?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
To determine the sorption potential and mechanisms of tetracycline (TC) on pristine and H2O2-modified biochars, rape stalk biochars were produced and modified by H2O2. The specific surface area and pore volume of biochars increased with improving pyrolysis temperature, while the acidic groups decreased. Moreover, modified by H2O2, much more oxygen-containing functional groups were introduced. The results of sorption dynamics and isothermals tests showed that RB300 had the largest TC sorption capacity of 35.90 mg/g, which was ascribed to a plenty of non-carbonized organic matter and surface functional groups were retained. Modified by H2O2, the sorption of TC on biochars was enhanced which indicated the dominated sorption mechanisms were H-bonding and π–π electron donor acceptor interaction. The pH value would impact on sorption, and the maximum sorption capacity was obtained at pH = 9.0. Both cation concentration and type would affect the TC sorption.
1. Introduction
Various pharmaceutical antibiotics such as tetracyclines (TCs), sulfonamides, macrolides and quinolones have been used extensively to prevent and control infections in animal and human medicine [Citation1]. Among the common used antibiotics, TCs are one of the most frequently used in the agriculture and livestock industry because of their relative effectiveness and low costs [Citation2]. However, about 70–90% of the ingested TCs can not be metabolized by humans and animals, thus the undigested antibiotic would be excreted and accumulated in the environment [Citation3]. Luo et al. [Citation4] reported that the average concentrations of TCs in the water and sediment of Haihe River were around 26 ng/L and 600 ng/kg, respectively. The bioaccumulation of TCs which passed along the food chain would induce the proliferation of antibiotic-resistant genes (ARGs) [Citation5], and might pose potential hazards to human health and ecosystems [Citation2,Citation6]. Thus, removal of TCs from wastewater has received increasing attention in recent years, and it is necessary to develop effective and low-cost technology for antibiotic removal.
Numerous antibiotics elimination techniques have been proposed and widely used, such as electrochemical treatments [Citation7], membrane filtration [Citation8], photocatalytic degradation [Citation9] and sorption onto porous materials. Specifically, the sorption technology was considered to be one of the most convenient and economical methods for its low cost, easy operation and no toxicities generated. However, the selection of adsorbents was the key factor on the application of this technology. Up to now, various adsorbents such as clay minerals [Citation10], graphene-based materials [Citation6], aluminum oxide [Citation11], functionalized carbon nanotubes [Citation12], and activated carbons had been used for the antibiotic sorption. Besides, the biochar had been considered as the most potential adsorbent candidate [Citation13,Citation14].
Biochar is a porous carbonaceous material with a high degree of aromatization and strong anti-decomposition ability. It is produced by the decomposition of carbon-rich biomass in an oxygen-limited environment [Citation15,Citation16]. Because of its high specific surface area, rich pore structure and surface functional groups, biochar has been widely used in environmental remediation, such as wastewater treatment, soil remediation [Citation17], waste management and greenhouse gas reduction [Citation18]. Biochar can adsorb both organic and inorganic pollutants in water and soils systems, such as heavy metals, dyes, ammonium, hydrophobic organic contaminants and antibiotics [Citation15,Citation16,Citation19]. As for the feedstock of biochar, all most any carbon containing biomass can be used as precursor of biochar [Citation20,Citation21]. Therefore, the biochar can be produced from a wide range of raw materials, including plant residues [Citation22], sewage sludge [Citation23], animal manures [Citation24] and even waste tires [Citation25]. Among the above mentioned materials, plant residues were the most widely used due to their abundance and low cost. In addition, the plant residues were used as feedstock to produce biochar can mitigate greenhouse effect by reducing the CO2 emitting. Therefore, in the present study, the plant residue of rape stalk, which had a large yield in China, was chosen as feedstock. Additionally, previous studies founded that the sorption ability of biochar was mainly determined by both the feedstock type and preparation methods, such as pyrolysis temperature [Citation26]. Therefore, three different pyrolysis temperatures of 300°C, 450°C and 600°C were used to produce the rape stalk biochars.
