on the right. The two light panels were affixed to the rear wall of the incubator. The incandescent light was attached to the door and centered. The magnets can be seen on the lower left side of the control flask and on the lower right side of the prototype flask. The picture was taken with the door open for convenience.
Assessment of biomass concentration
Prior to daily sampling, each culture was mixed manually to promote homogeneity. Each day, the cultures were tested for Dissolved Oxygen (RCYAGO Portable Dissolved Oxygen, Model#DO9100), pH (Milwaukee MC122Pro), Total Dissolved Solids and Electrical Conductivity (Vivosun TDS/EC Metre), and Light Transmittance and Absorption (Biolog Turbidimeter, Model#21907, 590 nm). Concentration (Cd g l−1) was calculated from %T590 using a standard curve.
The organisms were observed to form clumps of filaments, a sign of their previously documented colonial nature (Demoulin et al., Citation2019). The clumps were a source of minor error for the OD590 readings because they interfered with homogeneity. The clumps caused some minor, but noticeable, drift in the turbidity readings, as they circulated in the culture samples. To reduce the margin of error associated with this imperfect homogeneity, the turbidity metre was observed for a full minute for each reading. Time weighted means of the %T590 and ABS590 were recorded for each measurement, rather than single spot readings.
Standard curve preparation
A stock of A. platensis ARS was taken from a well-mixed culture that had previously plateaued in the incubator. Five samples of 10 ml each were pipetted from that stock, and each was measured using the dry weight protocol (supplementary material). Those five samples produced five different results for dry weight − 1.63 g l−1, 1.68 g l−1, 1.34 g l−1, 1.71 g l−1, and 1.57 g l−1 – which reflected various sources of error in the assay (supplementary material). The high and low values from the range were discarded, and the mean of the remaining three values was calculated to be 1.63 g l−1. The 95% confidence interval of the three sample dry weights was calculated to be ±3.83%. This was determined to be sufficiently accurate for the present study.
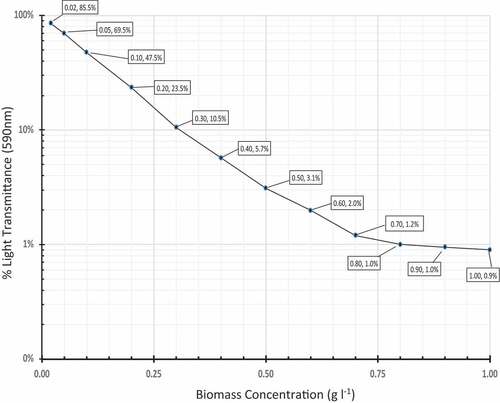
Another sample from the same stock was taken and measured in the turbidimeter, producing a %T reading of approximately 0.70%. From this sample, twelve dilutions were made to produce values of 1.00 g l−1 down to 0.02 g l−1, and each dilution was tested in the turbidimeter for %T with mass being calculated based on the dilution, using the 1.63 g l−1 figure as a reference point. These data are show in . A standard curve was made from the data (qryyig 3). The table was then used to convert each day’s %T readings into a corresponding Cd data point for each flask. For samples with particularly high C (generally anything above 0.70 g l−1), samples were diluted prior to determination of C, because %T readings were more difficult to discern at levels below 1%. Generally, the dilution rate was 1 : 5. Such samples were assessed at 1 : 5 dilution and that result was then multiplied by 5x to reach the final value.
Table 2. Dilutions for % Transmittance vs. Concentration standard curve.
The diluent for these dilutions was Zarrouk Media (ZM), because ZM was being used at the time the standard curve was prepared. Subsequent to the creation of this standard curve, a decision was later made to switch to MSM media. To examine the potential impact that the change in diluent might have on the OD readings, the OD590 for pure MSM was compared with the OD590 for pure ZM. It was determined that the difference in %T readings between the two media (<2%) was sufficiently small to justify continued use of the standard curve, without preparation of a new standard curve using MSM as the diluent.
. The data for the standard curve presented in . These data establish a correlation between biomass concentration (g l−1) of A. platensis and the optical density of the culture at 590 nm. “%T” stands for Percentage Light Transmission. “ABS” stands for Absorbance in absorbance units. %T and ABS are interrelated, according to the standard Beer-Lambert equation: ABS = 2 – log10 (%T × 100).
Calculation of production (Pd)
Pd is calculated by subtracting the previous day’s C from the current day’s C as follows.
P can be calculated for a multi-day time-period, in which case it is termed “Cumulative Production” (CP in g l−1), and it can also be done for each single day, in which case the value is termed Daily Production (DP in g l−1). In the time parsed data analysis presented in the results section, DP values are calculated for each day and then averaged together longitudinally for particular time periods. Those means are termed Mean Daily Production (MDP in g l−1) for the time-period in question.
As a metric, P (and its associated metrics: CP, DP, and ADP) focus on the absolute amount of biomass produced for a particular time period, regardless of the initial concentration of the culture. Other metrics, such as Specific Growth Rate (SGR), focus on the percentage of biomass growth per day, which depends on the initial C0. The difference between the two metrics is best observed by examining their respective units.
SGRd is presented as a fractional value per day (e.g., 0.20 day−1). This would mean that the culture grew at a compound mean rate of 20% per day during the measured time-period. On the other hand, Pd is presented in g l−1 day−1. Biomass is sold and consumed by weight, not by percentages or by fractions. Therefore, Pd may be more the more important metric for industry.
On the other hand, SGR is widely published in scientific journals, and academic readers may prefer this metric. When it is presented as a fraction of the Concentration, and for multi-day periods, then it takes the effect of daily compounding into account. Since different readers may have different preferences on how best to measure biomass growth, both metrics are presented herein, and readers are invited to decide for themselves which metric to use for their own purpose(s).
Calculation of specific growth rate (SGR)
SGR is based on the mathematical concept of the natural logarithm (Ln). The amount produced per day is presented as a fraction of the total concentration on the previous day, rather than as an absolute value. For multi-day time periods, it takes into account the compounding effect and is presented as a compound mean rate over multi-day time periods. The mathematical equation used to calculate SGR of biomass for a particular time-period (d0 to d10) is presented, here.
