 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Microalgal cultivation by small scale aquaculture farmers is limited by the high cost of synthetic culture media. The current study was conducted to investigate use of banana stem compost extract (BSCE) as an alternative medium for cultivation of the microalga Chlorella vulgaris. C. vulgaris was batch cultured for 24 days in the laboratory using synthetic Bold Basal Medium (BBM) as a control and BSCE at concentrations of 2%, 5% and 10% (by volume) as the treatments. Algal growth was evaluated by measuring dry cell weight and specific growth rate (SGR) during the experimental period. Chemical composition was analysed following standard analytical methods. Variations in growth trends among culture media were attributed to variations in nutrient concentration and lack of acclimatization period. Some macro- and micronutrients in BSCE-cultivated C. vulgaris were higher than or similar to those observed in BBM-cultivated algae. The macronutrients differed among BSCE treatments. It was concluded that BSCE can be used as the culture medium, providing similar nutritional value and supporting similar growth performance to synthetic media. However, selection of BSCE concentration should be based on macronutrients and take into account the intended use of cultivated microalgae.
Introduction
Microalgae are a diverse group of eukaryotic photosynthetic microorganisms that can produce amino acids, protein, minerals, vitamins, antioxidants and other bioactive substances (Mtaki, Kyewalyanga, & Mtolera, Citation2020; Sathasivam, Radhakrishnan, Hashem, & Abdallah, Citation2019). Their cultivation has received growing interest around the world due to their high growth rate, high nutritional value, carbon dioxide capture, ability to grow in different culture media and their use of different nutrition modes e.g., auto- and/or heterotrophic (Metsoviti, Papapolymerou, Karapanagiotidis, & Katsoulas, Citation2019; Ramaraj, Tsai, & Chen, Citation2015). Microalgae are used in multiple industries, for instance in aquaculture Chlorella vulgaris is utilized as live feed for larval and/or juvenile of some crustaceans and finfish, some bivalve molluscs (oysters, scallops, clams and mussels) and enrichment of zooplankton e.g., Artemia spp (Koyande et al., Citation2019; Rizwan, Mujtaba, Memon, Lee, & Rashid, Citation2018). It has been suggested that large-scale production of C. vulgaris could be used as the means to improve aquaculture production and address global food shortages (Abdullah et al., Citation2019; Chang, Nichols, & Blackburn, Citation2013).
Cultivation costs of C. vulgaris are too high for commercial applications in developing countries: mass production is frequently uses synthetic media such as BG 11, Zarrouk and Bold Basal media (BBM) which are very expensive and account for up to 50% of microalgal production costs (Michael, Kyewalyanga, & Lugomela, Citation2019; Mtaki, Kyewalyanga, & Mtolera, Citation2021; Xia & Murphy, Citation2016). These synthetic media are not easily accessible to small scale aquaculture farmers in developing countries (Michael, Kyewalyanga, & Lugomela, Citation2019). It is therefore imperative to replace expensive synthetic media with cheap and locally available media. Here we show how this can be produced from banana stem by-products so as to recycle nutrients and improve farmers’ livelihoods. Banana plants (Musa spp.) could be used as as cheap and widely available resource needed to make culturing medium for microalgal production.
Banana pseudo-stems are cut down after the fruit is harvested and are usually left in the field, creating an agri-waste problem and environmental nuisance (Gumisiriza, Hawumba, Okure, & Hensel, Citation2017; Gupta, Baranwal, Saxena, & Reddy, Citation2019). Banana pseudo-stems are reported to contain 58% carbohydrate, and high levels of nutrients including calcium and phosphorus (Aziz et al., Citation2011; Ho, Noor Aziah, & Bhat, Citation2012; Mtaki, Kyewalyanga, & Mtolera, Citation2020). These nutrients make banana stems an ideal candidate for a cheap alternative medium for microalgal cultivation. However, there are limited studies on the use of banana by-products or their derivatives as medium for microalgal cultivation. The current study aimed to evaluate application of banana stem compost extract (BSCE) as a potential replacement for synthetic media in C. vulgaris cultivation. The study also aimed to estimate the financial cost of preparing BSCE medium compared to the synthetic medium BBM. The hypothesis tested was that replacing BBM with BSCE culturing medium would result in similar growth and biochemical composition of C. vulgaris while reducing operational cost.
Materials and methods
Microalgal culture media preparation
The banana stems (Musa x paradisiaca) were harvested from a local farm in Dar es Salaam, Tanzania. The stems’ outer layers (epidermis) were removed manually by peeling and the inner part was taken to the Botany laboratory at University of Dar es Salaam, Tanzania for media preparation. The stems were laid on the ground, covered with dry grasses, and left for two weeks to allow decomposition. The compost was washed using running tap water, cut into small pieces and blended. Blended stem materials were filtered using 44 µm mesh nano-filter and extracts were centrifuged (Hettich Zentrifugen 168 Universal 1200, Germany) at 978.02 x g for 10 minutes. The collected compost supernatant was sterilized at 121°C for 15 min in an autoclave (SANYO, MLS-3750). The supernatant was diluted to 2%, 5% or 10% BSCE using sterilized (121°C for 15 min) and filtered (20 µm filter mesh) tap water. BBM medium was prepared as per standard operating procedure (Connon, Citation2007).