Although biochar was a potential candidate sorbent, low sorption capacity limited their commercial application. To improve the sorption ability of biochar, various modification methods, such as physical and chemical activation methods [Citation27] had been applied on biochars. Most of the modification methods could effectively change the surface physico-chemical characteristics of biochar, such as increase surface area and functional groups [Citation28], change porous structures, O/C and H/C ratios [Citation29]. H2O2 is a kind of strong oxidant that can generate much of hydroxyl radical. Besides, it is green chemical for it will become water after being used, no secondary pollution would be produced. Therefore, H2O2 was introduced in the present work to modify the biochar aim to improve their TC sorption ability.
In this study, three rape stalk biochars (RB300, RB450 and RB600) were produced by pyrolysis at 300°C, 450°C and 600°C, respectively. Then H2O2 was used to modify the obtained biochars. The sorption property of TC on both the pristine and H2O2-modified biochars was investigated. The overarching goals of this work were (1) evaluate the sorption capacity of TC on pristine and H2O2-modified rape stalk biochars, (2) elucidate their sorption mechanism and (3) investigate the effects of pH value and cation concentration on TC sorption by biochars.
2. Material and methods
2.1. Raw materials
TC (C22H24N2O8; purity > 99.0%; CAS Number: 60-54-8) was purchased from Beijing Tai Ze Jia Ye Technology Development Co., Beijing, China. All other reagents used in this work were purchased from Sinopharm Chemical Reagent Co. Ltd (China) and were all analytical grade (purity > 99%). All of the solutions were prepared with ultra-pure water (18.2 MΩ, Millipore USA). Rape stalk was collected from the farmland of Xuzhou in China. Before pyrolysis, the feedstock was cleaned, air-dried and cut to 1 cm in length.
2.2. Production of biochars and modified biochars
Pyrolysis of rape stalk was carried out in a tube furnace for 4 h at 300°C, 450°C and 600°C, respectively. The heating rate was 10°C/min with 50 ml/min N2 as shielding gas. According to the pyrolysis temperature, the produced biochars were labeled as RB300, RB450 and RB600, respectively. H2O2-modified biochars were produced in a 50-ml centrifuge tube where 1 g pristine biochar and 50 ml 30% H2O2 (w/w) were added. The centrifuge tube was placed in the thermostatic shaker and shaken at 25°C for 24 h. The obtained H2O2-modified biochars were named as RB300-H, RB450-H and RB600-H, respectively. All of the biochars were continuously washed with ultra-pure water until the filtrate reached stable pH, and then they were dried at 105°C overnight. At last, the biochars were ground and sieved to a uniform size fraction of 0.15–0.45 mm before being used.
2.3. Characteristics of biochars
Specific surface areas, pore volume of biochars were measured by aperture and specific surface area analyzer (Kubo X1000, Beijing bio Electronic Technology Co., Beijing, China) through multipoint N2 adsorption. The specific surface areas and pore volume were calculated by Brunaurer-Emmett-Teller (BET). The structure and surface morphology of biochars were characterized by scanning electron microscopy (SEM, Inspect S50, FEI USA). The surface functional groups on the biochars were determined by a Fourier transform infrared spectroscopy (FTIR, Nicolet iS10, Thermo Nicolet Corporation USA) in the range of 4000–400 cm−1 using the potassium bromide (KBr) disc technique.
2.3. Sorption experiments
2.4.1. Sorption kinetics
The sorption kinetics of TC on biochars was carried out by mixing 50 mg biochar with 1 L TC solution (20 mg/L) in brown bottle. At different time intervals, about 10 ml solution was taken out and then filtered by 0.22-μm millipore membranes. Thereafter, the filtrate was determined by UV-Vis spectrophotometer (L6S, INESA instrument China) at wavelength of 356 nm, which was the maximum absorption of TC by scanning within the range of 200–700 nm. In addition, the blank tests with only TC solution and biochar were carried out to eliminate the effect of TC decomposition and the distractions release from biochar. All of the experiments were carried out in triplicates, and the average value was calculated.