Mathematically, SGR can be calculated for any time-period in an experiment, but the common practice in phycology is to present SGR for the entire length of the experiment and to also include the presentation of SGRmax. SGRmax is the maximum of the daily SGR results for each culture. It is an indicator of the best single day’s performance.
The abbreviation we used for Specific Growth Rate is SGR, which is different from the abbreviation used in other studies (μ). The reason for breaking from convention is that the Greek letter “μ” is used in this study to describe light intensity in the units of μmol photons m−2 s−1. To avoid confusion in the present study, “μ” is restricted only to units of light intensity, and Specific Growth Rate is abbreviated as SGR.
Calculation of Delta (Δ)
To measure performance differences between the two vessels (control and prototype), Δ was calculated using the control datasets and the prototype datasets. Δ is expressed in percentage terms, relative to the control. It is not specific to any particular metric. Δ can be calculated for any metric and any time-period and labelled accordingly. For example, the difference between the prototype culture concentration and the control culture concentration (C in g l−1) on day #5 would be labelled ΔC5. The difference in cumulative production (CP in g l−1) on day #8 would be labelled ΔCP8. And the difference in Mean Daily Production (MDP in g l−1 day−1) during the first seven days would be labelled ΔMDP1-7. The equation for Δ of Cumulative Production on day #d (ΔCPd) is presented here.
It should be noted, here, that lower-case “c” and lower-case “p” denote control and prototype, respectively. They should not be confused with upper-case “c” and upper-case “p” which denote Concentration and Production, respectively.
Calculation of statistical significance (p-values)
The datasets were determined to follow non-normal distributions, which prevented the use of the standard t-test. Therefore, a non-parametric test was used. Statistical significance was assessed using the Wilcoxon Rank Sum Test (Mann-Whitney U Test; Corder, Foreman, Wiley, Sons, & Hoboken, Citation2014). The resulting p-value was considered statistically significant at p < 0.05.
Results
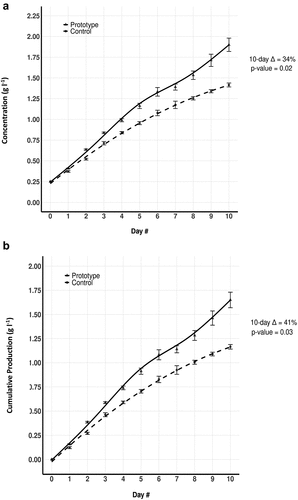
Both the control and prototype concentration data exhibited non-linear trends, and the two flasks each produced different shaped growth trends. A quadratic model was fit to the control data. A non-constant variance linear spline regression model was fit to the prototype data. The prototype data points from the four independent replicates showed a gradually increasing variance as the day number increased, a result that was not observed to a similar extent in the control flask. The means, however, still showed statistically significant growth differences in favour of the prototype. ().
Figure 4. (A) and 4(B). Daily Concentration and Cumulative Production values of prototype and control tubes. 4(a) Chart plotting the daily Concentration (Cd), averaged across the four independent replicates, for the AlgaTube™ prototype and cylindrical control, respectively. The prototype data points are represented by solid lines and black triangles (━▲━). ΔC10 = +34% (p = 0.02, Wilcoxon Rank Sum Test). 4(b) Chart plotting the daily Cumulative Production (CPd), averaged across the four independent replicates, for the two vessels, AlgaTube™ prototype and cylindrical control, respectively. δcp10 = +41% (p = 0.02, Wilcoxon Rank Sum Test). In both 4(a) and 4(b), the prototype data points are represented by solid lines and black triangles (━▲━). The control data points are represented by dashed lines and black circles (—□—).
Specific Growth Rate (SGR) was calculated as 0.17 day−1 for the control flask and 0.20 day−1 for the prototype flask, a difference of 17% in favour of the AlgaTube™. SGRmax was also calculated for both flasks, and the results were 0.40 day−1 and 0.49 day−1 for the control and prototype, respectively. Both SGRmax datapoints occurred on Day #1, when the cultures were at their lowest concentrations. Daily Production (DPd) was calculated, and the resulting dataset focuses attention on the amount of biomass produced per day, rather than the cumulative total. The Maximum Daily Production (MxDP) in the prototype, was 0.29 g l−1 day−1, 38% greater than the comparable figure for the control, 0.21 g l−1 day−1. The MxDP results were both achieved on different days than SGRmax, which highlights the difference between the two metrics. While theoretical phycologists may be interested in the greatest daily percentage growth, industrial producers may be more focused on the greatest daily mass growth, because they sell product by weight. These two metrics are different, and they do not occur on the same day.
To compare the Mean Daily Production (MDP) for different time periods, the Mean Daily Production dataset was parsed into three separate time periods: (1) the full 10 days, (2) the first seven days, and (3) the last three days. A longitudinal mean was calculated for each time period. Between the first seven days and the last three days, Mean Daily Production (MDP) in the control flask dropped by 38% from 0.13 g l−1 day−1 down to 0.08 g l−1 day−1; and this caused a large increase in the Delta (Δ) during the final three days. At +113%, ΔMDP8-10 was nearly five times higher than ΔMDP1-7. ().
Figure 5. Time-Parsed Mean Daily Production. The Mean Daily Production (MDP) data were, themselves, averaged together longitudinally, for each of three different time periods within the 10-day experiment. The respective Delta (∆mdp) values and p-values for different time periods are shown. The mean culture concentration during each of those time periods is shown below the x axis labels. The bars representing the control flask are white, and the bars representing the prototype flask are shaded grey.