All culture media were chemically analysed for their nutrient composition at the beginning of the experiment. The media were analysed for ammonium-nitrogen, phosphorus and nitrate-nitrogen using indophenol blue, ascorbic acid and cadmium reduction methods. Calcium, magnesium, zinc, manganese, boron, potassium and molybdate were determined calorimetrically using an Atomic Absorption Spectrophotometer (AA240 Varian, USA).
Experimental design
A culture of C. vulgaris isolated from fish pond water of pH 8.5 (for details see Mtaki, Kyewalyanga, & Mtolera, Citation2021) was grown in standard BBM medium for seven days prior to the start of the experiment. Thereafter, C. vulgaris was cultured for 24 days using BBM medium as control and BSCE media at 2%, 5% and 10% concentrations as the treatments. The microalgae were batch cultured in 2 l Erlenmeyer flasks (in triplicate per treatment) using 800 ml of the respective culture medium and 200 ml of C. vulgaris with an initial count of 0.3 x 106 cells ml−1. The experiment was conducted in a controlled environment with pH set at 9.0 and maintained throughout by adding appropriate quantities of 5 M sodium hydroxide and 1 M hydrochloric acid. Temperature was maintained at 28 ± 1°C; compressed air was provided; light intensity was set at 5000 ± 10 lux (c. 500 µmol m−2 s−1) with 16:8 photoperiods (light:dark cycle) monitored using a vertex VXLM-636 light metre and automatic timer respectively. Microalgae were evaluated for growth performance every three days throughout the experimental period and were analysed for chemical composition at the end of the experiment.
Microalgal growth evaluation
Growth of C. vulgaris was determined by measuring dry cell weight and expressed as specific growth rate (SGR). The algal dry weight (biomass concentration, g l−1) was measured by taking 10 ml from the culture flask and filtering it using pre-weighed Whatman GF/C filters. The filters were washed using distilled water to remove adsorbed salt and oven-dried at 105°C until constant weight was achieved. The microalgal dry weight was calculated as the difference between the weight of oven-dried filters containing microalgae and the empty filter. SGR was measured as an increase in biomass over time, and was determined using Equation 1 below.
Chemical analysis
Sample preparation and protein analysis
The C. vulgaris biomass was harvested after the individual treatments had reached the stationary growth phase which was day 18, 21, 21 and 24 for 2% BSCE, 5% BSCE, BBM and 10% BSCE respectively, by centrifugation at 978.02 x g for 10 min. The centrifuged mass was washed using distilled water and air-dried in a darkroom for about a week. The dried biomass was ground to powder and used for all biomass chemical composition analyses. The crude protein content in C. vulgaris biomass was determined using the Kjeldahl standard method (Barbano, Clark, Dunham, & Flemin, Citation1990).
Total lipids
Total lipid extraction followed Bligh & Dyer’s (Citation1959) procedures, whereby sampled C. vulgaris (~5 g) was mixed with chloroform, methanol and water in a 1:2:0.8 ratio. The mixture was homogenized using a Soxhlet apparatus for 2 min, then chloroform and water were added to give a solvent ratio of 2:2:1.8. The solvent was filtered using Whatman filter paper No. 44 to remove biomass residues. The filtered solvents were separated into two layers, chloroform (bottom phase) and aqueous methanol (top phase), using a separating funnel and the volume of the chloroform layer was recorded. The chloroform layer was collected, pipetted and weighed using a pre-weighed, clean oven-dried evaporation dish. The aliquot was oven-dried at 40°C for 60 minutes, cooled in a desiccator and weighed. The percentage lipids in C. vulgaris biomass was analysed gravimetrically, based upon starting and end mass.
Total carbohydrates
Total carbohydrates were quantified using Allen’s (Citation1989) method whereby 5 g of sampled biomass was mixed with 30 ml of distilled water in a 100 ml conical flask and boiled at 100°C for two hours. The boiled sample was allowed to cool to room temperature and filtered through a Whatman filter paper No. 44. A clear sample solution (aliquot) was put into a test tube and anthrone reagent prepared with boiled hot water was added. The mixed solution was allowed to cool, and its absorbance was measured at 625 µm. The percentage soluble carbohydrate was calculated based on the formula given in EquationEquation 2(2)
(2) below.
Where C = mg of glucose in the sample aliquot obtained from a calibration graph of the standard.
Mineral composition
The sampled biomass was digested using nitric perchloric acid to determine mineral composition as described by Jones (Citation1984). The sampled C. vulgaris (0.5 g) was placed in a beaker followed by a mixture of 5 ml concentrated nitric acid (70% HNO3) and 1 ml perchloric acid (72% HClO4). The solution was heated at 120°C until brown fumes disappeared which indicated complete digestion of organic matter. The solution was then cooled and distilled water added to make a volume of 100 ml. The Ca, Mg, Fe, Mn, Zn and Cu concentrations of the digested solution were determined using Atomic Absorption Spectrophotometer (AA240 Varian, USA).