2.4.2. Sorption isotherms
About 5 mg of each biochar was placed in a 50-ml centrifuge tube. A series of TC solutions with different initial concentrations of 1, 3, 5, 8, 10 and 15 mg/L were added to the centrifuge tube and shaken at 25℃ for 48 h. Afterward, the following procedures such as filtration, measurement and blank tests were the same as Section 2.4.1.
2.4.3. Effect of pH value and cation concentration
The initial TC aqueous concentration was 15 mg/L. The initial pH value was adjusted to 3, 5, 7, 9 and 11 by using 0.01 mol/L HCl and 0.01 mol/L NaOH solution. The cation concentration was adjusted to 0.01–0.1 mol/L by adding NaCl, MgCl2 and CaCl2. The other procedures were the same way as described in Section 2.4.2.
2.5. Sorption data analysis
Pseudo-first-order and pseudo-second-order were used to fit the sorption kinetics data. The equations were presented as follows:
pseudo-first-order: ,
pseudo-second-order: .
Where qt and qe are the amount of sorbate removed at time t and at equilibrium, respectively (mg/g); k1 and k2 are the first-order and second-order sorption rate constants (h−1), respectively.
The Langmuir and Freundlich models were used to fit the sorption isotherms. The equations were presented as follows:
Langmuir: ,
Freundlich: .
Where b and KF are the solute surface interaction energy-related parameter and the affinity coefficient of the Freundlich model (mg(1−n)Ln/g), respectively, KF is Freundlich affinity coefficient indicating sorption strength, and considered as a relative indicator of sorption capacity. qmax is the Langmuir maximal sorption capacity (mg/kg); Ce is the equilibrium solution concentration (mg/L) of the sorbate; and n is the Freundlich linearity index. The Langmuir model assumes monolayer sorption onto a homogeneous surface with no interactions between the adsorbed molecules. The Freundlich model is an empirical equation commonly used for heterogeneous surfaces.
3. Results and discussion
3.1. Characterization of biochars
The specific surface area and pore volume of pristine biochars ranged from 3.9 to 112.4 m2/g, and from 0.023 to 0.076 cm3/g (). Generally, both the specific area and pore volume increased with elevating the pyrolysis temperature, which was in coincident with previous observation [Citation26]. This suggested that temperature plays an important role in biochar production by controlling the creation of pores in the biochars basal-structural sheets. Modified by H2O2, the specific surface area of biochars did not improve significantly, the surface area of RB600 increased from 112.36 to 117.05 m2/g. On the contrary, there were a slightly decrease on RB300 and RB450, which decreased from 3.87 to 1.17 m2/g, and from 6.80 to 4.16 m2/g, respectively. Obviously, H2O2 modification did not enlarge the specific surface area of biochar effectively, which was consistent with the previous literature [Citation30]. The SEM images of pristine biochars () exhibit the layered structure and some rudimentary pores on rape stalk biochars. Additionally, with the increase of pyrolysis temperature, the cellulose, hemicellulose, and lignin in the feedstock decomposed gradually and much more pores emerged, which induced to the increase of internal surface area. Furthermore, the surface of RB300 was smooth while it became rough and irregular when the pyrolysis temperature increased to 600°C.
Table 1. Basic morphology characteristics of pristine and H2O2-modified rape stalk biochars.
Figure 1. Scanning electron micrographs (SEM) images of biochars: (a) and (d) RB300, (b) and (e) RB450, (c) and (f) RB600.

