?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Shooting ranges continue to pose environmental and human health risk due to the accumulation of toxic Pb emanating from munitions. Remediation of Pb pollution at shooting ranges has been carried out through application of various techniques of which chemical stabilization is the most common. In some instances, field chemical stabilization has achieved Pb decontamination of the soils to concentrations below the maximum contaminant limits as set by the United States Environmental Protection Agency (USEPA) and World Health Organization (WHO). However, the effectiveness of chemical additives to Pb stabilization depends to a great extent on the physicochemical properties of the soils. This review aims to: (i) discuss the effectiveness of chemical stabilization towards Pb remediation of polluted shooting range soils, (ii) establish the chemical reactions that take place between Pb and the chemical amendment and (iii) understand the influence of the soil physicochemical properties on the effectiveness of the chemical amendment.
1. Introduction
Lead (Pb) pollution of shooting range soils has been a problem for many years due to the continuous use of Pb in the manufacture of Pb bullets, shots and pellets [Citation1–Citation3]. Pb has been found to be the largest constituent of ammunitions with bullets containing up to 90–99% of Pb and traces of antimony (1–10.5%), arsenic (0.5%) and copper (0.1%) [Citation4]. As a result, areas such as shooting ranges where a lot of Pb is concentrated onto small pieces of land during military or recreational shooting activities need to be at the forefront of Pb-remediation measures. Numerous studies have been conducted to ascertain the extent of lead deposition and pollution in the environment. It has been reported that in countries such as the United States of America, Pb amounts exceeding 58,000 tons are deposited into the environment annually, in other countries including Netherlands, Switzerland, Sweden, Denmark, Canada and UK metallic Pb ranging between 200 and 6,000 tons are deposited into the environment annually [Citation5–Citation10]. In Africa countries such as Nigeria have documented concentrations of about 5,600 mg/kg whilst in Botswana lead concentrations reaching 38,386 mg/kg have also been reported [Citation11,Citation12]. Despite its many preferable features for ammunition manufacture, Pb has been found to be harmful to both human and animal life. It has a tendency to build up in bones and soft tissues; it acts as a neurotoxin, damages the nervous and renal systems and disrupts biological enzyme functions in the human body [Citation13,Citation14]. In adults, exposure to Pb leads to high blood pressure, neurological disorders, digestive and kidney complications and reproductive health problems including decreased fertility and miscarriages, still births and low birth weights in pregnant women [Citation15,Citation16]. The effects of Pb exposure are however even more apparent in children; concentrations as low as 10 µg/dL lead to damage to the brain and nervous systems, slowed growth, behavioural and learning problems, vision, hearing and motor skills impairment [Citation15]. The adverse health effects associated with exposure to Pb require that stringent measures should be taken to control and manage pollution from this toxic metal. These control measures should set off with a clear understanding of the chemical reactions and transformation of Pb shots and bullets once deposited into shooting range soils. Even though metallic Pb is perceived to be stable under normal soil conditions it undergoes a lot of transformation, both physical and chemical thereby enhancing its mobility, bioavailability, bioaccessibility, dissolution and fate in the environment [Citation4]. Physical weathering involves breaking down of new bullets into smaller fragments and even powder forms as the bullets experience abrasions upon striking targets and getting lodged into berm soils [Citation17]. Chemical weathering entails chemical transformation of metallic Pb. Below is a series of reactions that cause the transformation of Pb in the environment as proposed by Chen and Daroub (2002) [Citation18]. Equations (1–3) show the transformation of Pb into massicot, cerussite and hydrocerussite, respectively.
After transformation, the products of Equations (2,3) are easily dissolved by acid rain thereby mobilising Pb2+ as shown in Equations (4–6) below:
It is regrettable that many studies have and continue to focus on total Pb concentration deposited into shooting range soils and fail to address the soil remediation strategies and methods for polluted soils. In addition, few studies have tried to establish the speciation of Pb in shooting range soils in order to find out if Pb exists in the reactive chemical forms or in its inert chemical species. The speciation of Pb helps assess the mobility and bioavailability of Pb in shooting range soils, the degree of hazard posed and the best remediation strategies to undertake [Citation19]. Shooting range soil remediation methods such as physical remediation procedures, chemical stabilization and phytoremediation have been applied in mitigating Pb pollution of shooting range soils. The objectives of this review are: (i) to discuss the distribution, mobility and bioavailability of Pb in shooting range soils; (ii) extensively compare chemical stabilization Pb remediation strategies and methods applied to contaminated shooting range soils and finally to understand the mechanistic pathways through which chemicals stabilize and immobilize Pb in shooting range soils.
2. Distribution, mobility and bioavailability of Pb at shooting range soils
2.1. Spatial and temporal distribution of Pb in shooting range soils
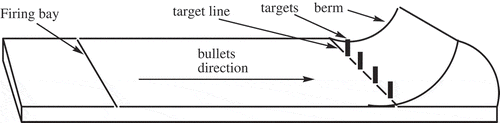
The general layout of the shooting range includes a backstop berm, target lines and firing lines or firing bay. The firing line is usually a certain distance from the target line and it is the point where shooters stand and discharge bullets and shots towards the targets [Citation20]. The target line on the other hand is an area closest to the impact berm where targets are erected. The berm is normally a heap of soil used to absorb and contain bullets and shots that have penetrated the targets. The schematic diagram in shows the layout of a shooting range.
The Pb may be found as spent bullets, shattered fragments, small particles, and lead smears on larger soil particles [Citation21]. Studies have shown that the berm soils contain the highest concentrations of Pb mainly because this section of the shooting range acts as storage for spent bullets and shots. For example, Chen et al. (2001), recovered 180 g Pb shots per kg of soil from the middle of the berm soil of a shooting range in Florida, USA [Citation22]. In Nothern Zealand, Denmark, a study by Astrup et al. (1999) unearthed about 70 kg Pb pellets from shooting range berm soil [Citation7]. Recently, a similar study by Sehube et al. (2017) determined total Pb concentration of 38,386 mg/kg in the berm soils of Thebephatshwa shooting range found in Botswana [Citation12]. Other recent studies include those carried out in Norway [Citation23], Nigeria [Citation24] and South Korea [Citation25] and total Pb concentrations in these studies exceeded the WHO set critical limit by 330, 169.8 and 186.1 times, respectively. summarizes some studies carried out in the past 10 years to quantify Pb pollution in shooting range berm soils in several countries. The magnitude through which the concentrations of Pb deposited into shooting range soils exceeds the set World Health Organization (WHO) and United States Environmental Protection Agency (USEPA) Pb set critical limits for polluted soils are also indicated in . All of the shooting ranges indicated in accumulated total Pb concentrations exceeding the WHO and USEPA set critical limits.
Table 1. Recent studies carried out in the past 10 years on Pb pollution of shooting range soils.
The spatial distribution of Pb pollution of shooting ranges is depicted clearly in a study by Cao et al. (2003) where a drastic decrease in total Pb concentration was observed when moving furthest away from the impact berm towards the firing lines [Citation4]. It was shown that at both TRR and MPR shooting ranges total Pb concentration was low at 1.5 m from the firing line with concentrations of 7.3 and 1,066 mg/kg, respectively, compared to 736 mg/kg (TRR) and 1,066 mg/kg (MPR) at 91.5 m away from the firing line and closer to the impact berm. In contrast, the berm soils accumulated the highest concentration of total Pb of 12,710 and 48,400 mg/kg at TRR and MPR shooting ranges, respectively. In 2003 when this study was undertaken, the TRR shooting range had been operational for only 3 years whilst the MPR had been operating for 16 years. The temporal distribution of Pb was displayed between TRR and MPR shooting ranges with the MPR that had been operational for a longer period of time (16 years) having accumulated higher concentrations of Pb (48,400 mg/kg) relative to TRR (12,710 mg/kg) which had only been operational for 3 years. In addition, substantial amounts of Pb were found in surface soils closer to the firing line in the older MPR (1,066 mg/kg) shooting range. This may be caused by fine/particulate Pb that is generated during weapon discharge especially for older shooting ranges like the MPR. Atomization of Pb may be caused by the heat generated during bullet firing leading to some Pb-vapor, which can then precipitate or condense on soil particles at the firing line [Citation57]. Besides the age of a shooting range, the type of ammunition and frequency of use also has a bearing on Pb concentrations found at shooting ranges [Citation58]. A similar study carried out by Sehube et al. (2017) observed the same spatial distribution of Pb [Citation12]. As anticipated, the berm soils collected the highest concentration of total Pb (38,386 mg/kg) while the target line accumulated 13,204 mg/kg and only 71 mg/kg was determined at the 50 m firing line [Citation12]. However, the 100 m firing line defied the normal trend and accumulating total Pb concentrations (896 mg/kg) higher than at the 50 m firing line even though the 50 m firing line is closest to the impact berm. It was established that shooting activities took place mostly at the 100 m shooting line and as bullets travel through the rifle barrel and hit the wall of the barrel containing riflings, friction is created which produce Pb powder that leaves the rifle barrel and settles at the 100 m firing line. This event does not take place at the 50 m shooting line and hence low concentrations of Pb at this point of the shooting range bed. It is important to note that, background soils samples taken near the TRR and MPR shooting ranges contained 1.83 mg/kg and 4.28 mg/kg of Pb, respectively. These values are comparable to Botswana (Southern Africa) studies whose background samples were found to have concentrations ranging between 2.5 and 17 mg/kg [Citation12,Citation58]. The background concentrations of Pb found are low and consistent with the composition of the earth’s crust which normally occurs at 10–30 mg/kg giving an indication that the Pb pollution is still contained within the shooting ranges [Citation59].