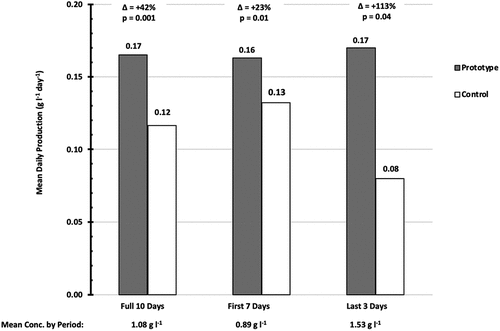
The time-parsed data analysis clearly shows that the biomass growth in the control flask decreased in the final three (3) days of the experiment, whereas there was no such decrease in the prototype flask. This was most likely due to a negative impact caused by the shadow effect. The filaments shaded each other when their concentrations increased. On the other hand, the prototype did not exhibit the same negative impact during that same time-period (). When viewed alongside the Delta (∆) values noted in , this observation suggests that the enhanced surface area of the prototype reduced the shadow effect and enabled the culture to maintain a higher rate of growth and for a longer time-period, when compared to the cylindrical control flask.
Discussion
Growth conditions
The purpose of this experiment was to isolate and evaluate the shape of the sidewalls of the AlgaTube prototype flask for its effect on growth rates. This required all other variables to be held constant and identical between the control flask and the prototype flask. It also required that no other variable be set to a level that was rate limiting or restrictive of the growth of the organism. The existence of any single rate limiting factor, other than the PSA of the respective culture flasks, could inhibit both cultures growth rates to a degree that it would override and conceal the hypothesized positive effect of the enhanced surface area. Thus, to evaluate the singular effect of the shape of the sidewalls, the value or setting of the other variables was a matter of careful consideration and subject to pre-testing via preliminary experiments.
Other researchers have shown large variations in growth curves and production rates of A. platensis by using different ratios of red and blue light, while holding total intensity constant at 100 μmol photons m−2 s−1. Maximum growth was achieved with a red-to-blue ratio of 70: 30. (Lima, Teixeira, Teixeira, Filócomo, & Lage, Citation2018). The ratio of red to blue in the present study is calculated from the manufacturer’s data as approximately 60:40. To enhance stimulation of P700, a photoreceptor that absorbs 700 nm light, a 15-Watt incandescent bulb (General Electric) was added (Webber & Lubitz, Citation2001). Incandescent bulbs produce a broad spectrum of radiation that is heavily weighted towards the red, far red, and infra-red regions of the visible spectrum (Azizi, Golmohammadi, & Aliabadi, Citation2016). All of the light sources were on 24 hr day−1, according to the method of Prates, Radmann, Duarte, de Morais, & Costa (Citation2018).
Using an enhanced silicon photodiode assembly (LI-190 R Quantum Sensor), the light intensity was measured at 24 spot locations around the perimeter of the flasks, or 12 locations per flask. Spot readings ranged from 22–105 µmol photons m−2 s−1. This wide range of light intensity was the natural outcome of the physical placement of the light sources and the culture flasks within the incubator. The sides of the culture flasks that faced directly towards the light sources experienced a higher intensity than the sides not facing the light sources directly. This kind of intensity imbalance is consistent with what is found in outdoor tubular photobioreactors (tPBRs) that are illuminated by the sun.
Li, Guo, Li, & Cai (Citation2007) found that an electromagnetic field enhanced the growth rate of A. platensis at an intermediate light intensity of 252 μmol photons m−2 s−1. Deamici, Costa, and Santos (Citation2016) also reported positive effects on A. platensis growth rates from magnetic fields, as did de Costa Menestrino et al. (Citation2021). Based on those two studies, it was determined to use magnets in this experiment. The temperature was derived from a previously published range of preferred temperature for A. platensis (Oliveira, Monteiro, Robbs, & Leite, Citation1999; Soni, Sudhakar, & Rana, Citation2019).
As we observed during the preliminary studies that preceded these trials, humidity can have two effects on cultures. First, it impacts the rate of evaporation of water from the cultures (data not included). Second, humidity can interfere with the transmission of light to the cultures. This latter effect can also be seen in the natural world by examination of the solar insolation data in different parts of the world, where significant variation in annual solar insolation data at ground level is found to correlate inversely with the mean humidity in different areas at the same latitude (National Solar Radiation Database, National Renewable Energy Laboratory, U.S. Government).
The light attenuating effect of humidity can also be observed in small, experimental systems in a laboratory. During pre-testing for this experiment, tubs of water were included to reduce evaporation inside the incubator. With two large tubs of water in the incubator, relative humidity was maintained at approximately 55% and evaporation from the cultures was greatly reduced. However, at 55% relative humidity, the growth rates of the cultures were also significantly reduced, compared to the growth rates observed at 10% relative humidity, without the water tubs. This was attributed to the light attenuating effect of humidity, the same effect observed in the solar insolation data of the National Solar Radiation Database of the NREL. To avoid the inhibitory effect of high humidity in the present study, it was determined not to use the tubs of water. In lieu of tubs to reduce evaporative water loss, deionized water was added daily to replace evaporative water loss in the cultures.
Kazbar et al. (Citation2019) showed that, when dissolved oxygen (DO) levels reach approximately 300% of atmospheric concentration, significant inhibition of the growth rate of photosynthetic cultures of Chlorella vulgaris was observed. Torzillo & Vonshak (Citation2013) described a cascade of inhibiting effects triggered by excess O2, and its effect on the concentration of Reactive Oxygen Species (ROS) such as superoxide radical (O2–), hydrogen peroxide (H2O2), and hydroxyl radical (·OH). Ganesh, Manoharan, & Suraishkumar (Citation2007) showed that large increases in ROS correlated strongly with reductions in biomass growth rates in Spirulina maxima.
Using the cyanobacterium Synechocystis sp. PCC 6803, Nishiyama, Allakhverdiev, Yamamoto, Hayashi, & Murata (Citation2004) demonstrated that another ROS, singlet oxygen (1O2), appears to inhibit the repair of photodamaged PSII by inhibiting production of a key component of PSII – the D1 protein (Aro, Ivar, & Andersson, Citation1993) - at the translational level. This feedback loop has the effect of shutting down the functionality of PSII, which is to split water molecules into hydrogen atoms and O2 (Aro, Ivar, & Andersson, Citation1993). This being the first step of the photosynthetic pathway, the rate of photosynthesis is reduced by these events. These biochemical events also reduce the production of O2 by PSII, thus countering the excess O2 levels that caused them in the first place. This is a self-fulfiling and self-protective feedback loop.