Vitamins
Vitamin extraction was by mixing 0.5 g of sampled biomass with 100 ml 95% ethanol into a conical flask that was shaken vigorously for 15 min. The mixture was centrifuged for 10 min and filtered using Whatman No. 1 filter paper. The sample was put into a rotary evaporator (Gmbh & Co.KG, Germany) at 40°C and set under reduced pressure so as to remove ethanol and obtain clear extracts. Vitamin A (as beta-carotene) was determined by vigorously stirring 100 mg of dried extract with 10 ml of acetone-hexane mixture (4:6) for 1 minute and filtered using Whatman No.4 filter paper. The filtrate absorbance was measured at 453, 505 and 663 µm, and the obtained values were used to calculate beta carotene in EquationEquation 3(3)
(3) .
Vitamins B1 (thiamine), B2 (riboflavin), B3 (niacin), B6 (pyridoxine) and C (ascorbic acid) were determined using thiamine hydrochloride, riboflavin, nicotinamide, pyridoxine hydrochloride and ascorbic acid stock solutions respectively as described by Rajput, Kumar, Kumar, & Res (Citation2011). Individual stock solutions were prepared by dissolving a known weight of standard vitamin in a known volume of distilled water and the working solution was prepared by dissolving a known weight of each C. vulgaris extract in a known volume of distilled water. The stock solutions, working solutions and blank were used to determine vitamin B1 at 430 µm, vitamin B2 at 444 µm, vitamin B3 at 450 µm, vitamin B6 at 650 µm and vitamin C at 450 µm using a UV/visible spectrophotometer (Jenway 6305).
Estimation of microalgal media cost
The cost of making 1 l of the medium was calculated. The costs of BBM medium included price of each reagent, taxation and consumed electricity. The price of BBM reagents were obtained from https://www.alibaba.com and https://www.sigmaaldrich.com. Value added tax was set at 18% of the reagent price as indicated by Tanzania Revenue Authority while electricity cost was calculated from the electricity used to prepare medium. The product was then multiplied by the price of one unit of electricity which was 350 TSh (~0.15 USD) per kWh in Tanzania. The estimation of BSCE media cost considered collection, transport and processing (sterilization and blending) costs only since banana stem was obtained free in the current study.
Statistical analysis
Collected data were analysed using R software (version 4.0.3), and data were checked for normality using the Shapiro-Wilk test and homogeneity of variances using Levene’s tests. One-way analysis of variance (ANOVA) and Kruskal Wallis were used to analyse differences in parameters measured among culture media for normally and not normally distributed data respectively. Tukey’s (ANOVA) and Dunn (Kruskal Wallis) post hoc tests were used for mean pairwise comparison among culture media. Results were presented as mean ± SE (standard error of the mean) and difference was considered significant when p ≤ 0.05.
Results
Growth parameters and media cost
There were variations in growth trends () among culture media whereby C. vulgaris cultured in high BSCE concentrations (5% and 10%) had a slow initial growth rate. Exponential growth phases were delayed in 5% and 10% BSCE compared to other culture media. Moreover, 10% BSCE took longer to reach stationary phase (day 24) compared to BBM (day 21), 5% BSCE (day 21) and 2% BSCE (day 18). There were no statistical differences in specific growth rate (SGR) between control (BBM) and treatments (BSCE). However, the difference was significant (p = 0.046) among treatments whereby SGR was higher in 2% BSCE (0.18 day−1) than in 10% BSCE (0.15
day−1) while 5% BSCE (0.17
day−1) was not significantly different from either of the two. It cost more to prepare 1 l of BBM medium compared to the BSCE treatments ().
Figure 1. Mean dry cell weight of Chlorella vulgaris cultivated using Bold’s Basal Medium (BBM) (asterisks), and media formulated with 2% (filled triangle), 5% (empty circle), or 10% (empty triangle) volume/volume additions of Banana Stem Compost Extract (BSCE) (n = 3, error bars = standard error).
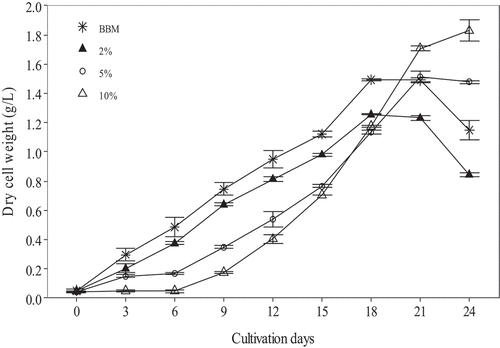
Table 1. Production cost in US$ (converted from Tanzanian Shillings) per unit (1 l) of Bold Basal Media (BBM) and Banana Stem Compost Extract (BSCE) culture media.