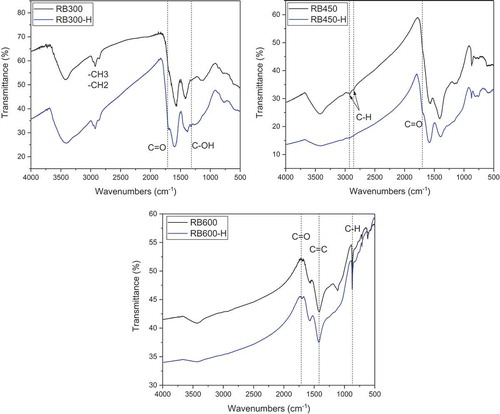
With the increase of pyrolysis temperature, the C-H bending vibration peaks of methyl (-CH3) and methylene (-CH2) in alkanes on biochars decreased gradually (), which was attributed to the decomposition of cellulose, hemicellulose and other organic matter in the rape stalk. As reported the alkyl groups on the surface of the biochar would decompose at higher temperature and might form gaseous hydrocarbons, such as CH4, C2H4 and C2H6 [Citation26]. In addition, the absorption peak caused by bending vibration of aromatic ring C-H (around 875 cm−1) increased significantly, which indicated the aromatization degree of biochar increased with elevating the pyrolysis temperature. Lian et al. [Citation31] reported the similar findings that intense deoxygenation and dehydrogenation reactions occurred during the pyrolysis process, and the decreasing H/C and O/C ratios as well as polarity index [(O + N)/C] suggested the biochars become more hydrophobic and aromatic during pyrolysis at high temperatures.
As for H2O2-modified biochars, the bending vibration of carboxyl C = O (around 1710–1690 cm−1) were enhanced, which indicated H2O2 modification increased the carboxyl groups on the biochars. In addition, a new peak appeared near 1300 cm−1 in RB300-H, was assigned to the bending vibration of phenolic hydroxyl groups. Nevertheless, it was not found on RB450-H and RB600-H, which could be suggested that the formation of phenolic hydroxyl groups was related to the reaction between H2O2 and uncarbonated organic matter in RB300.
3.2. Sorption kinetics
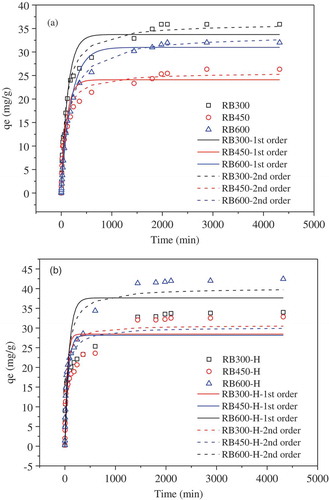
The result of sorption kinetics experiments () showed that the sorption rate of TC on biochar was faster in the first 400 min. Then the sorption rate gradually reduced to zero until the sorption reached equilibrium at about 33 h for pristine biochars and 22 h for H2O2-modified biochars. The sorption equilibrium can be reached quickly on the H2O2 modified biochars indicated that the H2O2 modification could effectively enhance the sorption performance of biochar for TC.
The sorption process was analyzed by two kinetic models of pseudo-first-order and pseudo-second-order equation. The R2 (0.898–0.999) values of the pseudo-second-order model were higher than those of the pseudo-first-order model (0.765–0.988). Besides, the theoretical qe values calculated by the pseudo-second-order kinetics model were more closer to experimental sorption capacity (). Therefore, the pseudo-second-order model could describe the experimental data better, which implied the TC sorption on biochar was dominated by the binuclear surface adsorption mechanism [Citation32]. The equilibrium sorption capacities of TC on RB300, RB450 and RB600 were 35.90, 26.33 and 32.00 mg/g, respectively. However, the order of biochars sorption capacity was not in agreement with the order of their specific surface area. It was different from some previous research, such as Inyang et al. [Citation13], who reported that the sorption capacity of TC on biochar increased with the increase of preparation temperature and specific surface area of carbon materials. In this study, RB600 with the high-surface area and pore volume had higher TC sorption capacity of 32.0 mg/g than RB450 (26.33 mg/g), which indicated high-surface area was conducive to the sorption. However, RB300 with the lowest surface area had the largest TC sorption capacity of 35.90 mg/g. Therefore, other mechanism would govern the TC sorption besides the adsorption dominated by high-surface area, e.g. partition. As Chen et al. reported [Citation33], the non-carbonized organic matter retained in biochar could effectively absorb contaminants in the manner of partition. Such as the sorption of naphthalene, nitrobenzene and m-dinitrobenzene on pine needle biochar [Citation33]. The similar observation was reported by Zhang et al. who studied the sorption of cyclohexane, acetone and toluene on 15 biochars [Citation34]. Likewise, the partition mechanism might dominate the TC sorption on RB300.