Another impact of excess oxygen levels in photosynthetic microorganisms is the stimulation of a secondary metabolic pathway, photorespiration, in which the Rubisco enzyme utilizes oxygen in lieu of carbon (Vonshak, Torzillo, Accolla, & Tomaselli, Citation1996; Fernie & Bauwe, Citation2020; Sforza et al., Citation2020). There is some evidence that the O2 : CO2 ratio, more-so than the absolute level of dissolved oxygen, governs this pathway (Kitaya, Azuma, & Kiyota, Citation2005). Nevertheless, the net effect of excess oxygen can be summarized as a combination of shutting down photosynthesis and turning on photorespiration. Based on observations of reduced biomass production rates at high dissolved oxygen levels (Kazbar et al., Citation2019 and preliminary testing for the present study), the photorespiratory pathway was assumed to be much slower, kinetically, than photosynthesis.
During pre-testing for the present study, cultures were also observed without supplemental CO2. The pH of those cultures drifted steadily higher from a starting level of 9.0 up to a level of 11.0, or higher in some cases. A concomitant plateauing of daily growth was observed as the pH increased. These observations were consistent with a report of the optimal pH for A. platensis total biomass growth being pH 9.0 (Ismaiel, El-Ayouty, & Piercey-Normore, Citation2016).
OD590 and phycobiliprotein expression
The use of OD590 to measure optical density of cultures in this study raises the possibility of errors in the measurement of Cd, because 590 nm is near the spectral absorbance peak of the phycobiliproteins, an important class of photopigments expressed by A. platensis (Barber & Richards, Citation1977; Kronick, Citation1986). Differential expression of these photopigments between the control culture and the prototype cultures could have been, theoretically, a source of error in the OD590 readings, because changes in OD590 could be attributed to differences in photopigment expression, rather than differences in total biomass concentration. However, this possible source of error can most likely be ruled out by the following methods and logic.
In the present study, the control and prototype cultures were established from a common culture, the “starter” culture. Prior to division of the starter culture into the control flask and the prototype flask, it was mixed thoroughly to ensure uniformity between the control and prototype cultures on Day #0. Any differences in the rate of expression of the phycobiliproteins, therefore, would have to have emerged during the 10-day trial period, not prior to it. Since the cultures were identical on Day #0, the only possible causes for differential expression of the phycobiliproteins during the 10-day trials were: (1) differences in Cd as the cultures grew at different rates and (2) differences in the light intensity caused by the PSA difference between the two flasks. All of the other variables, and inputs, without exception, were fixed and controlled so that they were equal between the two flasks.
As the growth data show, the Cd in the prototype flask was consistently higher than the Cd in the control flask. Therefore, it is theoretically possible that the difference in concentrations between the two cultures could have triggered an internal, biochemical pathway in the organism, which, in turn, caused a difference in the expression rate of the phycobiliproteins between the control and the prototype. However, the standard curve covered a broad range of culture concentrations from 0.02 g l−1 up to 1.0 g l−1. Therefore, the standard curve is believed to have fully accounted for any differences that may have been caused by differences in concentration.
In terms of light intensity, Nomsawai, de Marsac, Thomas, Tanticharoen, & Cheevadhanarak (Citation1999) did show significant variability in phycobiliprotein expression in A. platensis C1 when the light intensity was changed by a large factor of 10x from 50 μmol photons m−2 s−1 to 500 μmol photons m−2 s−1. However, Rizzo et al. (Citation2015) showed that smaller changes in light intensity from 50 to 100 to 150 μmol photons m−2 s−1 did not have a statistically significant effect on the level of total protein content (%mg mg−1, p < 0.05) or on the phycobiliprotein content (%mg mg−1, p < 0.05) in cultures of A. platensis. The present study was conducted at low light intensity, and well below 150 μmol photons m−2 s−1. Thus, differences in phycobiliprotein expression most likely did not impact the Cd results.
Phycological consistency of results
The higher rate of biomass production observed in the prototype compared to the control is conceptually consistent with results reported by other researchers in the field of phycology. The application of a higher PSA vol−1 ratio caused improvement in both Cd and Pd of A. platensis UTEX 1926, according to da Silva et al. (Citation2016). Converti, Lodi, Del Borghi, & Solisio (Citation2006) showed that cultures of A. platensis UTEX 1926 grew faster and to higher plateaus in tPBRs with greater PSA vol−1 ratios than in an open pond. The same phenomenon was also observed in Chlorella vulgaris CCAP211 by Frumento et al. (Citation2013). In all of these studies, including the present study, a higher PSA vol−1 ratio helped to reduce the self-shading effect and to expose greater numbers of cells, filaments, and/or photoreceptor pigment molecules to incoming photons. In our time parsed data (qryyig. 5), we elaborate on the previously known self-shading effect by showing that it actually has two components: (1) a slower rate of growth during the first 7 days and (2) avoidance of the substantial growth rate reduction seen in the control flask during days #8–#10.
In all of these studies, compelling evidence and comparisons are presented, and we can learn more about the performance of the AlgaTube™ by comparing the various studies to each other and to our own data. It is noteworthy, for example, that da Silva et al. (Citation2016) showed a Cmax of 8.44 g l−1 after only 9 days of growth. At +344%, this Cmax was far higher than the present study, which showed a Cmax of 1.90 g l−1 after 10 full days of growth. There were several important differences in configuration, which may explain the difference.
The da Silva study used a higher starting concentration on Day #0 of 0.40 g l−1 (60% higher than 0.25 g l−1 for the present study). It was run with a higher light intensity of 100 μmol photons m−2 s−1 (82% higher than 55 μmol photons m−2 s−1 for the present study). The reported PSA vol−1 ratio was 1.94 cm−1 (70% higher in than 1.13 cm−1 for the present study). There were also differences in the quality of the light (fluorescent vs. LED), the vessel materials (glass vs. 3-D printing resin), and the supplemental hardware (the hose clamps on the AlgaTube™ blocked 7% of the PSA). Finally, and perhaps most importantly, there was a very important difference in the mixing techniques between these aqueous cultures.