Chemical analysis
There were variations in nutritional values of culture media (). BBM had higher nitrate (45.5 mg l−1) but lower ammonium and potassium (21 mg l−1) than other culture media. The 10% BSCE had higher nitrate, potassium, phosphorus and ammonium than the other concentrations. Phosphorus was higher in 10% BSCE (18.2 mg l−1) than in BBM medium (13.3 mg l−1). The culture media significantly affected (p < 0.05) crude protein (CP), carbohydrate and lipids contents of C. vulgaris biomass (). The BBM-cultured C. vulgaris had higher CP (45%) than algae cultured in 2% (21.4%) and 5% BSCE (24.5%). CP values did not differ between BBM and 10% BSCE (34.8%). The carbohydrate content in C. vulgaris biomass did not vary among treatments, however, it was relatively higher in 10% BSCE (36.4%) than in BBM (20.8%, p < 0.05). Lipid contents did not differ between treatments and controls, however, there were differences among treatments whereby it was lower in 10% BSCE (13.9%) than in 2% (30.8%) and 5% BSCE (28.1%).
Table 2. Chemical composition of Bold Basal Media (BBM) and Banana Stem Compost Extract (BSCE).
Table 3. Chemical composition of Chlorella vulgaris biomass cultured in Bold Basal Media (BBM) and Banana Stem Compost Extract (BSCE).
The Ca, Zn and Mn contents of C. vulgaris biomass did not differ significantly (p > 0.05) between treatments and controls but the differences among treatments were significant (see ). Ca and Zn were lowest in 2% BSCE (67.1 mg 100 g−1 and 1.0 mg 100 g−1 respectively), while Mn was highest in 10% BSCE (1.6 mg 100 g−1). The 10% BSCE media had the highest Mg (182.4 mg 100 g−1) and Fe (26.5 mg 100 g−1) contents (p < 0.05) among active treatments. The K content did not vary among treatments but it was lower in BBM (1113.6 mg 100 g−1) than in 5% BSCE (1219.7 mg 100 g−1) and 10% BSCE (1235.2 mg 100 g−1). Culture media had a significant effect (p < 0.05) on Vitamin A, C, B1, B2, B3 and B6 contents. Vitamins A, C, B2 and B3 were significantly higher in treatments than control. There were no variations in Vitamins A, B1, B2, B3 and B6 contents among treatments but Vitamins C was significantly higher in 10% BSCE (7.6 mg 100 g−1) compared to 2% (6.6 mg 100 g−1) and 5% (6.4 mg 100 g−1) BSCE. The BBM-cultured C. vulgaris had significantly (p < 0.05) higher Vitamin B1 (1.8 mg 100 g−1) than 2% (1.3 mg 100 g−1) and 5% (1.5 mg 100 g−1) BSCE.
Discussion
Nutritional composition
Similar crude protein (CP) values in BBM and 10% BSCE () could be explained by the high nitrate supply in both culture media (). Nitrogen is an essential nutrient for protein synthesis and there is a positive relationship between CP values in microalgal biomass and nitrogen concentration in the culture media (Hodaifa, Sánchez, Martínez, & Órpez, Citation2013; Ji et al., Citation2014; Li et al., Citation2020; Zhang et al., Citation2018). The CP values observed in BBM and 10% BSCE were within ranges reported for C. vulgaris cultured using synthetic media, kitchen waste or monosodium glutamate wastewater, i.e., 36–51% (Ji et al., Citation2014; Prabakaran et al., Citation2019). The similarity in CP values for BBM and 10% BSCE showed the potential of using banana stem compost to produce high quality protein products which can be used in aquaculture production (Barros, Gonçalves, Simões, & Pires, Citation2015; Koller, Muhr, & Braunegg, Citation2014; Sathasivam, Radhakrishnan, Hashem, & AbdAallah, Citation2019). Additionally, variations in CP among culture media in this study are attributed to different nitrogen concentrations in the media as C. vulgaris cultures were harvested when they reached stationary growth phase. Lipid concentration declined with an increase in BSCE concentration while carbohydrate content seemed to increase with an increase in BSCE concentration ().
C. vulgaris increases lipids and carbohydrate concentration under environmental stress since polysaccharides and lipids are used as an energy source when there is nitrogen deficiency (Cho et al., Citation2015; Cointet et al., Citation2019; Illman, Scragg, & Shales, Citation2000; Wang, Xiong, Hui, & Zeng, Citation2012). However, it should be noted that C. vulgaris are mixotrophic (both auto- and heterotrophic) hence carbon to nitrogen ratio (C:N) in culturing media can influence biomass chemical composition. Increasing C:N changes carbon partitioning which increases lipids and carbohydrate concentration while reducing protein content (Cointet et al., Citation2019; Metsoviti, Papapolymerou, Karapanagiotidis, & Katsoulas, Citation2019; Wang, Xiong, Hui, & Zeng, Citation2012). Organic carbon was not measured in BSCE media due to limited resources, but it should be analysed in future studies to determine the potential for mixotrophy. It is likely that variation in observed lipids and carbohydrate among treatments relates to C:N ratios, with nitrogen levels increasing with BSCE concentration. These results could be useful in selecting the appropriate BSCE concentration and manipulation of C:N ratios in culture media to favour heterotrophy by increasing organic carbon when the end product is lipids or carbohydrate.