Table 2. Sorption kinetics parameters of TC on rape stalk biochars at 25 °C.
Comparing the sorption ability of pristine and H2O2-modified biochars, the pristine RB300 had slightly higher TC sorption amount of 35.90 mg/g than RB300-H (34.0 mg/g), which might be related with the loss of non-carbonized organic matter on H2O2 modified biochar. As for the higher pyrolysis temperature biochars, both RB600-H (42.45 mg/g) and RB450-H (32.83 mg/g) had higher sorption capacity than the pristine RB600 (32.0 mg/g) and RB450 (26.33 mg/g). Given that both the surface area and pore volume of RB600-H and RB450-H did not changed obviously, thus it was reasonable to attributed the enhancement of sorption capacity on the increase of oxygen functional groups, such as carboxyl C = O, etc. Generally, H2O2 can be used as an oxidation reagent to modify/activate the high-temperature pyrolysis biochar by creating more oxygen functional groups on the biochars, while it has adverse effect on the low-temperature biochar by removal the non-carbonized organic matter.
3.3. Sorption isotherm
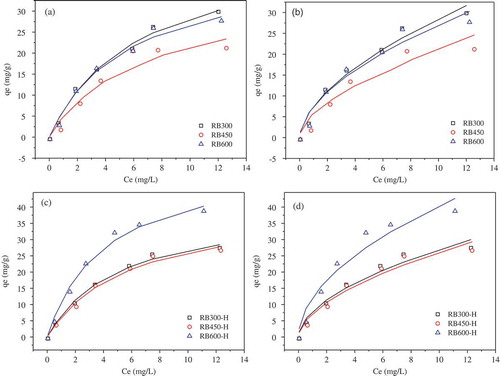
The most commonly used two-parameter isotherms models of Langmuir and Freundlich were used to fit the test data (), and the fitting parameters were listed in . The Langmuir sorption isotherm model assumes that a large number of adsorbed active sites are present on the adsorbent surface, and the sorption equilibrium is reached when all of the adsorbent active sites are occupied. Thus Langmuir model is suitable for describing the monolayer sorption. While the Freundlich model is usually used to describe the multi-molecular layers sorption. In this study, both Freundlich and Langmuir model could fit the TC sorption on all of the six biochars well with the correlation coefficients R2 > 0.9047. Comparatively, the Langmuir model fitted most of the data a little better than the Freundlich model.
Table 3. Sorption isotherm parameters of TC on rape stalk biochars at 25°C.
Antibiotics have certain polarity and hydrophobicity. Therefore, π–π electron donor acceptor (EDA) interaction, cation exchange, hydrogen bonding and surface complexation were possible sorption mechanisms for the TC sorption on biochar [Citation35]. There would be a π–π interactions between graphite-like structure (act as a π-electron-donor) and aromatic ring structure (act as a π-electron-acceptor) [Citation36]. Thus, the polarized electron-rich graphite surface of biochar can act as a π-electron-donor, while TC molecules act as the effective π-electron-acceptor for their strong electron-withdrawing ability on ketone group and aromatic ring structure [Citation31]. Additionally, High temperature carbonization would promote the formation of graphene structures in biochar, which would further enhance the π–π EDA interaction between biochar and TC [Citation37]. This may be an important reason why the sorption capacity of TC on RB600 was larger than RB450. In addition, the hydrogen bonding would be formed between TC and biochar for a plenty of oxygen-containing functional groups was present on the biochar. After modification by H2O2, the amount of oxygen-containing functional groups increased significantly, which would contribute to the increase of TC sorption capacity on biochar.