To whit, by circulating their cultures continuously through a looped pipe network, da Silva et al. (Citation2016) used a different mixing technique and introduced another variable to their comparisons. They compared a horizontal tPBR with a circulating loop configuration against a simple shaken flask and a paddled raceway. Not only were the PSA vol−1 ratios different between those three configurations, but there were also three different mixing techniques used: continuous circulation, flask shaker machine, and paddle wheel. Their conclusions, therefore, should be understood in light of these important differences in mixing technique and not solely as a reflection of the different PSA vol−1 ratios. Indeed, the different mixing techniques in that study raise an important question about which variable was most important to their exceptionally high growth rate: the increased PSA vol−1 ratio, the continuous circulation, or the combination of the two.
In the present study, there was no continuous circulation in either culture. The only mixing applied to both cultures in the present study was provided by the energy of the air bubblers, and that was identical in both cultures. Thus, the present study was more precisely controlled for PSA vol−1 ratio as the sole variable in the experiment. The important question of the relative effects of PSA vol−1 vs. mixing technique arising out of da Silva et al. (Citation2016) remains unanswered, empirically. However, it is possible to derive an estimated answer to that question, mathematically, based on data that are currently available.
First, the differences in configuration between the da Silva study and the present study are quantified and added together to reach an estimate of their cumulative total effect on Cmax as shown in .
Table 3. Quantification of primary differences between present study vs. da Silva et al. (Citation2016).
Assuming that the effect of these differences on the growth metrics are additive (they may not actually be), the three primary variables listed in can be estimated to have accounted for a total of +214% out of the +344% difference in Cmax between the two studies. The remaining difference, +130%, can be attributed to the combined effect of the other remaining variables, a list that includes: light quality (fluorescent vs. LED), vessel material (glass vs. 3-D printing resin), supplemental hardware (the hose clamps on the AlgaTube™ blocked 7% of the PSA), and mixing technique.
Some of these variables can be approximated using known information. Geometrically, the hose clamps block about 7% of the PSA; so, that effect is estimated at 7%. The difference in the light transmission of glass vs. the 3-D printing resin is known to be about 10% (with some variation depending on wavelength); so, that effect is estimated at 10%. Based on analysis of two different studies (Chainapong et al., Citation2012; Thaweedet et al. Citation2012; Lima, Teixeira, Teixeira, Filócomo, & Lage, Citation2018), light quality is estimated to have an impact of 30%. That leaves approximately 83% as the remainder, which can be attributed to the mixing technique. This analysis concludes, then, that the mixing technique is the single most important difference between the two studies, though the next three variables are not far behind. The final determination of the relative effect of all of the differences between the da Silva study and the present study are presented and summarized in .
Table 4. Ranked summary of all differences between present study vs. da Silva et al. (Citation2016).
NB: Strain differences were not considered in this table or in the related discussion.
Light containment
Light containment is not considered as a separate variable unto itself. Rather, it is a means to increase the light intensity without increasing the power input to the lights. Since light intensity is already included in the evaluation of the key variables above in Section 4.2, our discussion of light containment is presented here separately, not as part of the list of variables in the previous section.
The light intensity inside the incubator of this study was considerably different with the door open, compared to the door being closed. With the door open, the iPPFDavg was measured to be 29 µmol photons m−2 s−1, or 55% lower than the iPPFDavg of 55 µmol photons m−2 s−1 when the door was closed. This observation is noteworthy for the field of PBR design, because it shows that light containment has a large impact on the mean radiation received by phycological cultures in artificial systems. Without containment, a large percentage of PAR photons can be lost to the surrounding environment without ever being used for biomass growth. The horizontal tPBR of Converti, Lodi, Del Borghi, & Solisio (Citation2006), for example, did not include any light containment features and might be improved upon by including a housing made of white walls which reflects and contains stray photons.
This concept of light containment has been successfully used for land plants for many years (Warman & Mayhew, Citation1979); and therefore, it is likely to have similar, positive effects on aqueous photosynthetic cultures. At present, however, state-of-the-art, outdoor PBRs generally do not include any light containment features.
It is reasonable to hypothesize that the inclusion of light containment features on outdoor systems may increase the utilization of physically limited solar radiation by, in effect, “recycling” stray photons. This effect may be dependent, however, on the angle of incidence of the solar radiation on the light containment housing, an effect that has been very thoroughly studied in the field of photovoltaics (PV). Solar panels are well known to perform best when angled continuously towards the sun with a device that rotates throughout the day to follow the sun, or when set at a fixed angle called “Latitude Tilt” which maximizes the intensity of the sun by averaging out its different seasonal phases and daily paths (Nicolás-Martín, Santos-Martín, Chinchilla-Sánchez, & Lemon, Citation2020). It is expected that outdoor PBRs with light containment housings may behave analogously. Indeed, this may be one of the key reasons that outdoor systems are widely known to underperform lab studies.
Summary of key metrics
This study demonstrates that the AlgaTube™ clearly outperformed the control flask in all the key metrics used for comparison. In , the Delta (Δ) values are presented relative to the control, meaning that positive Delta (Δ) values show that the AlgaTube™ performed better than the control. Across the entire spectrum of growth metrics, the AlgaTube™ consistently outperformed the control flask ().
Table 5. Summary of key metrics.
Finally, the decrease in the biomass growth in the control flask during the final three days of the experiment, which was not seen in the prototype flask, supports the hypothesis that the prototype flask’s gains are due to overcoming the shadow effect. In the control flask, the filaments shaded each other, when their concentrations increased. On the other hand, the prototype did not exhibit the same negative impact during that same time-period (). When viewed alongside the Delta (∆) values noted in , this observation suggests that the enhanced surface area of the prototype reduced the shadow effect and enabled the culture to maintain a higher rate of growth and for a longer time-period, when compared to the cylindrical control flask.
The comparative analysis of different time periods showed also that the prototype improved the Average Daily Production by 113% during the final three days of the trial, when the cultures were at their thickest.
Future directions
The present study serves as compelling proof-of-concept for the novel shaped walls of the AlgaTube™. The prototype performed better than the control on all six growth metrics as summarized in . This study is considered a successful first step, but there remains considerable potential to optimize the performance of such tubes, as shown by the comparisons to other studies summarized in .