Future studies should also investigate effects of using BSCE levels of above 10% on protein, lipids and carbohydrates values. Carbohydrate extracted from microalgal biomass could find uses in the bioethanol and biohydrogen industries (Behera et al., Citation2019). Moreover, high phosphorus contents observed in BSCE (3.6–18.2 mg l−1) could also make banana stem a cheap phosphorus source for cultivation of other crops. Some trace elements (Zn, 28.4–141.8 mg l−1) were higher in the BSCE media (), but not in biomass of BSCE-cultured C. vulgaris (). Zn concentrations (1.0–3.1 mg 100 g−1) in BSCE cultured C. vulgaris were within WHO/FAO recommended amounts for human and livestock consumption as was noted by Elbagermi, Edwards & Alajtal (Citation2012). The differences in Zn between culture media and cultured biomass is because only a small proportion of the nutrient is transferred between trophic levels and the rest could have remained in the culture media.
Contents of minerals and vitamins (A, B1 and B3) reported in this study were within ranges reported elsewhere for C. vulgaris (Andrade, Andrade, Dias, & Nascimento, Citation2018; Panahi et al., Citation2012; Prabakaran et al., Citation2019; Tokuşoglu & Üunal, Citation2003). The results indicated that replacing BBM with BSCE did not affect most of the micronutrient concentrations. The C. vulgaris produced can be used as the source of microminerals for humans and livestock so as to ensure normal body functioning (Gatlin, Citation2003; Marsan, Conrad, Stutts, Parker, & Deeds, Citation2018; Paul & Mukhopadhyay, Citation2016). The biochemical composition of C. vulgaris from different media were report here accounted for only 80–83% of total biomass (), because other components such as chlorophyll, carotenoids and antioxidants were not analysed as they were of limited interest in the current study. Moreover, the current study did not determine nutrient uptake or cultivation rate among microalgae as our primary objective was to establish if C. vulgaris can grow successfully on BSCE media. Future studies should investigate further the composition of BSCE media post-cultivation so as to determine carrying capacity for effective utilization of BSCE and customization of the medium.
Chlorella vulgaris growth and cost of culture media
Observed differences in growth rates () among culture media could be attributed to several factors. C. vulgaris was cultured in BBM before the start of the experiment and there was no acclimation period prior to culture in BSCE. This could have initially favoured algal growth in BBM than in BSCE hence explaining the fast initial growth rate in BBM. However, C. vulgaris in 2% BSCE also had rapid initial growth compared to algae in 5% and 10% BSCE which is attributed to its lower levels of ammonium (1.2 mg l−1) compared to 2.9 mg l−1and 5.9 mg l−1 observed in 5% and 10% BSCE respectively. High ammonium concentration in culture media is toxic and reduces growth rate of C. vulgaris (De Lourdes, Josefina, Ulises, & De Jesús, Citation2017). Therefore, use of high BSCE concentrations would require an acclimation period of at least 6 days, after which a fast growth rate was noted in 5% and 10% BSCE (), or other means of reducing ammonium levels in the media. Nonetheless, observed growth trends in this study were similar to those reported by Singh, Babcock, & Radway (Citation2000), Venckus, Kostkevičienė, & Bendikienė (Citation2017) and Abu-rezq et al. (Citation2010) who noted similar growth pattern in different culture media.
The C. vulgaris dry weight obtained in this study was within the range (0.67–4.23 g l−1) reported for this species cultivated using aquaculture waste water or effluent from sewage sludge (Cho et al., Citation2015; Mtaki, Kyewalyanga, & Mtolera, Citation2021). The high biomass could provide large amount of raw materials for livestock feed, in fuel production and in pharmaceutical industries. The lower specific growth rate (SGR) in 10% BSCE (0.15 day−1) than in 2% BSCE (0.18
day−1) was a result of the longer exponential growth phase in the former than the latter (day 24 versus day 18). The delay to achieving exponential growth phase in 10% BSCE could indicate that a longer acclimatization period is needed for this medium to achieve maximum biomass concentration. The higher organic carbon in this medium facilitates mixotrophy which in turn increases the exponential growth rate. Higher SGR in 2% BSCE than in 10% BSCE indicated that many cultivation cycles per year could make 2% BSCE more productive in terms of annual yield. The SGR reported in the current study was higher than the 0.06
day−1 reported for microalgae cultured in hydroponic systems (Supraja, Behera, & Balasubramanian, Citation2020). The differences in SGR between the current and previous studies could be due to differences in the strain of C. vulgaris (unidentified in this study), media composition and nutrient concentration. Since we did not carry out any molecular characterization (e.g., DNA barcoding) of our strain, future experiments should address this limitation and assess potential use of BSCE for cultivation of specified C. vulgaris strains. However, similar SGR in control and treatments showed that banana stem can be used to replace expensive synthetic media for microalgal cultivation without affecting productivity.