3.4. Effect of pH
TC is an amphoteric molecule with multiple ionizable functional groups, such as tricarbonylamide (C-1:C-2:C-3), phenolic diketone (C-10:C-11:C-12) and dimethylamine (C-4) groups [Citation36]. Moreover, the TC has three values of pKa (3.3, 7.7 and 9.7), which make it exist as a cation (TCsH3+), zwitterion (TCsH2°) or anion (TCsH− and TCs2−) based on different pH values [Citation38]. In this study, the impact of pH value on the TC sorption by biochar was explored, and the results were illustrated in . The sorption capacity decreased slightly when the initial pH increased from 3.0 to 5.0, and then it increased significantly with the pH increasing from 5.0 to 9.0. The maximum TC sorption amount on biochar was achieved at pH = 9.0.
Figure 5. Effect of pH value on the TC sorption by (a) pristine biochars and (b) H2O2-modified biochars at 25°C.
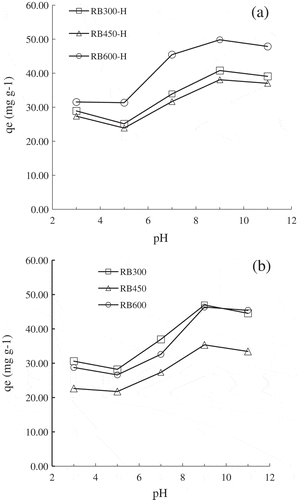
TC was mainly present in the form of TCsH3+ and TCsH2° during 3.0 < pH <5.0, while the biochar was overwhelmingly positive. Therefore, the sorption of TC on biochar would be inhibited by the electrostatic repulsion between cationic TC and positively charged biochar. With the increase of pH, TC was gradually deprotonated and the fractions of zwitterion and anionic species increased, which resulted in higher sorption capacity due to the enhanced electrostatic interaction between TC and biochar. In addition, excess π-electrons on the surface of biochar would strengthen the π–π EDA interaction between TC and biochar [Citation31], which would greatly promote TC sorption on biochar. When the pH was further increased to higher than the isoelectric point of biochar, the biochar would become negatively charged. Thus the electrostatic repulsion between anionic TC and biochar would result in the decrease of sorption capacity. In conclusion, the sorption of TC on biochar was mainly attributed to π–π EDA interaction and the electrostatic interaction.
3.5 Effect of initial cation concentration
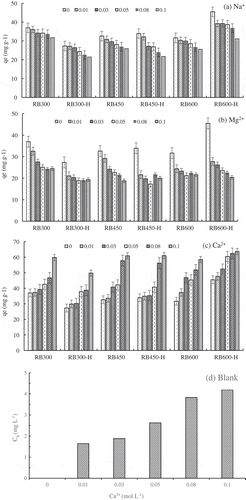
The effect of cation concentration on the TC sorption by RBx and RBx-H (x = 300, 450, 600°C) at 25°C were illustrated in . The sorption capacity of TC on biochars tend to decrease when the Na+ and Mg2+ concentration increased from 0.01 to 0.1 mol/L (,), on the contrary, the sorption would be enhanced with the increase of Ca2+ concentration ()). The Na+ and Mg2+ would suppress the TC sorption on biochars for they would occupy the sorption sites on biochar. Moreover, the competition between TC and cation increases with improving the valence of cation, thus, Mg2+ presents greater adverse effect than the Na+. However, the Ca2+ facilitated the sorption significantly, and for example, the adsorption capacity increased from 45.45 mg/g to 63.64 mg/g on RB600-H. To further explore the effect of Ca2+ on the TC sorption, a blank experiment on the reaction between Ca2+ and TC was carried out without adding biochar. The result ()) showed that the TC concentration decreased gradually with the increase of Ca2+ concentration. Thus, the promotion of Ca2+ on TC removal was related with the complexation between Ca2+ and TC. The TC has multiple ionizable functional groups and various species in solution, thus exhibits strong complexing capability with Ca2+. Therefore, Ca2+ would facilitate the removal of TC by the formation of TC-Ca2+ complexes.