2.2. Mobility and bioavailability of lead in shooting range soils
Soil properties such as soil moisture, temperature, pH, cation exchange capacity (CEC) and organic matter play a significant role in Pb solubility and transformation in shooting range soils [Citation60,Citation61]. Soil moisture content and temperature also affect the bioavailability of lead in soils [Citation62]. The water layer that forms on the Pb shot surface though non-corrosive to the metal; facilitates the corrosion of Pb shot as it acts as a means of diffusion of atmospheric gases such as oxygen and carbon dioxide that attack the surface of the metal to form secondary minerals [Citation62]. The transformation of metallic lead to more reactive and available species in the soil was found to be a speedy process that is influenced by soil moisture. After 5 days of incubation, both hydrocerussite [Pb3(CO3)2(OH)2] and litharge (PbO) were formed and verified using XRD in a study carried out in Florida, USA [Citation62]. A combination of high moisture content of soil with other factors such as low soil pH, low clay and organic matter accelerate weathering of Pb shots and bullets which causes concern for areas with shallow groundwater levels especially during the rainy season posing serious threats of leaching and downward migration of Pb as mentioned by Bricka, (2000) [Citation63].
Soil pH plays a vital part in Pb solubility, mobility and transformation in shooting range soils [Citation61,Citation64]. It has been recommended that soil pH at shooting ranges should lie between 6.5 and 8.5, since the mobility of Pb increases outside this range [Citation15] as low pH affects the mobilization intensity of heavy metals in soils. Mobility of Pb is much higher in acidic than neutral and alkaline soils [Citation15,Citation64,Citation65]. High soil pH’s have been associated with soils contaminated with Pb shot at a shooting range in Denmark and were ascribed to the corrosion of the Pb bullets and shots [Citation61]. Rooney and McLaren (2007) incubated Pb shot for up to 24 months in soils and found that crusts developed on all samples but larger ones developed on samples under higher soil pH conditions [Citation66]. Minerals such as cerussite, hydrocerussite, thorikosite and galena were contained in all these crusts but cerussites dominated especially at higher pH values. Massicot and cerussite have been found to be stable in soils with high pH, but these may become soluble and more mobile in acidic sandy soils and may be leached away during precipitation [Citation4]. Cation exchange capacity (CEC) is another property of soil that affects the mobility and bioavailability of Pb in shooting range soils. Soils with high CEC values have a high binding ability and vice versa [Citation4]. Cationic metal binding increases with an increase in valence, atomic weight and ionic potential [Citation64]. The affinity of metal cations relative to clay minerals is arranged in a series of Cu2+ > Cd2+ > Fe2+ > Pb2+ > Ni2+ > Co2+ > Mn2+ > Zn2+ [Citation64]. Soils with high pH, CEC and a wide variety of minerals in their clay fractions have shown the highest sorption capacities for Pb2+ ions [Citation67]. In addition, Pb shot incubated in Temuka silt loam soil of CEC 31 cmol/kg displayed greater corrosion as compared to Pb shot in Waimakariri sandy loam soil of CEC 11 cmol/kg and this was attributed to the high sorption capacity as indicated by the sorption values [Citation66]. The higher the CEC the higher the soil’s binding capacity leading to reduced Pb mobility and bioavailability. Cao et al. (2003) found total Pb concentrations as high as 48,400 mg/kg in berm soils of MPR shooting range with a matching high CEC of 43.2 cmol/kg whilst a total Pb concentration of 12,710 mg/kg with a CEC of only 8.51 cmol/kg was found in the TRR shooting range soil [Citation4]. S/PIST range in Botswana had the lowest CEC and pH values of 8.20 cmol/kg and 6.81, respectively, with a corresponding low Pb concentration of 685 mg/kg; rendering a favourable environment for Pb mobility in this range whereas in the MAT R2 range with a high CEC of 21.87 cmol/kg, high pH and high soil organic matter high Pb concentrations reaching 20,882 mg/kg were found in the berm soil [Citation58]. The presence of soil organic matter is required for the transformation of metallic Pb to more reactive Pb compounds in the soil. Soil with little amounts of organic matter (0.15%) showed no evidence of secondary Pb minerals even after 12 days of incubation [Citation62]. These findings suggest that low organic matter levels hinder the transformation of metallic Pb to Pb oxides and carbonates [Citation62]. Organic carbon also plays a significant role on interactions between metal concentrations and microbial parameters [Citation68]. A study conducted on five shooting range soils over 5 years in storage revealed that the total inorganic carbon increased whilst total organic carbon decreased [Citation69]. This suggests a conversion of organic carbon into inorganic carbon. This was confirmed by the presence of primary Pb carbonates which were the predominant products found in soils, indicating that soil organic matter does play a vital role in Pb bullet and shot weathering and also controls Pb migration within the soil [Citation69]. Besides soil properties and leaching tests, other measures such as risk assessment indices can be applied to determine the mobility, bioavailability and bio-accessibility of Pb at shooting ranges to the ecosystem; these have been detailed by Dinake et al. (2018) whose research showed that all of the seven shooting ranges studied presented ‘high risk’ to ‘very high risk’ to the ecological system [Citation70].
2.3. Lead partitioning in soil and leachability risks
Toxicity characteristic leaching procedure (TCLP) and synthetic precipitation leaching procedure (SPLP) are simulations for leaching of heavy metals such as Pb through a typical landfill and mimicking in situ acid rain leaching, respectively. Both simulations employ acidic media [Citation4]. The two are used to determine the potential risk posed by hazardous materials such as Pb to both surface and underground waters. Studies carried out have shown that almost all shooting ranges studied are well over the USEPA threshold of 0.015 mg/l and 5 mg/l for SPLP-Pb and TCLP-Pb, respectively, revealing possible risk of pollution of the water table. summarizes some of the studies that have been carried out on the two procedures.
Table 2. Toxicity characteristic leaching procedure and synthetic precipitation leaching procedures simulating leaching of Pb in landfill and leaching of Pb due to acid rain.
It is worth noting that there is direct proportionality between the total Pb concentration found in shooting range soils and both the TCLP-Pb and SPLP-Pb concentrations. Shooting range soils that accumulated high concentrations of Pb in turn experienced high concentrations of TCLP-Pb and SPLP-Pb as shown in . In addition, the TCLP and SPLP procedures reiterate the importance of soil properties such as soil pH in the weathering and transformation of Pb shots and bullets and their subsequent leaching into the soil. In acidic soil conditions, the dissolution and transformation of Pb shots and bullets is enhanced compared to alkaline soil conditions [Citation73]. It has also be found that the high TCLP-Pb and SPLP-Pb concentrations can give insight into the speciation of Pb in the soil as in most studies carried out Pb was predominantly held in the carbonate fraction as purported by Chen and Daroub (2002) [Citation18], Dermatas et al. (2006) [Citation74] and Kelebemang et al. (2017) [Citation58]. It is recommended that soils that have failed both TCLP and SPLP procedures be treated before their disposal.