The BSCE had lower preparation cost (0.02–0.04 USD) compared to BBM (0.19 USD, ) because banana stem was free hence BSCE cost might vary depending on access to banana stems (free or purchased). There is a chance that banana stem could be assigned monetary value and become very expensive due to an increase in its demand for culture medium. Also, environmental stress like drought, diseases e.g., black Sigatoka leaf disease, and pests such as banana weevils (Batte et al., Citation2019; Ndayihanzamaso et al., Citation2020) could lower banana productivity and reduce the relevance of using BSCE as culture media. We remain optimistic despite the potential challenges that BSCE could be an ideal and cheap culture medium especially among small scale aquaculture producers in developing countries. Its relevance among small scale aquaculture producers is due to integrated aquaculture practices i.e., keeping fish with horticultural crops like banana and national banana breeding programs aiming to improve production (Batte et al., Citation2019; Ndayihanzamaso et al., Citation2020). It was clearly established in this study that BSCE can be used to replace synthetic media although the study was unable to determine the appropriate BSCE concentration for best results across all parameters in cultivated C. vulgaris. Lower SGR in 2% BSCE could give relatively high biomass per annum compared to 10% BSCE. However, 2% BSCE had lower CP and other micronutrients than those seen in BBM (). We recommend that the choice of BSCE concentration should be based on targeted macronutrient (protein, lipids or carbohydrate) and intended use of the cultivated microalgae (nutrient supplement, livestock feeding, biodiesel production).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on request.
Acknowledgment
The authors wish to thank the Swedish International Development Cooperation Agency and Institute of Marine Science (IMS), University of Dar es Salaam for financial support which led to successful completion of this work. We thank Mr. Charles Kweyunga of Department of Botany, University of Dar es Salaam for his assistance in data collection and Dr. Peter R. Ruvuga for assisting with reviewers’ comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdullah, B., Muhammad, S. A. S., Shokravi, Z., Ismail, S., Kassim, K. A., Mahmood, A. N., & Aziz, M. M. A. (2019). Fourth generation biofuel: A review on risks and mitigation strategies. Renewable and Sustainable Energy Reviews, 107, 37–50. doi:10.1016/j.rser.2019.02.018
- Abu-Rezq, T. S., Al-Hooti, S., Dangly, A., & J. (2010). Optimum Culture Conditions Required for the Locally Isolated Dunaliella Salina. Journal of Algal Biomass Utilization, 1, 12–19.
- Allen, S. E. (1989). Analysis of vegetation and other organic materials. In Chemical analysis of ecological materials (2nd ed., pp. 46–60). Oxford London Edinburgh, Boston Melbourne: Blackwell Scientific Publications.
- Andrade, L.M., Andrade, C.J., Dias, M., & Nascimento, C.A.O. (2018). Chlorella and Spirulina Microalgae as sources of functional foods, nutraceuticals, and food supplements; An overview. MOJ Food Processing & Technology, 6, 45–58. doi:10.15406/mojfpt.2018.06.00144
- Aziz, N.A.A., Ho, L.H., Azahari, B., Bhat, R., Cheng, L.-H., & Ibrahim, M. N. M. (2011). Chemical and functional properties of the native banana (Musa acuminata balbisiana Colla cv. Awak) pseudo-stem and pseudo-stem tender core flours. Food Chemistry, 128, 748–753. doi:10.1016/j.foodchem.2011.03.100 Kjeldahl
- Barbano, D. M., Clark, J. L., Dunham, C. E., & Flemin, R. J. (1990, November 1). Kjeldahl method for determination of total nitrogen content of milk: Collaborative study. Journal of Association of Official Analytical Chemists, 73, 849–859. doi:10.1093/jaoac/73.6.849
- Barros, A.I., Gonçalves, A.L., Simões, M., & Pires, J.C.M. (2015). Harvesting techniques applied to microalgae: A review. Renewable and Sustainable Energy Reviews, 41, 1489–1500. doi:10.1016/j.rser.2014.09.037