4. Conclusions
Rape stalk biochar can effectively adsorb the TC in aqueous solution with the sorption capacity ranged from 26.33 to 35.90 mg/g. The RB300 with low surface area had the largest TC sorption capacity, which may be related with the partition mechanism. After modification by H2O2, the sorption ability was enhanced and the highest sorption capacity of 42.45 mg/g was observed on RB600-H, which was caused by the introduction of quantity of oxygen functional groups. The pseudo-second-order model and Langmuir model described the experimental data better. The sorption amount of TC on biochars was significantly increased along with pH increasing from 5.0 to 9.0. Both the cation concentration and type would affect the TC sorption on biochar by the competitive sorption, specially, the Ca2+ would facilitate the removal of TC by the formation of TC-Ca2+ complexes. Generally, both the pristine and H2O2-modified rape stalk biochars are potential adsorbent for TC removal.
Acknowledgments
This work was partially supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 18KJA610003, 17KJB610012), the Science and Technology Plan Projects of Xuzhou City (Grant No. KC18150), the Opening Project of Jiangsu Key Laboratory of Vehicle Emissions Control (Grant No. OVEC035), the Natural Science Foundation of China (Grant NO. 41807368, 31701566), the Natural Science Foundation of Jiangsu Province (Grant No. BK20170249), and Xuzhou University of Technology (Grant NO. XKY2018136, XKY2017121, XKY2016121).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Yu F, Li Y, Han S, et al. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere. 2016;153:365–385.
- Chen Z, Zhang W, Wang G, et al. Bioavailability of soil-sorbed tetracycline to escherichia coli under unsaturated conditions. Environ Sci Technol. 2017;51(11):6165–6173.
- Acosta R, Fierro V, Yuso AMD, et al. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere. 2016;149:168–176.
- Luo Y, Xu L, Rysz M, et al. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ Sci Technol. 2011;45(5):1827–1833.
- Rui W, Chen M, Feng F, et al. Effects of chlortetracycline and Copper on tetracyclines and copper resistance genes and microbial community during swine manure anaerobic digestion. Bioresour Technol. 2017;238:57–69.
- Huang H, Chen Y, Zheng X, et al. Distribution of tetracycline resistance genes in anaerobic treatment of waste sludge: the role of pH in regulating tetracycline resistant bacteria and horizontal gene transfer. Bioresour Technol. 2016;218:1284–1289.
- Dirany A, Sirés I, Oturan N, et al. Electrochemical treatment of the antibiotic sulfachloropyridazine: kinetics, reaction pathways, and toxicity evolution. Environ Sci Technol. 2012;46(7):4074–4082.
- Kovalova L, Siegrist H, Singer H, et al. Hospital wastewater treatment by membrane bioreactor: performance and efficiency for organic micropollutant elimination. Environ Sci Technol. 2012;46(3):1536–1545.
- Sousa JM, Macedo G, Pedrosa M, et al. Ozonation and UV 254 nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J Hazard Mater. 2017;323:434–441.
- Thue PS, Sophia C, Lima EC, et al. Synthesis and characterization of a novel organic-inorganic hybrid clay adsorbent for the removal of acid red 1 and acid green 25 from aqueous solutions. J Clean Prod. 2018;271:30–44.
- Chen WR, Huang CH. Adsorption and transformation of tetracycline antibiotics with aluminum oxide. Chemosphere. 2010;79(8):779–785.
- Li H, Zhang D, Han X, et al. Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere. 2014;95(1):150–155.
- Inyang M, Gao B, Zimmerman A, et al. Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars. Environ Sci Pollut Res. 2015;22(3):1868–1876.
- Zwieten LV, Kimber S, Morris S, et al. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil. 2010;327(1–2):235–246.