3. Remediation techniques applied to polluted shooting range soils
A wide range of technologies and methods have been suggested by numerous researchers for the remediation of Pb contamination of shooting range soils [Citation75,Citation76]. An extensive investigation of these methods and procedures has revealed that their effectiveness and efficiency depend to a large extent on the physical and chemical characteristics of the shooting range soil, cost, length of time allowed for remediation, land availability, future land use and many other factors [Citation76,Citation77]. As a result, it is important to investigate the effectiveness and efficacy of the remedial alternative in order to assess: (i) its financial implications and viability in field scale implementations; (ii) the risk to the environment and biota [Citation78]. In addition, the mechanisms and kinetics of Pb stabilization/immobilization have to be determined in order to predict with certainty the short-term and long-term treatment under different environmental conditions [Citation78]. It is also important to note that the fate of Pb in shooting range soils is directly influenced by its reactions with soil fractions and partitioning between soil compartments such as clay minerals, Fe and Mn oxides, organic matter and the residual component [Citation79]. Pollution remediation techniques that have been applied to polluted shooting range soils include the physical remediation methods such as; (i) soil replacement, (ii) mechanical sieving, and (iii) Vitrification. On the other hand, chemical remediation techniques have also been used to control and manage Pb pollution of shooting range soils and such methods include; (i) liming, (ii) encapsulation, (iii) phosphate addition (iv) soil washing (v) biochar and (vi) iron oxides. In addition, other techniques such as phytoremediation and coupled-phytoremediation techniques have also been found to alleviate the impact of Pb to shooting range soils. Some of these technologies have proven to be successful suggesting that with proper planning and design successful remediation and reclamation of polluted shooting range soils can be achieved. indicates the impact that soil remediation techniques and methods can have on controlling and managing pollution of shooting range soils from Pb.
Table 3. Different chemical remediation methods and other methods applied for amendment of Pb pollution of shooting range soils.
3.1. Chemical remediation of polluted shooting range soils
Chemical remediation methods have been found to be potentially cost-effective and efficient measure in mitigating Pb contamination of shooting range soils [Citation62,Citation79,Citation85–Citation87]. There are some considerations to take into account when selecting chemical remedial strategies for Pb impacted soils such as in situ viability and environmental sustainability of the applied chemical [Citation78]. Previous studies have shown that chemical stabilization has the capability to reduce the bioavailability, bioaccessibility, mobility, ecotoxicity and leaching of Pb in shooting range soils [Citation86,Citation88,Citation89]. The stabilization and immobilization of Pb in shooting range soils are based mainly on the modification Pb chemical and mineralogical form such as speciation and valence and soil properties such as sorption capacity and buffering potential [Citation90]. These chemical remedial additives enhance physicochemical processes which make Pb pollutant less mobile and less bioavailable due to the formation of leaching resistant Pb minerals. It is also worth noting that these soil amendments methods are in situ immobilization strategies and their application does not change total Pb concentration in the soil [Citation91]. The immobilization processes involve a wide range of chemical reactions such as precipitation and adsorption. Chemicals that have been applied towards shooting range soil amendments include both inorganic and organic chemicals. Inorganic chemicals such as phosphate rock (X5Y(PO4)3 where X can be Ca2+ or Pb3+ and Y is normally F−, Cl−, or OH−) [Citation62], magnesium oxide (MgO) [Citation79], hydroxyapatite (Ca₁₀(PO₄)₆(OH)₂) [Citation92], ferrihydrite (5Fe2O3 · 9H2O) [Citation93], natural iron oxides (Fe2O3) [Citation91], calcium carbonate (CaCO3) [Citation86] and zero-valent iron [Citation23] have been used. Organic substances have been found to be the most cost-effective soil amendment techniques for Pb-polluted shooting range soils [Citation91,Citation94,Citation95]. Biochar is a carbon-rich material that is obtained when biomass, such as wood, manure, or leaves is combusted in a closed container under oxygen-deficient conditions [Citation96]. Biochar immobilisation of Pb in shooting range soils has been achieved through the application of organic substances such as oak wood biochar (via pyrolysis at 400°C) [Citation91,Citation94], soybean stover derived biochar [Citation95], biodegradableethylenediamine-N,N′-disuccinic acid (EDDS) followed by phosphate amendment [Citation97] and green waste biochar [Citation87]. It is of paramount importance that the environmental risks from the application of chemical remediation techniques are well understood and addressed [Citation98]. The application of chemical additives for in situ Pb immobilisation helps treat and control pollution on site. However, the pitfall with these in situ remedial techniques is that the contamination stays within the soil. Prior to application of chemical amendments to affected shooting range soils, evidence has to show that the degree of stabilization has reduced the risk associated with Pb to an acceptable regulatory level commensurate with proposed land use [Citation98].
3.1.1. Phosphate addition
The stabilizing ability of phosphate in removing soluble Pb from aqueous solutions was first coined by Nriagu in 1974 in a study involving the formation and properties of Pb-orthophosphates [Citation99]. This culminated in an extensive research on phosphate remedial alternative to Pb-polluted soils and this has resulted in a wide acceptance of phosphate as a stabilizing and immobilization agent for Pb-contaminated soils and solid waste [Citation98]. Phosphate soil remediation technologies have been incorporated into the shooting range best management practices (BMPs) manual as one of the recommended methods for the control and management of Pb pollution of shooting range soils [Citation15,Citation100]. Phosphate has been found to be a stabilizing agent through the formation of the thermodynamically stable Pb-phosphates and pyromorphites (Pb5(PO4)3X where X = Cl−, OH−, F−) which are insoluble over a wide range of pH and redox potential (Eh) [Citation100]. The stability of the pyromorphites follow the order chlorinated>hydroxylated>fluorinated, with the chlorinated component being the most stable at a pH range of 3–11 [Citation99]. When carrying out phosphate remediation strategies, it is important to be cautious of the Pb-phosphate reaction kinetics which are highly dependent on the pH of the applied material and the solubility of the original sources of phosphate and Pb [Citation78]. Other factors that determine the reaction thermodynamics of Pb-contaminated shooting range soils include such factors as moisture availability, solution chemistry and particle size of Pb and phosphate compounds [Citation78]. The fate and environmental risk of unreacted high concentrations of phosphate should also be well understood. Sources of phosphate that have been applied for Pb remediation of shooting range soils include phosphoric acid, mono-calcium phosphate, di-calcium phosphate, tri-calcium phosphate, triple super phosphate, diammonium phosphate, hydroxyapatite, phosphate rock, Apatite II, phosphatic clay, commercial fertilisers, poultry waste, bone meal and bone char. These phosphate sources fall in three main categories depending on their solubility: (i) readily soluble phosphates (e.g. phosphoric acid); (ii) moderately soluble phosphates (e.g. mono-, di- and tricalcium phosphate); and (iii) less soluble phosphate (e.g. synthetic hydroxyapatite and phosphate rock) [Citation78]. The pH of the soil has a great influence on the dissolution, transformation and insolubility of Pb minerals and phosphate sources. Acidic pH conditions support the dissolution of Pb minerals and phosphate bearing materials while pH values of more than six are unfavourable to both Pb minerals and phosphate sources solubility [Citation101]. In a study by Spuller et al. (2007), the application of commercially available diammonium phosphate (DAP: (NH4)2HPO4), and calcium phosphate monobasic (CPM: Ca(H2PO4)2) were applied to shooting range soil highly contaminated with Pb leading to reduction in Pb concentrations by 45% (DAP) and 99% (CPM) [Citation90]. Pb stabilization in this case was perceived to have been through the formation of the slightly soluble Pb-phosphates, Pb3(PO4)2 or Pb4O(PO4)2. The immobilisation of Pb was lower in DAP compared to CPM due to the limited availability of phosphate in DAP and the accompanying increase of dissolved organic carbon in the DAP additive which results in the formation of soluble organo-Pb complexes [Citation90]. The limited availability of phosphate in DAP is due to the fact that in the presence of calcium in the soil, the DAP is converted to the semi soluble calcium hydrogen phosphate which in turn dissolves liberating phosphoric acid as shown in Equations (7,8) below:
Sanderson et al. (2015) have used and compared the effectiveness of three phosphate containing substances such as soft rock phosphate (10% phosphorus), phosphoric acid (85% H3PO4) and commercial phosphorus for remediation of four Pb-polluted shooting range soils of varying physicochemical properties [Citation79]. The researchers studied the effectiveness of the amendments on water-extractable Pb and the toxicity characteristic leaching procedure Pb (TCLP-Pb) of the four shooting ranges Murray Bridge, Townsville, Darwin and Perth. Due to the different physicochemical properties of the four shooting range soils, the three amendments displayed different efficacies towards Pb stabilisation. For example, soft rock phosphate decreased water-extractable Pb by over 90% at the Murray Bridge shooting range and only minimally at Darwin and Perth. In addition, phosphoric acid effectively reduced water-extractable Pb of the three shooting range soils, Murray Bridge, Townsville and Darwin [Citation79]. In contrast, the application of phosphoric acid to Perth soils caused the mobilisation of Pb. Commercial phosphorus was also able to lower water-extractable Pb at Murray Bridge and Townsville and mobilised Pb at Perth and Darwin shooting range soils. The TCLP-Pb exceeded the 5 mg/l USEPA regulatory set limit in untreated soils of three shooting ranges reaching highs of 200 mg/l in the Murray Bridge shooting range. Upon application of soft rock phosphate, significant reduction in the TCLP-Pb concentration in all four shooting ranges to below the regulatory set limit of 5 mg/l was observed [Citation79]. The physiologically based extraction test (PBET) was performed in order to establish the effectiveness of amendments on bio-accessible Pb and it was found that the amendments were able to lower bio-accessible Pb by over 50%. Other sources of phosphate such as hydroxyapatite (Ca5(PO4)3OH) have also been found to be effective in stabilising Pb in shooting range soils [Citation93]. In a study by Ogawa et al. (2015), the total Pb concentration found in the shooting range soil was around 23 000 mg/kg with water exchangeable Pb concentration exceeding 100 mg/kg. After application of the phosphate containing hydroxyapatite, the concentration of the water-soluble Pb was significantly reduced to 0.9 mg/kg – a 99.2% reduction [Citation93]. This implies that most of the water-soluble Pb was transformed into insoluble Pb phosphate minerals upon addition of the hydroxyapatite. The transformation of Pb into stable Pb phosphate minerals was confirmed through the sequential extraction experiments in which the total percentages of the labile or soluble exchangeable and carbonate fractions in the shooting range soil decreased from 78.4% to 45.4% after application of the hydroxyapatite amendment. In contrast, the inert, unreactive and immobile residual fraction in the shooting range soil increased by 37.8% after hydroxyapatite amendment [Citation93]. The efficacy of phosphate amendment can also be monitored using the X-ray absorption spectroscopy in which the predominant Pb minerals can be identified in untreated soils and compared with those found in the remediated shooting range soils [Citation102]. In a study by Sanderson et al. (2015), the untreated soil was found to contain high concentrations of the mineral litharge (PbO), iron-oxide bound Pb and humic acid bound Pb. In contrast, X-ray absorption spectroscopy studies of the phosphate-amended soils indicated that the Pb minerals have been transformed into considerable amounts of pyromorphite (17%) [Citation102]. In a similar study by Ogawa et al. (2018), the XRD patterns indicated that 95% of Pb had been immobilised into pyromorphite after application of hydroxyapatite during Pb migration in the water-unsaturated soil of different Pb mobilities [Citation103]. Environmental conditions and the soil physicochemical properties such as soil moisture, temperature and pH play a pivotal role in the effectiveness of the phosphate amendment to stabilize Pb in shooting range soil [Citation103,Citation104]. The existence of moisture in soil pores exceeding 50% water-holding capacity of the soil was found to inhibit the water-soluble Pb level by more than 99.8% relative to conditions of no water in the soil pores [Citation103]. To add to this observation, the XRD analysis indicated that up to 27% of Pb was transformed into pyromorphite in the soil with 50% water-holding capacity relative to less than 3.0% in the soil with 0% water-holding capacity. It can be deduced from these determinations that elevated soil moisture conditions are suitable to the transformation of bioavailable Pb into insoluble Pb phosphate minerals such as pyromorphite. However, the addition of excessive water should be avoided as this could make the Pb scatter during the Pb immobilisation reactions [Citation103].
It is worth noting that the cost of soil amendment additives has put a barrier in effectively controlling and managing Pb pollution especially when enormous amounts of contaminated soils are involved such as shooting ranges. Scientists and researchers have shifted their attention to more cost-effective yet efficient measures of phosphate bearing industry by-products such as poultry waste [Citation88]. Poultry waste has been found to be an excellent source of hydroxyapatite, Ca5(PO4)3OH and studies have shown that it immobilises and stabilises Pb better than the commercially available pure hydroxyapatite [Citation105]. In a study by Hashimoto et al. (2008), the application of poultry waste to shooting range soils polluted with Pb was able to reduce the water-extractable Pb by about 43% compared to the control. Furthermore, sequential extraction procedures indicated that Pb fractions in soils remediated with poultry waste contained more of the less soluble Pb mineral species as revealed by an increase in the residual fraction (20%) and a decrease in reactive fractions of exchangeable and carbonate compartments (22%) as compared to the control soil [Citation105]. The increase in the residual fraction after poultry waste amendment has also been confirmed in other studies using poultry waste and after application of other phosphate containing materials such as pure hydroxyapatite and phosphate rock [Citation93,Citation106–Citation108]. The efficacies of hydroxyapatite obtained from poultry and gypsum wastes relative to pure hydroxyapatite were evaluated for Pb immobilization of a shooting range soil in Gifu, Japan [Citation92]. The researchers investigated the dissolution rate of Pb minerals upon the application of soil amendments. It was established that the concentration of dissolved Pb declined upon addition of amendments and the Pb concentrations were 66% (gypsum waste) and 50% (poultry waste) lower than that of the pure hydroxyapatite treatment. The pH dependency of Pb dissolution was also evaluated and it was found out that the attenuation of Pb by the amendments was more effective at higher pH values than in acidic media. At higher pH values, dissolution kinetics of poultry and gypsum wastes occurred at a faster rate compared to pure hydroxyapatite leading to the release of phosphate anions and providing a favourable condition for the formation of insoluble chloropyromorphite precipitates. The lower pH values are favourable towards the dissolution of original Pb minerals in shooting range soils while higher pH values favour the dissolution poultry and gypsum wastes making the phosphate anions available for reaction with the dissolved Pb from the original Pb material resulting in the formation of insoluble pyromorphites [Citation92]. Hashimoto et al. (2009) explained the Pb stabilisation by the amendments through the ionic exchange mechanism between Ca in the poultry and gypsum wastes (constituent of hydroxyapatite) and the Pb in Pb source materials [Citation92]. The solubility of the formed Pb-phosphate becomes low with an increase in the pH and thereby achieving the Pb immobilisation goal. In the first step, Pb exchanges for Ca in the hydroxyapatite as shown in Equation (9); the concomitant dissolution of the hydroxyapatite leads to the release of the dihydrogen phosphate anion (H2PO4−) as depicted in Equation (10). Lastly, the precipitation of Pb-phosphate takes place as shown in Equation (11).
In Switzerland, a study of shooting range soil characterized by basic soil condition (pH ~ 8.2) was studied for Pb extractability after application of soil amendments including potassium phosphate dibasic (K2HPO4) [Citation109]. The application of phosphate revealed a decrease in the extractability of Pb using water and NaNO3 single extraction procedures. However, undesirable consequences were encountered in this study since the soil amendment using phosphate increased the extractability of other toxic heavy metals in the soil such as antimony (Sb). The applied phosphate additive has the tendency to compete for the same binding sites on the surfaces of pedogenic iron hydroxides with antimony anions such as antimonite giving rise to the release of antimony into the soil solution [Citation110].