- Batte, M., Swennen, R., Uwimana, B., Akech, V., Brown, A., Tumuhimbise, R., & Ortiz, R. (2019). Crossbreeding east African highland bananas: Lessons learnt relevant to the botany of the crop after 21 years of genetic enhancement. Frontiers in Plant Science, 10, 81. doi:10.3389/FPLS.2019.00081/BIBTEX
- Bligh, E.G., & Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917. doi:10.1093/nq/s9-III.75.427-d
- Bunushree, B., Acharya, A., Akhil Gargey, I., Aly, N., & Balasubramanian, P. (2019). Bioprocess Engineering Principles of Microalgal Cultivation for Sustainable Biofuel Production. Bioresource Technology Reports, 5, 297–316. doi:10.1016/J.BITEB.2018.08.001
- Cho, H.U., Kim, Y.M., Choi, Y.N., Xu, X., Shin, D. Y., & Park, J. M. (2015). Effects of pH control and concentration on microbial oil production from Chlorella vulgaris cultivated in the effluent of a low-cost organic waste fermentation system producing volatile fatty acids. Bioresource Technology, 184, 245–250. doi:10.1016/j.biortech.2014.09.069
- Cointet, E., Wielgosz-Collin, G., Bougaran, G., Rabesaotra, V., Gonçalves, O., & Méléder, V. (2019). Effects of light and nitrogen availability on photosynthetic efficiency and fatty acid content of three original benthic diatom strains. PLoS One, 14, 1–28. doi:10.1371/JOURNAL.PONE.0224701
- Connon, R. 2007. “Culturing of Chlorella Vulgaris - Standard Operating Procedure.” Reading, United Kingdom. http://www.biosci.rdg.ac.uk/Research/eb/daphnia.htm
- De Lourdes, F.M.M., Josefina, R. D., Ulises, M.M.C., & De Jesús, M.R.A. (2017). Tolerance and nutrients consumption of Chlorella vulgaris growing in mineral medium and real wastewater under laboratory conditions. Open Agriculture, 2, 394–400. doi:10.1515/opag-2017-0042
- Elbagermi, M. A., Edwards, H. G. M., & Alajtal, A. I. (2012). Monitoring of Heavy Metal Content in Fruits and Vegetables Collected from Production and Market Sites in the Misurata Area of Libya. International Scholarly Research Notices, 827645. doi:10.5402/2012/827645
- Gatlin, D.M., & Hardy, R.W. (2003). Nutrition and fish health. In Fish Nutrition (3rd ed., pp. 671–702). USA: Elsevier Science. Copyright 2002.
- Gumisiriza, R., Hawumba, J.F., Okure, M., & Hensel, O. (2017). Biomass waste-to-energy valorisation technologies: A review case for banana processing in Uganda. Biotechnology for Biofuels, 10, 1–29. doi:10.1186/s13068-016-0689-5
- Gupta, G., Baranwal, M., Saxena, S., & Reddy, M.S. (2019). Utilization of banana stem juice as a feedstock material for bioethanol production. CLEAN – Soil, Air, Water, 47, 1–5. doi:10.1002/clen.201900047
- Hodaifa, G., Sánchez, S., Martínez, M.E., & Órpez, R. (2013). Biomass production of Scenedesmus obliquus from mixtures of urban and olive-oil mill wastewaters used as culture medium. Applied Energy, 104, 345–352. doi:10.1016/j.apenergy.2012.11.005
- Ho, L.H., Noor Aziah, A.A., & Bhat, R. (2012). Mineral composition and pasting properties of banana pseudo-stem flour from Musa acuminata x balbisiana cv. Awak grown locally in Perak, Malaysia. International Food Research Journal, 19, 1479–1485.
- Illman, A.M., Scragg, A.H., & Shales, S. W. (2000). Enzyme and microbial technology: Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme and Microbial Technology, 27, 631–635. doi:10.1016/S0141-0229(00)00266-0
- Ji, Y., Hu, W., Li, X., Ma, G., Song, M., & Pei, H. (2014). Mixotrophic growth and biochemical analysis of Chlorella vulgaris cultivated with diluted monosodium glutamate wastewater. Bioresource Technology, 152, 471–476. doi:10.1016/j.biortech.2013.11.047
- Jones, J.J.B. (1984). Plants. In S. Williams (Ed.), Official methods of analysis of the association of official analytical chemists (pp. 38–64). Arlington, Virginia: Association of Official Analytical Chemists.