- O‘Laughlin J, Mcelligott K. Biochar for environmental management: science and technology, JohannesLehmannStephen M.Joseph Earthscan, London UK (2009), 448 p. For Policy Econ. 2009;11(7):535–536.
- Wang B, Gao B, Fang J. Recent advances in engineered biochar productions and applications. Crit Rev Environ Sci Technol. 2018;47(22):2158–2207.
- Li Y, Hu S, Chen J, et al. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: a review. J Soils Sediments. 2018;18(2):546–563.
- Deng W, Zwieten LV, Lin Z, et al. Sugarcane bagasse biochars impact respiration and greenhouse gas emissions from a latosol. J Soils Sediments. 2017;17(3):1–9.
- Liu Z, Xue Y, Gao F, et al. Removal of ammonium from aqueous solutions using alkali-modified biochars. Chem Speciation Bioavailability. 2016;28(1–4):26–32.
- Fernández AM, Barriocanal C, Alvarez R. Pyrolysis of a waste from the grinding of scrap tyres. J Hazard Mater. 2012;203(203–204):236–243.
- Islam MR, Haniu H, Beg MRA. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: product yields, compositions and related properties. Fuel. 2008;87(13–14):3112–3122.
- Zhang X, Gao B, Fang J, et al. Chemically activated hydrochar as an effective adsorbent for volatile organic compounds (VOCs). Chemosphere. 2019;218:680–686.
- Xu X, Cao X, Zhao L, et al. Comparison of sewage sludge- and pig manure-derived biochars for hydrogen sulfide removal. Chemosphere. 2014;111:296–303.
- Wang Z, Han L, Sun K, et al. Sorption of four hydrophobic organic contaminants by biochars derived from maize straw, wood dust and swine manure at different pyrolytic temperatures. Chemosphere. 2016;144:285–291.
- Betancur M, Martínez JD, Murillo R. Production of activated carbon by waste tire thermochemical degradation with CO2. J Hazard Mater. 2009;168(2):882–887.
- Zhang X, Gao B, Creamer AE, et al. Adsorption of VOCs onto engineered carbon materials: A review. J Hazard Mater. 2017;338:102–123.
- Wang S, Zhao M, Zhao Y, et al. Pyrogenic temperature affects the particle size of biochar-supported nanoscaled zero valent iron (nZVI) and its silver removal capacity. Chem Speciation Bioavailability. 2017;29(1):179–185.
- Wu W, Li J, Lan T, et al. Unraveling sorption of lead in aqueous solutions by chemically modified biochar derived from coconut fiber: A microscopic and spectroscopic investigation. Sci Total Environ. 2017;576:766–774.
- Ma Y, Liu WJ, Zhang N, et al. Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour Technol. 2014;169(5):403–408.
- Xue Y, Gao B, Yao Y, et al. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: batch and column tests. Chem Eng J. 2012;200–202(34):673–680.
- Lian F, Song Z, Liu Z, et al. Mechanistic understanding of tetracycline sorption on waste tire powder and its chars as affected by Cu(2+) and pH. Environ Pollut. 2013;178(1):264–270.
- Fang J, Gao B, Mosa A, et al. Chemical activation of hickory and peanut hull hydrochars for removal of lead and methylene blue from aqueous solutions. Chem Speciation Bioavailability. 2017;29(1):197–204.
- Chen B, Zhou D, Zhu L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol. 2008;42(14):5137–5143.
- Zhang X, Gao B, Zheng Y, et al. Biochar for volatile organic compound (VOC) removal: sorption performance and governing mechanisms. Bioresour Technol. 2017;245:606–614.
- Peiris C, Gunatilake SR, Mlsna TE, et al. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour Technol. 2017;246:150–159.
- Liu P, Liu WJ, Jiang H, et al. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour Technol. 2012;121(121):235–240.
- Luo J, Li X, Ge C, et al. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresour Technol. 2018;263:385–392.
- Jang HM, Yoo S, Choi YK, et al. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour Technol. 2018;259:24–31.