Waste cow bone has been found to contain high amounts of phosphorus (18.5 wt. % P2O5) and owing to its low cost and ready availability have been considered as a cheap substitute for Pb stabilization and immobilisation in shooting range soils [Citation111]. However, it is imperative that the ease of availability of the phosphorus in the waste cow bone is well understood. In a study by Moon et al. (2013), the sole application of waste cow bone was not as effective as in a combination with calcined oyster shells which contain high concentrations of calcium oxide, CaO (84.3 wt.%). The leachability of Pb was significantly reduced upon combined application of 5 wt.% of both waste cow bone and calcined oyster shells: a drastic 99% reduction in Pb leachability was encountered compared to the control sample [Citation111]. In particular, initial Pb concentration of 3613 mg/kg in the control soil sample was reduced to 16.2 mg/kg (>99% reduction) upon combined application of calcined oyster shells and waste cow bone [Citation111]. The researchers assessed the efficacy of the combined amendments relative to the Korean Standard leaching test of 100 mg/kg as warning standards residential areas. The effectiveness of cow bone (25.63 P2O5 and 34.81 CaO wt. %) in immobilising Pb was also assessed by Ahmad et al. (2014) [Citation94]. In this study, the researchers evaluated the potency of cow bone amendment in stabilising the bioavailable and exchangeable concentrations of Pb in which 93% of exchangeable Pb was significantly reduced by the application of the material. In addition, the application of cow bone increased the concentration of the dominant residual fraction, an indication of the transformation of Pb to the immobile geochemically stable residual fractions arising from the application of the cow bone amendment. The XRD pattern of the cow bone amended soil indicated the presence of hydroxyapatite which may have led to the formation of the geochemically stable and immobile Pb-phosphate minerals. The phytoavailability of Pb was also greatly reduced by 70.47% upon addition of cow bone soil amendment. However, it was established that the cow bone amendment increased the bioavailability of a co-existing pollutant in antimony (Sb) and its application increased uptake of Sb to 3.46 mg/kg from 1.3 mg/kg [Citation94]. The research findings by Ahmad et al. (2014) corroborate the results obtained by Conesa et al. (2010) in which the addition of phosphate mobilised Sb and increased its bioavailability and leachability instead of immobilising and stabilising it [Citation109].
In their quest to overcome the cost commercially available phosphate sources, researchers have also found that pyrolytic biomass (biochar) has proved to be an excellent source of phosphate [Citation91,Citation112]. Rajapaksha et al. (2015) studied the efficacies of buffalo weed (Ambrosia trifida L.) biomass and its pyrolytic biochar at 300°C and 700°C. The original buffalo weed biomass contained phosphate (PO43-) concentration of 3.89 mg/kg while its resultant biochars at 300 and 700 oC carried phosphate concentrations of 79.96 and 70.72 mg/kg, respectively. The addition of these soil amendments to shooting range soil contaminated with 17 468 mg/kg of Pb demonstrated high efficacy towards Pb stabilisation. The ammonium acetate extraction of Pb in the soil was greatly reduced by 35.5%, 92.6% and 94.7% upon application of biomass, biochar (700°C) and biochar (300°C), respectively [Citation91]. In addition, the TCLP-extractable Pb was also reduced by up to 77.2% after the addition of biochar. Many other studies have also been carried out in recent years that confirmed the effectiveness of pyrolytic biomass (biochar) in immobilization of Pb in shooting range soils [Citation95]. Biochar derived from soybean stover and pine needle has demonstrated high potency towards immobilisation of Pb by reducing exchangeable Pb by up to 88% and a decrease in TCLP-Pb by 65.14% in contaminated shooting range soil [Citation95]. An array of mechanisms has been suggested for the interaction of biochar with Pb in shooting range soils. In some cases, the Pb immobilisation ability of biochar has been ascribed to increase in the water-soluble PO43- upon addition of the biochar, an indication of the release of available phosphate from the biochar [Citation91]. Pyrolysis of biochar plays a key role in adjusting the biochar properties [Citation113]. As the pyrolysis temperature increase, a decrease in the mobile components and an increase in the stable matter due to loss of volatile components during thermal decomposition of biomass. The pyrolysis process contributes to an increase in the ash content of biochar due to the collection and accrual of inorganic minerals and organic matter pyrolytic residue with increasing pyrolysis temperature [Citation113]. The pyrolysis of biomass also results in high surface areas in biochar due to the generation of micropores [Citation113].
The effectiveness of the biochar in Pb immobilisation is sometimes attributed to the ability of biochar to induce changes in soil properties such as the soil pH, electrical conductivity (EC), cation exchange capacity (CEC), dissolved organic carbon (DOC), and water-soluble PO43- which have significant impact on the leachability, mobility and bioavailability of Pb in shooting range soils [Citation91]. An improvement in the soil CEC and strengthening of the soil fertility by aiding the retention of water and nutrients after application of biochar have been reported [Citation113]. In addition, the abundance of carboxyl functional groups in biochar has also been attributed to the formation of strong complexes with Pb on the surfaces of biochar [Citation114]. Biochar materials with high O/C molar ratios have been found to effectively complex divalent heavy metals such as Pb due to their high O-containing carboxyl functional groups [Citation114]. It has also been reported that the surfaces of biochar material are normally negatively charged which present a favourable environment for the electrostatic attraction between the positively charged Pb and the biochar and thereby enhancing the π-π electron donor–acceptor interaction between electron-rich graphene surface of the biochar and the π-electron-deficient positively charged Pb cations [Citation115,Citation116]. The changes in the soil pH to basic conditions upon application biochar have also provided favourable environment for the formation of insoluble Pb phosphates, carbonates and hydroxides leading to the immobilisation of Pb [Citation117].
Researchers have also shifted their attention to the capability of phosphate-containing amendments in reducing the ecotoxicity of Pb found in shooting range soils towards ecological receptors such as vegetation, small living organisms and micro-organisms [Citation118]. In a study of shooting range soil contaminated with Pb concentrations of 2330–12,167 mg/kg, the application of phosphate-containing soil amendments soft rock phosphate and commercial phosphate enhanced the per cent survival and weight-loss reduction of earthworms in the most contaminated soils with little effect in soils with lower concentration of Pb [Citation118]. The concentration of Pb was also found to be low in soil organisms notwithstanding the high concentrations of Pb in shooting range soils, an indication of suppressed bioavailability of Pb. The accumulation of Pb in the studied earthworms was greatly reduced by up to 96% after addition of the phosphate-containing soil remediation additives. In addition, the average uptake of Pb by lettuce was also reduced by up to 70% after treatment of the Pb-contaminated soils with phosphate bearing amendments. It is noteworthy that the microbial activity in the amended soil was enhanced by more than 50% after application of soft rock phosphate. In a similar study by Seshadri et al. (2017), the bioaccumulation of Pb in earthworms was reduced by up to 54.6% after application of soft phosphate rock. The bioaccessibility of Pb also depicted long-term stability phosphate immobilised Pb [Citation119].
3.1.2. Liming
The USEPA has recommended the use of lime in conjunction with phosphate sources for the immobilization of Pb in shooting range soils. Liming also helps in controlling the pH of soils when amendments are added to acidic soils through elevation of the pH as shown in Equation 12 [Citation15];
In addition, liming is also efficient in reducing water-soluble Pb through the formation of Pb carbonates with concomitant lowering of the H+ ion concentration which in effect reduces the solubility of the formed Pb carbonate minerals [Citation62]. Formation of pozzolanic products has also been reported in soils amended with lime-containing materials [Citation117]. Mechanisms for the formation of pozzolanic products include sorption, phase mixing and substitution and such products may include calcium silicon hydrates, calcium aluminium hydrates and ettringite (Ca6Al2(SO4)3(OH)12 · 26H2O) as shown in Equations (13–16) below;
These pozzolanic products are responsible for Pb immobilisation through the entrapping of Pb in their chemical lattice [Citation85]. In some instances, liming has been found to activate soil microbial activity [Citation86,Citation120]. Application of lime amendment has effectively increased dissolved organic carbon in pine forest soils and in some cases promoted the accumulation of soil organic matter which boosted Pb adsorption [Citation121,Citation122]. The effectiveness of two liming agents, CaCO3 and blast furnace slag, was evaluated for immobilization of Pb in shooting range soils, the findings of which indicated a drastic decrease in the water-extractable Pb by 69% and 61% after amendment with CaCO3 and blast furnace slag, respectively, [Citation86]. The supremacy of Pb stabilization by CaCO3 over rock phosphate, at 80% field moisture capacity, was observed by Ma et al. (2007) in which the CaCO3 amended soils contained the lowest concentration of water-soluble Pb compared to the phosphate rock amended soil [Citation62]. In Australia, the application of 5% lime at 60% moisture holding capacity to contaminated soils obtained from four different shooting ranges showed a significant reduction in water-extractable Pb by over 90% [Citation79]. In a study by Yin et al. (2010), liming of shooting range soils reduced total Pb by 20–80% [Citation89]. It was shown that after 15 months of liming, the total Pb concentrations in the berm soils of the control was 497–777 mg/kg whereas in the limed soils was in the range 302–362 mg/kg.