The AlgaTube™ should be tested, for example, in a circulating loop, horizontal configuration such as the tPBR configuration demonstrated by da Silva et al. (Citation2016). It should be tested in smaller diameter sizes, such as those used by Converti, Lodi, Del Borghi, & Solisio (Citation2006), and in a circulating loop vertical configuration. The important factor of light containment should be studied further. Vertically oriented tubes contained in a containment housing, for example, might perform better than stand-alone vertical tubes in a fully transparent greenhouse. Continuous feeding and fed-batch methodologies should also be tested in the AlgaTube™. And, finally, as with any new PBR concept, the transition to larger scale, outdoor environments must also be made.
Author contributions
The idea for the AlgaTube™ was conceived by JLG, individually. The experiment was designed collaboratively by JLG and SMJ. Laboratory space, certain laboratory equipment, and general laboratory supplies were provided by SMJ. All laboratory experiments were performed by JLG, who also wrote the first draft of the manuscript. NRC and DLE performed the statistical analyses, created the charts, and wrote the statistical language. Subsequent drafts were reviewed and edited by all four authors and by both reviewers and an editor at the Journal of Applied Phychology.
Acknowledgements
We thank Brigham Young University for providing laboratory space, laboratory equipment, and general laboratory supplies. We thank the first author, JLG, for his personal contribution of financial support, which was used for prototyping manufacturing expenses and the purchase of certain laboratory instruments. We thank Brigham Young University’s Engineering Department for use of its Prototyping Lab. We thank Mr. Nicolas Miller, draftsman, who was hired by JLG to make the technical drawings of the prototype and Mr. Harlan Stevens, BYU undergraduate, who proofread the manuscript without pay. Xometry™ and Stratasys™ were hired by JLG for 3-D printing of the vessels.
Disclosure statement
No potential conflict of interest was reported by the author(s). Mr. Jonathan L. Gal, Dr. Steven M. Johnson, and the Brigham Young University Office of Technology Transfer each have a financial interest in the patent application(s) related to the present study.
References
- Adeniyi, O. M., Azimov, U., & Burluka, A. (2018). Algae Biofuel: Current status and future applications. Renewable and Sustainable Energy Reviews, 90, 316–335. doi:https://doi.org/10.1016/j.rser.2018.03.067
- Aro, E.-M., Ivar, V., & Andersson, B. (1993). Photoinhibition of photosystem ii: Inactivation, protein damage and turnover. Biochimica Et Biophysica Acta (BBA) – Bioenergetics, 1143, 113–134. doi:https://doi.org/10.1016/0005-2728(93)90134-2
- Arora, K., Kumar, P., Bose, D., Li, X., & Kulshrestha, S. (2021). Potential applications of algae in biochemical and bioenergy sector. 3 Biotech, 11, 1–24. doi:10.1007/s13205-021-02825-5
- Azizi, M., Golmohammadi, R., & Aliabadi, M. (2016). Comparative analysis of lighting characteristics and ultraviolet emissions from commercial compact fluorescent and incandescent lamps. Journal of Research in the Health Sciences, 16, 200–205. https://pubmed.ncbi.nlm.nih.gov/28087852
- Barber, D. J. W., & Richards, J. T. (1977). Energy Transfer in the Accessory Pigments R-Phycoerythrin and C-Phycocyanin. Photochemistry and Photobiology, 25, 565–569. doi:10.1111/j.1751-1097.1977.tb09129.x
- Benemann, J. R. (1979). Production of nitrogen fertilizer with nitrogen-fixing blue - green algae. Enzyme and Microbial Technology, 1, 83–90. doi:https://doi.org/10.1016/0141-0229(79)90103-0
- Chainapong, T., Traichaiyaporn, S., & Deming, R. L. (2012). Effect of light quality on biomass and pigment production in photoautotrophic and mixotrophic cultures of Spirulina platensis. Journal of Agricultural Technology, 8, 1593–1604.
- Chong, J. W. R., Khoo, K. S., Yew, G. Y., Leong, W. H., Lim, J. W., Lam, M. K. … Show, P. L. (2021). Advances in production of bioplastics by microalgae using food waste hydrolysate and wastewater: A review. Bioresource Technology, 342, 125947. doi:10.1016/j.biortech.2021.125947
- Christaki, E., Karatzia, M., & Florou-Paneri, P. (2010). The use of algae in animal nutrition. Journal of the Hellenic Veterinary Medical Society, 61, 267–276. doi:10.12681/jhvms.14894
- Clippinger, J., & Davis, R. (2019). Techno-Economic analysis for the production of algal biomass via closed photobioreactors: future cost potential evaluated across a range of cultivation system designs. National Renewable Energy Laboratory, Golden, CO. NREL/TP-5100–72716. https://www.nrel.gov/docs/fy19osti/7271.
- Converti, A., Lodi, A., Del Borghi, A., & Solisio, C. (2006). Cultivation of spirulina platensis in a combined airlift-tubular reactor system. Biochemical Engineering Journal, 32, 13–18. doi:https://doi.org/10.1016/j.bej.2006.08.013
- Corder, G. W., & Foreman, D. I. (2014). Nonparametric Statistics: A Step-by-Step Approach (Vol. 4, 2nd ed., pp. 69–75). Hoboken, NJ, USA: John Wiley & Sons, Inc.
- da Costa Menestrino, B., Sala, L., Costa, J. A. V., Buffon, J. G., & Santos, L. O. (2021). Magnetic Fields Exhibit a Positive Impact on Lipid and Biomass Yield During Phototrophic Cultivation of Spirulina Sp. Bioprocess and Biosystems Engineering, 44, 2087–97. doi:10.1007/s00449-021-02585-9
- da Silva, M. F., Casazza, A. A., Ferrari, P. F., Perego, P., Bezerra, R. P., Converti, A., & Porto, A. L. F. (2016). A new bioenergetic and thermodynamic approach to batch photoautotrophic growth of Arthrospira (Spirulina) platensis in different photobioreactors and under different light conditions. Bioresources Technology, 207, 220–228.