- Koller, M., Muhr, A., & Braunegg, G. (2014). Microalgae as versatile cellular factories for valued products. Algal Research, 6, 52–63. doi:10.1016/j.algal.2014.09.002
- Koyande, A.K., Chew, K.W., Rambabu, K., Tao, Y., Chu, D.-T., & Show, P.-L. (2019). Microalgae: A potential alternative to health supplementation for humans. Food Science and Human Wellness, 8, 16–24. doi:10.1016/j.fshw.2019.03.001
- Lee Chang, K.J., Nichols, P.D., & Blackburn, S.I. (2013). Hydroformylation of vegetable oils and the potential use of hydroformylated fatty acids. Lipid Technology, 25, 199–203. doi:10.1002/lite.201300295
- Li, S., Ji, L., Chen, C., Zhao, S., Sun, M., Gao, Z., & Fan, J. (2020). Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresource Technology, 309, 123362. doi:10.1016/j.biortech.2020.123362
- Marsan, D.W., Conrad, S.M., Stutts, W.L., Parker, C. H., & Deeds, J. R. (2018). Evaluation of microcystin contamination in blue-green algal dietary supplements using a protein phosphatase inhibition-based test kit. Heliyon, 4, e00573. doi:10.1016/j.heliyon.2018.e00573
- Metsoviti, M.N., Papapolymerou, G., Karapanagiotidis, I.T., & Katsoulas, N. (2019). Comparison of growth rate and nutrient content of five microalgae species cultivated in greenhouses. Plants, 8, 1–13. doi:10.3390/plants8080279
- Michael, A., Kyewalyanga, M.S., & Lugomela, C.V. (2019). Biomass and nutritive value of Spirulina (Arthrospira fusiformis) cultivated in a cost-effective medium. Annals of Microbiology, 69, 1387–1395. doi:10.1007/s13213-019-01520-4
- Mtaki, K., Kyewalyanga, M.S., & Mtolera, M.S.P. (2020). Applied sciences assessment of antioxidant contents and free radical-scavenging capacity of Chlorella vulgaris cultivated in low cost media. Applied Sciences, 10, 8611. doi:10.3390/app10238611
- Mtaki, K., Kyewalyanga, M.S., & Mtolera, M.S.P. (2021). Supplementing wastewater with NPK fertilizer as a cheap source of nutrients in cultivating live food (Chlorella vulgaris). Annals of Microbiology, 71, 1–13. doi:10.1186/s13213-020-01618-0
- Ndayihanzamaso, P., Mostert, D., Matthews, M.C., Mahuku, G., Jomanga, K., Mpanda, H. J., & Viljoen, A. (2020). Evaluation of mchare and matooke bananas for resistance to Fusarium oxysporum f. Sp. cubense race 1. Plants, 9, 1–15. doi:10.3390/plants9091082
- Panahi, Y., Pishgoo, B., Jalalian, H.R., Mohammadi, E., Taghipour, H. R., Sahebkar, A., & Abolhasani, E. (2012). Investigation of the effects of Chlorella vulgaris as an adjunctive therapy for dyslipidemia: Results of a randomised open-label clinical trial. Nutrition & Dietetics, 69, 13–19. doi:10.1111/j.1747-0080.2011.01569.x
- Paul, B.N., & Mukhopadhyay, P.K. (2016). Importance of trace minerals in aquaculture nutrition. Fish Chimes, 21, 34–36.
- Prabakaran, G., Moovendhan, M., Arumugam, A., Matharasi, A., Dineshkumar, R., & Sampathkumar, P. (2019). Evaluation of chemical composition and in vitro antiinflammatory effect of marine Microalgae Chlorella vulgaris. Waste and Biomass Valorization, 10, 3263–3270. doi:10.1007/s12649-018-0370-2
- Rajput, G., Kumar, A., Kumar, A., & Res, G.S. (2011). To develop a simple (UV-Vis spectrometric) method for the estimation of water soluble vitamins. International Journal of Drug Research, 2, 232–239.
- Ramaraj, R., Tsai, D.D.W., & Chen, P.H. (2015). Biomass of algae growth on natural water medium. Journal of Photochemistry and Photobiology B, Biology, 142, 124–128. doi:10.1016/j.jphotobiol.2014.12.007
- Rizwan, M., Mujtaba, G., Memon, S.A., Lee, K., & Rashid, N. (2018). Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renewable and Sustainable Energy Reviews, 92, 394–404. doi:10.1016/j.rser.2018.04.034
- Sathasivam, R., Radhakrishnan, R., Hashem, A., & Abdallah, E. F. (2019). Microalgae metabolites: A rich source for food and medicine. Saudi Journal of Biological Sciences, 26, 709–722. doi:10.1016/j.sjbs.2017.11.003
- Singh, E., Babcock, R., & Radway, J. A. (2000). Photobioreactor modification for Dunaliella salina. Marbec Summer Undergraduate Research Fellowship, Marine Bioproducts Engineering Center. Berkeley, California: University of Hawaii at Manoa and University of California.
- Supraja, K.V., Behera, B., & Balasubramanian, P. (2020). Performance evaluation of hydroponic system for co-cultivation of microalgae and tomato plant. Journal of Cleaner Production, 272, 122823. doi:10.1016/j.jclepro.2020.122823
- Tokuşoglu, O., & Üunal, M.K. (2003). Biomass nutrient profiles of three microalgae. Journal of Food Science, 68, 1144–1148. doi:10.1111/j.1365-2621.2003.tb09615.x
- Venckus, P., Kostkevičienė, J., & Bendikienė, V. (2017). Green algae Chlorella vulgaris cultivation in municipal wastewater and biomass composition. Journal of Environmental Engineering and Landscape Management, 25, 56–63. doi:10.3846/16486897.2016.1245661
- Wang, H., Xiong, H., Hui, Z., & Zeng, X. (2012). Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresource Technology, 104, 215–220. doi:10.1016/j.biortech.2011.11.020
- Xia, A., & Murphy, J.D. (2016). MIcroalgal cultivation in treating liquid digestate from biogas systems. Trends in Biotechnology, 34, 264–275. doi:10.1016/j.tibtech.2015.12.010
- Zhang, L., Cheng, J., Pei, H., Pan, J., Jiang, L., Hou, Q., & Han, F. (2018). Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renewable Energy, 115, 276–287. doi:10.1016/j.renene.2017.08.034