Industrial lime containing byproducts have been shown to be a cheap substitute to commercially available lime materials. Researchers and scientists have used lime-containing materials such as egg shells [Citation85], Mussell shell [Citation94], cow bone [Citation94], oyster shells [Citation117]. A more pronounced 99% decrease from an initial Pb concentration of 3613 mg/kg in the contaminated soil to 16.2 mg/kg was observed in soils amended with 5 weight percent of (5 wt.%) calcined oyster shells compared to un-amended soil [Citation117]. Eggshells and calcined eggshells containing CaCO3 weight percent of 47% and 93%, respectively, were able to decrease TCLP-Pb by up to 68.8% [Citation85]. The TCP-Pb in the un-amended soil was found to be almost 8 times higher than the USEPA set regulatory limit of 5 mg/l. Similarly, a positive from the application of the two amendments to polluted shooting range soils saw a decrease in the exchangeable Pb fraction to less than 1% of the total Pb concentration while the carbonate bound Pb fraction on the other hand increased to up to 47% [Citation85]. The SEM-EDS, XAFS and elemental dot mapping from this same study also revealed the interaction between Pb and the Si and Ca, an indication that Pb was entrapped into the calcium silicate hydrate and thereby restricting its mobility. Immobilization of Pb through formation of Pb(OH)2 and lanarkite (Pb2O(SO4)) were also observed in the study by Ahmad et al. (2012) [Citation85]. In addition, an increase in the soil pH was also observed after soil amendment with eggshell and calcined eggshell and this effect is similar to when CaCO3 and CaO are used to remediate Pb impacted soils. This increase in pH is an important factor in the immobilization of Pb in shooting range soils through elevating the number of negatively charged sites on soil particles, increase in ion exchange and provision of favourable conditions for the precipitation of Pb hydroxides, oxides and carbonates. Conversely, the increase in pH due to liming in soils with significant amounts of dissolved organic matter/carbon can lead to the mobilization of Pb due to formation of complexes that are soluble at alkaline pH conditions [Citation123]. In addition, lime amendment is best suited to areas containing acidic soil, it requires frequent application and excessive use can have adverse effects to water sources in the vicinity of the area under treatment [Citation124].
3.1.3. Iron-based sorbents
Remediation of Pb pollution of shooting range soils using iron-based sorbents has attracted the attention of scientists and researchers due to the non-intrusive nature of the method [Citation125]. Iron-based sorbents are used for the control and management of Pb in shooting range soils through the immobilization of Pb and thereby reducing its mobility, solubility, leaching and bioavailability [Citation93]. The iron sorbents immobilize Pb through its sorption by iron (III) hydroxides found in iron-based sorbents such as ferrihydrite, goethite and gibbsite [Citation90]. Zerovalent iron (Fe°) will undergo weathering and corrode in the presence of oxygen and moisture leading to a variety of iron (hydr)oxides which provides new sorption and binding sites for Pb as shown in Equations (17–19) below [Citation126].
The H+ produced in Equation (19) will react with Pb adsorbed onto the soil mineral surfaces such as the kaolinite via ion exchange resulting in the removal of Pb from the kaolinite surfaces and replaced by the iron (oxy)hydroxide precipitates. Consequently, the Fe(III) solution will adsorb more Pb from the soil at such a lower solution pH due to the continuous ion exchange between Pb in the soil and the re-generated hydrogen ions (H+) [Citation127]. Furthermore, the formation of ferric (oxy)hydroxides from Fe(III) such as ferrihydrite (Fe(OH)3), goethite (α-FeO(OH)) and akaganeite (with the formula Fe(OH)2.7Cl0.3 in the presence of chloride) can separate water molecules and can also produce protons (H+) during precipitation leading to lowering of soil solution pH and enhancement of Pb extraction [Citation127]. The chemical reaction leading to the formation of the ferric hydroxide akaganeite is given in Equation (20);
In the end, the iron (oxy)hydroxides can be transformed to more chemically stable and crystalline iron oxides, such as hematite [Citation127]. The sorption stabilization mechanism may also arise from ligand exchange processes. The ligand exchange mechanism may also involve dissolved organic carbon (DOC) in which the hydroxide anions from iron sorbents could be exchanged for the carboxylic and phenolic functional groups of DOC. As a result, the carboxylic and phenolic groups attached to the iron sorbents now act as binding sites for Pb leading to the formation of insoluble Pb complexes [Citation128]. However, the sorption ability of iron oxides and hydroxides is greatly influenced by the type of metal being sopped and the net electric charge of the adsorbent which varies with solution pH [Citation93]. In other related studies, Pb complexation in the inner sphere by iron hydroxides bearing hydroxyl groups such as ≡ FeO−, ≡ FeOH° and ≡ FeOH2+ have been reported [Citation129,Citation130].
In a study by Spuller et al. (2007), the addition of iron hydroxides (goethite and deferrisation sludge) to Pb-polluted shooting range soil led to a highly noticeable decrease in total concentration of water-extractable Pb of up to 97% in the amended soil compared to the control [Citation90]. The sorption mechanism was confirmed by the higher surface area of deferrisation sludge (262.9 m2/g) which immobilized higher concentrations of Pb compared to goethite (23.5 m2/g). In addition, the predominance of the permeable ferrihydrite over the crystalline fraction allowed for the diffusion of Pb ions into the binding sites located on the inner surfaces of the amendment leading to the immobilization of the Pb contaminant. The concentration of water-soluble Pb decreased from about 100 mg/kg to 3.6 mg/kg after single application of ferrihydrite to a skeet shooting range located in Japan, leading to a 96.8% reduction of water-extractable Pb compared to the shooting range soil without the immobilization material [Citation93]. Likewise, the total percentage of the reactive or labile fractions of the soil being the exchangeable and carbonate fractions dropped by 62.8% while that of the inert Fe-Mn oxide bound fraction surged by 27.2%. This implies that the single application of ferrihydrite additive stabilized Pb towards formation of insoluble Pb minerals resulting in low mobility and reduced bioavailability of Pb. The efficacy of iron oxide amendments was also investigated in the soil collected from a shooting range in Gangwon-do, South Korea [Citation91]. The addition of natural iron oxide resulted in a 13.6% reduction of ammonium acetate extractable Pb in the sampled Gangwon-do shooting range soil with acute Pb pollution (17,468 mg/kg) and TCLP-Pb concentration of 5190 mg/kg which by far exceeded the TCLP-Pb critical level of 5 mg/L. A study by Mariussen et al. (2018), used two amendments ferric oxyhydroxide powder and zerovalent iron for stabilization of Pb mobility and bioavailability in soils collected from four shooting ranges found in Norway [Citation23]. The application of ferric oxyhydroxide showed a 54% decrease in Pb leachability in one of the shooting ranges studied compared to the untreated soil. In contrast, the zerovalent iron displayed the greatest efficacy towards Pb stabilization as 24–98% reduction of Pb leaching was achieved in soils amended with zerovalent iron compared to un-amended soils [Citation23].
Iron bearing materials such as red mud have successfully immobilized Pb in shooting range soils [Citation79]. In a study by Sanderson et al. (2015), the application of red mud containing 28–45% iron oxide was very effective in stabilizing Pb mobility in four shooting range soils. The total Pb concentration levels in untreated soils of four shooting ranges was in the range 341–51,820 μg/kg and after application of the red mud amendment, water-extractable Pb was greatly reduced by over 99%. In addition, red mud was able to reduce TCLP-Pb from around 15 mg/l to around 7 mg/l. The bio-accessible Pb measured in terms of the physiologically based extraction test (PBET) was also reduced by up to 50% in the four shooting range soils investigated [Citation79]. Red mud (as a commercially available derivative reagent ViroSoilTM) has also been used by Tandy et al. (2017) in the immobilization of Pb in moderately contaminated shooting range soils [Citation131]. The ViroSoilTM additive resulted in a drastic increase in the concentrations of iron (oxy)hdroxides, cancrinite and sodalite and thereby increasing the Pb sorption sites. In effect, the adsorption of Pb to the iron (oxy)hydroxides was therefore enhanced in addition to its binding to the negatively charged cancrinite and sodalite channels and cages. Another amendment, iron sulphate, was able to reduce the concentration of exchangeable Pb from 15,700 µg/kg to 7.5 µg/kg in 1 week. The application of FeSO4 treatment is believed to have influenced the release of Pb from the soil Fe oxides due to the reductive dissolution process and transformation into the Fe-Mn oxide bound inert fraction by co-precipitation [Citation131].