- Deamici, K. M., Costa, J. A. V., & Santos, L. O. (2016). Magnetic fields as triggers of microalga growth: Evaluation of its effect on Spirulina sp. Bioresources Technology, 220, 62–67. doi:10.1016/j.biortech.2016.08.038
- Demoulin, C. F., Lara, Y. J., Cornet, L., François, C., Baurain, D., Wilmotte, A., & Javaux, E. J. (2019). Cyanobacteria evolution: insight from the fossil record. Free radical biology & medicine, 140, 206–223. doi:10.1016/j.freeradbiomed.2019.05.007
- Fernie, A. R., & Bauwe, H. (2020). Wasteful, essential, evolutionary stepping stone? The multiple personalities of the photorespiratory pathway. The Plant Journal, 102, 666–677. doi:https://doi.org/10.1111/tpj.14669
- Frontasyeva, M. V., Pavlov, S. S., Mosulishvili, L., Kirkesali, E., Ginturi, E., & Kuchava, N. (2009). Accumulation of trace elements by biological matrix of Spirulina platensis. The Journal of the Society of Ecological Chemistry and Engineering, 16, 277–285.
- Frumento, D., Casazza, A. A., Al Arni, S., & Converti, A. (2013). Cultivation of Chlorella vulgaris in Tubular Photobioreactors: A Lipid Source for Biodiesel Production. Biochemical engineering journal, 81, 120–25. doi:10.1016/j.bej.2013.10.011
- Ganesh, A. B., Manoharan, P. T., & Suraishkumar, G. K. (2007). Responses of the photosynthetic machinery of Spirulina maxima to induced reactive oxygen species. Biotechnology and Bioengineering, 96, 1191–1198. doi:https://doi.org/10.1002/bit.21217
- González-Camejo, J., Viruela, A., Ruano, M. V., Barat, R., Seco, A., & Ferrer, J. (2019). Dataset to assess the shadow effect of an outdoor microalgae culture. Data in Brief, 25, 104143. doi:10.1016/j.dib.2019.104143
- Hoseini, S. M., Almodares, A., Afsharzadeh, S., Shahriari, A. R., & Montazeri, F. (2014). Growth response of Spirulina platensis PCC9108 to elevated CO2 levels and flue gas. Biological Journal of Microorganism, 2, 29–36. University of Isfahan, Iran https://www.sid.ir/en/journal/ViewPaper.aspx?id=491905
- Ismaiel, M. M. S., El-Ayouty, Y. M., & Piercey-Normore, M. (2016). Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Brazilian Journal of Microbiology, 47, 298–304. doi:10.1016/j.bjm.2016.01.003
- Joshi, S. (2018). Applications of Algae in Cosmetics: An Overview. International Journal of Innovative Research in Science, Engineering and Technology, 7, 1269–1278.
- Kazbar, A., Cogne, G., Urbain, B., Marec, H., Le-Gouic, B., Tallec, J. … Pruvost, J. (2019). Effect of dissolved oxygen concentration on microalgal culture in photobioreactors. Algal Research, 39, 101432. doi:10.1016/j.algal.2019.101432
- Kendirlioglu, G., & Cetin, A. K. (2017). Effect of different wavelengths of light on growth, pigment content, and protein amount on Chlorella vulgaris. Fresenius Environmental Bulletin, 26, 7974–7980.
- Kitaya, Y., Azuma, H., & Kiyota, M. (2005). Effects of Temperature, CO2/O2 concentrations and light intensity on cellular multiplication of microalgae, Euglena gracilis. Advances in Space Research, 35, 1584–1588. doi:https://doi.org/10.1016/j.asr.2005.03.039
- Kronick, M. N. (1986). The Use of Phycobiliproteins as Fluorescent Labels in Immunoassay. Journal of immunological methods, 92, 1–13. doi:10.1016/0022-1759(86)90496-5
- Kumar, K., Dasgupta, C. N., Nayak, B., Lindblad, P., & Das, D. (2011). Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresource Technology, 102, 4945–4953. doi:https://doi.org/10.1016/j.biortech.2011.01.054
- Li, Z.-Y., Guo, S.-Y., Li, L., & Cai, M.-Y. (2007). Effects of electromagnetic field on the batch cultivation and nutritional composition of Spirulina platensis in an air-lift photobioreactor. Bioresource Technology, 98, 700–705. doi:10.1016/j.biortech.2006.01.024
- Lima, G. M., Teixeira, P. C. N., Teixeira, C. M. L. L., Filócomo, D., & Lage, C. L. S. (2018). Influence of spectral light quality on the pigment concentrations and biomass productivity of Arthrospira platensis. Algal Research, 31, 157–166. doi:https://doi.org/10.1016/j.algal.2018.02.012
- Liu, J., Pemberton, B., Lewis, J., Scales, P., & Martin, G. J. O. (2020). Wastewater treatment using filamentous algae – a review. Bioresource Technology, 298, 122556. doi:https://doi.org/10.1016/j.biortech.2019.122556
- Nicolás-Martín, C., Santos-Martín, D., Chinchilla-Sánchez, M., & Lemon, S. (2020). A global annual optimum tilt angle model for photovoltaic generation to use in the absence of local meteorological data. Renewable Energy, 161, 722–735. doi:10.1016/j.renene.2020.07.098
- Nishiyama, Y., Allakhverdiev, S. I., Yamamoto, H., Hayashi, H., & Murata, N. (2004). Singlet oxygen inhibits the repair of photosystem ii by suppressing the translation elongation of the D1 Protein in Synechocystis sp. PCC 6803. Biochemistry-US, 43, 11321–11330. doi:10.1021/bi036178q
- Nomsawai, P., de Marsac, N. T., Thomas, J. C., Tanticharoen, M., & Cheevadhanarak, S. (1999). Light regulation of phycobilisome structure and gene expression in Spirulina platensis C1 (Arthrospira sp. PCC 9438). Plant & Cell Physiology, 40, 1194–1202. doi:10.1093/oxfordjournals.pcp.a029507
- Olaizola, M., & Duerr, E. O. (1990). Effects of light intensity and quality on the growth rate and photosynthetic pigment content of Spirulina platensis. Journal of Applied Phycology, 2, 97–104. doi:10.1007/BF00023370
- Oliveira, M. A. C. L. D., Monteiro, M., Robbs, P. G., & Leite, S. G. F. (1999). Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquaculture International, 7, 261–275. doi:https://doi.org/10.1023/A:1009233230706
- Poonam, S., & Sharma, N. (2017). Industrial and biotechnological applications of algae: A review. Journal of Advances in Plant Biology, 1, 01–25. doi:https://doi.org/10.14302/issn.2638-4469.japb-17-1534
- Prates, D. D., Radmann, E. M., Duarte, J. H., de Morais, M. G., & Costa, J. A. V. (2018). Spirulina cultivated under different light emitting diodes: Enhanced cell growth and phycocyanin production. Bioresource Technology, 256, 38–43. doi:10.1016/j.biortech.2018.01.122
- Priyadarshani, I., & Biswajit, R. (2012). Commercial and industrial applications of micro algae–a review. Journal of Algal Biomass Utilization, 3, 89–100.