Ferric salts such as FeCl3 and Fe(NO3)3 have also shown some potency towards Pb extraction and remediation of Pb-contaminated shooting range soils [Citation127]. In a shooting range soil contaminated with 5700 mg/kg Pb, well over the 200 mg/kg Korean set standard, around 80% of Pb was partitioned in the exchangeable and Fe-Mn oxide fractions [Citation127]. However, upon application of FeCl3, a drastic decrease in total Pb concentration in the exchangeable and Fe-Mn bound Pb occurred with percent removal efficiency of Pb reaching highs of 86% after application of 100 mM of FeCl3 [Citation127].
3.1.4. Combined chemical amendments
Scientists and researchers have applied a combination of two or more chemical amendments to improve their efficacies towards immobilization and stabilization of Pb pollutant in shooting range soils [Citation90,Citation93,Citation129,Citation131–Citation133]. In a study by Spuller et al. (2007), the combined application of diammonium phosphate ((NH4)2HPO4) and calcium phosphate monobasic (Ca(H2PO4)2) increased Pb stabilization by up to 97% compared to the single application of (NH4)2HPO4 that immobilized Pb by only 45%. The inefficient Pb stabilization by the single application of (NH4)2HPO4 amendment can be attributed to the low number phosphate ions in this compound. The addition of the (Ca(H2PO4)2) to this additive increases the availability of the phosphate in the compound and the resulting enhanced Pb stabilization effect [Citation90]. An increase in the dissolved organic carbon (DOC) was also observed in the single application of the (NH4)2HPO4 which may have led to the formation of soluble DOC-Pb complexes. In contrast, the addition of (Ca(H2PO4)2) avails some Ca2+ that react with (NH4)2HPO4 leading to the formation of calcium hydrogen phosphate which further reacts as per Equation (7) discussed earlier in this review. The efficacies of a single application of either calcined oyster shells or waste cow bone towards Pb immobilization and stabilization were found to be very low [Citation111]. However, combined application of the two amendments (5 wt.% calcined oyster shells and 5 wt.% waste cow bone) reduced Pb leachability by 99.6% compared to the 98.3% and 98.2% for a single application of calcined oyster shells and waste cow bone, respectively. In particular, the total Pb concentration of 3613 mg/kg in the untreated soil was reduced to 16.2 mg/kg after combined application of calcined oyster shells and waste cow bone compared to reduction to 63 and 64 mg/kg for the single application of the calcined oyster shells and waste cow bone, respectively, [Citation111]. By inference, the effectiveness towards Pb stabilization by the combined application of the two additives was mainly controlled by the synergistic effect of the phosphate from the waste cow bone and the pozzolanic products from the calcined oyster shells. The sole application of either hydroxyapatite or ferrihydrite has been found to be effective towards Pb immobilization in shooting range soils due to the presence of phosphate and iron (oxy)hydroxides in hydroxyapatite and ferrihydrite, respectively [Citation93]. However, the integrated application of hydroxyapatite and ferrihydrite resulted in pronounced suppression of the level of water-extractable Pb by 99.9% compared 99.2% and 96.8% arising from the single addition of hydroxyapatite and ferrihydrite, respectively. In addition, the combined application of the two amendments resulted in a 44.5% decrease in the total percentages of the labile or reactive exchangeable and carbonate fractions while the percentages of the inert and insoluble residual fraction increased by 33.2%. Specifically, the effects from the single application of the two amendments were lower compared to when combined [Citation93]. This implies that the combined application of amendments effectively transformed Pb in the shooting range soils to more stable and less soluble Pb minerals. The immobilization of Pb in shooting range soils through the addition of phosphate and the mechanisms involved are well known [Citation90,Citation100]. Notwithstanding the foregoing, coupling phosphate amendment with another chemical such as magnesium oxide (MgO) enhances Pb stabilization of shooting range soils [Citation102]. The combined application of phosphate and MgO at Perth (PE) shooting range in Australia increased the concentration of Pb confined to the inert and non-reactive residual fractions where Pb concentration increased to 10.54 mg/kg and 12.47 mg/kg after application of phosphate and phosphate plus MgO, respectively, compared to Pb concentration of only 0.14 mg/kg in the residual fraction of the untreated soil. The single application of MgO only caused a slight increase of Pb partitioned in the residual fraction (2.09 mg/kg) compared to the untreated soil (0.14 mg/kg). In the same way, bio-accessibility of Pb was greatly reduced after the combined phosphate and MgO treatment. The combined treatment resulted in 56% decrease of percent bio-accessible Pb relative to the control (75%) while single applications resulted in a 68.8% (phosphate) and 65% (MgO) decrease, respectively. The addition of MgO to the phosphate amendment augmented the Pb immobilization and stabilization through co-precipitation of metal hydroxides and Mg(OH)2 on the MgO mineral surface coupled with sorption in the newly formed precipitates. The mechanism for MgO Pb stabilization is outlined in Equations (21–24) below. In the first step, (Equation (21)) the hydration of MgO takes place forming the mineral Mg(OH)2 (brucite) [Citation102].
The formed brucite mineral dissolves under aqueous conditions producing hydroxyl ions that are a good source for Pb hydroxide precipitation (Equation (23)). The dissolution of the MgO in a two-step fashion with the liberation of the hydroxyl ion makes this amendment effective for long-term immobilization of Pb than lime [Citation102]. In a field study in Norway by Okkenhaug et al. (2016), a mixture of 2% ferric oxyhydroxide powder and 1% limestone led to 97% decrease of pore water Pb concentration. The total concentration of Pb in pore water of the un-mended soils was in the range 7–1495 µg/l which exceeded the Norwegian water quality guideline of 0.5 µg/l for Pb in freshwater. In addition, the concentration of Pb in the labile fractions substantially decreased while that of the inert fractions increased after the combined application of the amendments [Citation126]. The cooperative effect of phosphate rock and phosphoric acid was observed in a study by Fayiga and Saha (2017), in which the TCLP-Pb concentration was reduced to below the USEPA TCLP-Pb regulatory level of 5 mg/l [Citation134]. The combined application of the phosphate rock and phosphoric acid was able to reduce the TCLP-Pb concentration from 8.2–358 mg/l to 0.43–8.36 mg/l. A mixture of 2% ferric oxyhydroxide powder and 2% lime caused a 28% decrease of Pb concentration in the pore water compared to 22% decrease when only 2% lime was applied to one of the four shooting range soils, Evjemoen [Citation23]. In contrast, a drastic increase of Pb concentration in pore water by over 1000% was observed when a single application of 2% ferric oxyhydroxide powder was used. The mobilisation of Pb in pore water after single addition of ferric oxyhydroxide is mainly attributed to its acidic properties that led to a decrease in the soil pH to a more acidic condition resulting in the dissolution of Pb minerals. Hence, higher concentrations of Pb in pore water observed at the Evjemoen shooting range.