- Rasala, B. A., & Mayfield, S. P. (2015). Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynthesis Research, 123, 227–239. doi:10.1007/s11120-014-9994-7
- Rizzo, R.F., Santos, B.D., Castro, G.F., Passos, T.S., Nascimento, M.D., Guerra, H.D. … Lima-Araújo, K.G. (2015). Production of Phycobiliproteins by Arthrospira platensis under different light conditions for application in food products. Food Science and Technology -Brazil, 35, 247–252. doi:10.1590/1678-457x.6463
- Sforza, E., Pastore, M., Franke, S. M., & Barbera, E. (2020). Modeling the Oxygen Inhibition in Microalgae: An Experimental Approach Based on Photorespirometry. New biotechnology, 59, 26–32. doi:10.1016/j.nbt.2020.06.003
- Shahi, K. A., Behnaz, E. K., Dehghanian, Z., Pandey, B., Lajayer, A., Price, G. W., & Astatkie, T. (2022). Removal of organic and inorganic contaminants from the air, soil, and water by algae. Environmental Science and Pollution Research. 06/2022 online. doi: 10.1007/s11356-022-21283-x
- Sheehan, J., Dunahay, T. G., Benemann, J. R., Roessler, P. G., & Weissman, J. C. (1998). A Look back at the U.S. department of energy’s aquatic species program—biodiesel from algae. National Renewable Energy Laboratory, Golden, CO, US Department of Energy, Close-out Report. https://www.nrel.gov/docs/legosti/fy98/24190.pdf
- Shigesada, N., & Okubo, A. (1981). Analysis of the self-shading effect on algal vertical distribution in natural waters. Journal of Mathematical Biology, 12, 311–326. doi:10.1007/BF00276919
- Soni, R. A., Sudhakar, K., & Rana, R. S. (2017). Spirulina – from growth to nutritional product: A review. Trends Food Science & Technology, 69, 157–171. doi:10.1016/j.tifs.2017.09.010
- Soni, R. A., Sudhakar, K., & Rana, R. S. (2019). Comparative study on the growth performance of Spirulina platensis on modifying culture medium. Energy Reports, 5, 327–336. doi:10.1016/j.egyr.2019.02.009
- Srinivasan, T., & Illanjiam, S. (2021a). Extraction and purification of phycocyanin and their radical- scavenging activity from multi - stress spirulina isolated from marine water. Applied Ecological and Environmental Sciences, 9, 73–75.
- Srinivasan, T., & Illanjiam, S. (2021b). Optimization studies of multistress spirulina isolated from marine water. Applied Ecological and Environmental Sciences, 9, 76–78.
- Stanley, J. G., & Jones, J. B. (1976). Feeding Algae to Fish. Aquaculture, 7, 219–23. doi:10.1016/0044-8486(76)90140-X
- Tacon, A. G. J., & Metian, M. (2008). Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture, 285, 146–158. doi:10.1016/j.aquaculture.2008.08.015
- Tayebati, H., Shariati, F. P., Soltani, N., & Tehrani, H. S. (2020). The effect of different light variables on spirulina growth and its component. Iranian International Conference. Chemical Engineering Congress, Exhibition 11. https://www.researchgate.net/profile/HaniehTayebati/publication/345775863
- Thaweedet, C., Traichaiyaporn, S., & Deming, R. L. (2012). Effect of light quality on biomass and pigment production in photoautotrophic and mixotrophic cultures of Spirulina platensis. Journal of Agricultural Technology, 8, 1593–1604.
- Torzillo, G., & Vonshak, A. (2013). Environmental Stress Physiology with Reference to Mass Cultures. Handbook of microalgal culture: applied phycology and biotechnology (pp. 901–13). New Jersey, USA: Blackwell Publishing Ltd. doi:10.1002/9781118567166.ch6
- Uslu, L. H., Oya, I., Sayin, S., Durmaz, Y., Göksan, T., & Gökpinar, Ş. (2009). The effect of temperature on protein and amino acid composition of Spirulina platensis. Journal of Fisheries and Aquatic Sciences, 26, 139–142.
- Vonshak, A., Torzillo, G., Accolla, P., & Tomaselli, L. (1996). Light and oxygen stress in Spirulina platensis (cyanobacteria) grown outdoors in tubular reactors. Physiologia Plantarum, 97, 175–179. doi:10.1111/j.1399-3054.1996.tb00494.x
- Wang, C. Y., Fu, C.-C., & Liu, Y.-C. (2007). Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochemical Engineering Journal, 37, 21–25. doi:10.1016/j.bej.2007.03.004
- Warman, P. R., & Mayhew, W. J. (1979). Effect of reflective surfaces on a greenhouse lettuce crop. Canada. https://www.osti.gov/etdeweb/biblio/8487983
- Webber, A. N., & Lubitz, W. (2001). P700: The primary electron donor of photosystem I. Biochimica Et Biophysica Acta, 1507, 61–79. doi:10.1016/S0005-2728(01)00198-0