4. Research pitfalls/shortfalls and future perspectives
Most studies have concentrated more on the efficacies of chemical amendments towards Pb stabilization or immobilization and less on the long-term effects of soil amendments to the ecosystem. Furthermore, most studies have focused more on ex-situ effectiveness of the chemical amendments towards Pb stabilization than in-situ application of the chemical additives [Citation90,Citation125,Citation135]. Chemical amendments applied on-site to shooting range soils indeed do transform Pb into more stable Pb minerals. However, over time the stabilized Pb minerals get subjected to the physical, chemical and biochemical weathering processes such as wear and tear, oxidation-reduction, acid-base reactions, association-dissociation, sorption-desorption and precipitation-dissolution [Citation136,Citation137]. As a result, the prolonged stability of the transformed Pb minerals such as hydrocerussite [Pb3(CO3)2(OH)2], cerussite (PbCO3), massicot (PbO) and anglesite (PbSO4) has to be fully comprehended. The use of solubility diagrams have been helpful in elucidating the long-term solubility of these Pb mineral phases, regrettably, these are only theoretical and do not take into consideration the complex reactions that take place in the soil system [Citation9,Citation138]. Scheckel and Ryan (2002) have also shown through high-resolution thermogravimetric analysis and crystal dynamics that as the Pb minerals age, their relative stabilities increase [Citation138]. The changes in the soil physicochemical properties such as soil pH, organic matter, moisture and cation exchange capacity have to be well understood [Citation7,Citation18,Citation62,Citation139]. The acidic soils and high moisture content provide a favourable condition for the dissolution of Pb minerals which may lead to the re-mobilisation of Pb in the soil [Citation62]. Shooting range soils with high content of organic matter are likely to contain high concentrations of humic and fulvic acids which can form soluble complexes with Pb from the stable Pb mineral at high pH ranges and thereby leaching out Pb into soil solution [Citation140]. It is also important to note that the presence of co-contaminant cations such as antimony (Sb), nickel (Ni), copper (Cu) and zinc (Zn) in shooting range soil may replace Pb in the stable Pb minerals such as Pb phosphate leading to the formation of less stable crystalline structures. For example, the presence of Zn metal reduced the stability of Pb-humic acid complex as Zn replaced Pb in the organo-complex leading to the mobilisation of Pb in the soil solution [Citation141]. In contrast, immobilization of Pb by fluoroapatite was enhanced by the presence of zinc due to the increased ion–ion interaction potential difference between Pb and Zn. Remediation of Pb-polluted soils using chemical amendments such as lime (CaO or Ca(OH)2), that have a direct influence on the soil pH are susceptible to weathering actions which can change the pH of the soil buffer and as such may not be good candidates for the long-term Pb immobilization. The soil pH is critical in the dissolution rates of Pb minerals. The dissolution rates of Pb minerals should be slowest when the soil solution pH is at the point at which the net charge equals zero [Citation98]. At lower pH, there is an increase in the proton concentrations that get sopped into the oxide surfaces and thereby polarizing the metal-oxygen bonds and resulting into a weakened bond on the underlying lattice [Citation142].
A study by Stanforth et al. (2005) has shown that the pH of the soil became more acidic after a one year of wear and tear of the MgO immobilized Pb and these resulted in the mobilization of Pb in the soil and an increase in TCLP-Pb [Citation136]. The study by Stanforth et al. (2005) has demonstrated that soil acidification has the potential to reverse sorption chemical reactions [Citation136]. These findings were further supported by Kumpiene et al. (2007), in which chemical amendment induced low pH decreased the extent of adsorption of Pb by the oxides and cations leading to dissolution of precipitates and leaching of Pb [Citation143]. Therefore, it should be borne in mind that the application of amendments are site-specific and that the physicochemical properties of a shooting range should be taken into consideration before chemical remediation.
The availability of government environmental protection guidelines and conservation policies on maximum contaminant limits (MCLs) help the range users identify the best management practices (BMPs) for remediation of Pb pollution of shooting range soils. Organizations like the USEPA have outlined BMPs for shooting ranges that give considerations to the site physicochemical properties, land use of nearby environment, presence of nearby surface and underground water sources and distance to sensitive receptors [Citation144]. This approach helps to identify areas that present potential environmental and human health risk from Pb pollution for further investigation. It also assists in targeting areas where the most needed natural resources are at a higher risk of being contaminated in order to establish priority for remediation and implementation of cost-effective measures.
Chemical amendment technique is not the only mechanism that has been applied to shooting ranges for the control and management of Pb pollution in shooting range soils, other techniques such as phytoremediation [Citation100,Citation145,Citation146], bioremediation [Citation147], physical [Citation89], electro-dialytic [Citation148] and hydrothermal treatment [Citation149]. Phytoremediation stabilize Pb in shooting range soils through a process called phytostabilization in which plants uptake Pb and accumulate it in their roots and in some case precipitation in the rhizosphere can occur [Citation146]. Physical remediation techniques may involve replacing berm soils with sand because sand traps low content of moisture, contains low organic matter and high pH which are good attributes to the slow transformation and low mobility of Pb in shooting range soils [Citation75]. In addition, electrodialytic remediation methods have also been found to be effective in the control and management of Pb in shooting range soils. In a study by Pedersen et al. (2018), application of a 2-compartment electrodialytic cell made up of NaNO3 (0.01 M) as the catholyte, platinum-coated titanium electrode (cathode) and titanium-coated mesh (anode) was able to transport more than 30% of the initial Pb content out of the soil [Citation148]. A decrease in the distance between the electrodes from 3.0 to 1.5 cm improved transport of Pb by more than 200%. After hydrothermal treatment under sub-critical conditions, a decrease in the Pb bioavailability factor from 41.33% to14.66% was observed [Citation149].
It is noteworthy that for a successful remediation of Pb-polluted shooting range soil, a number of important steps must be taken and followed such as 1) technology prescreening and treatability study scoping, 2) remedial investigation of the contaminated site, 3) feasibility study of prescreened remediation techniques, 4) determination of best remediation method, 5) design and implementation of remediation practices and 6) evaluation of remediation performance [Citation150]. The initial step of technology prescreening and treatability study scoping should be carried out at the start of the soil remediation project by searching the literature for some information on the amendment technique to employ as well as consulting profession experts in the field. In the second step, the effectiveness of the prescreened amendment technique towards Pb stabilization is tested ex-situ on soil samples collected from the contaminated shooting range [Citation151]. Once the selected remediation technique passes the feasibility study and the performance goals have been met, the implementation of the selected Pb-remediation technique will be conducted after which the performance of the amendment technique will be evaluated as the last step. When followed properly, these steps generate detailed information on the design, cost and performance of the remediation method.
5. Conclusion
Shooting ranges have been of great concern for many years as a source of Pb pollution being second to the battery industry. A number of soil amendment and remediation techniques have been suggested and applied towards the control and management of Pb pollution in soil. These amendment techniques exploit the chemical reactions that can take place between Pb and the amendment assisted by the physicochemical processes of the shooting range soils such as physical interactions, chemical reactions, biological activities and electrochemical processes. Amendment techniques may involve: 1) containment such as encapsulation and land filling, 2) extraction (soil washing and flushing), 3) immobilization (stabilization, solidification and vitrification). Immobilization by stabilization can further be divided into chemical stabilization, phytostabilization and biostabilization. Chemical stabilization is the most common form of soil remediation technique and is one of the techniques recommended by the USEPA for the control and management of Pb pollution in shooting range soils. In addition, chemical remediation is an ideal in-situ amendment technique since this method leaves the soil intact and does not disturb and disrupt the soil micro-organism community. It only exploits the soil physicochemical properties such as soil pH, organic matter, cation exchange capacity, electrical conductivity and soil texture. Furthermore, chemical remediation technique being an in-situ process is a more cost-effective technique than ex-situ remediation methods such as land filling and soil washing. In overall, there is an infinite list of waste materials that contain the reactive component found in commercially available chemicals that can work effectively to immobilize and stabilize Pb in shooting range soils. Materials such as 1) phosphate-containing materials such as poultry waste, waste cow bone and gypsum waste, 2) lime-containing waste such as egg shells, oyster shells and mussel shells and 3) Iron-based waste such as deferrisation sludge and red mud have been found to be effective in stabilization of Pb in shooting range soils. Combining amendments has also been found to have superior effect on Pb stabilization compared to single application of amendments. Lastly, it is again imperative to note that the suitability of a chemical amendment technique is site-specific and that its effectiveness is controlled by the shooting range physicochemical characteristics, time, amendment efficacy and cost implications. As a result, the feasibility and treatability studies are important in ascertaining the best remediation technique to use prior to carrying out a full-scale in-situ addition of amendment to polluted shooting range soils.
Acknowledgments
The authors would like to thank the Botswana International University of Science and Technology (BIUST) for the resources used in the preparation of this review.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Guberman DE. U.S. Department of the interior, United States Geological Survey (USGS), 2012 minerals year book – lead. 2016. p. 42.8.
- Camobreco VJ, Richards BK, Steenhuis TS, et al. Movement of heavy metals through undisturbed and homogenized soil columns. Soil Sci. 1996;161:740–750.
- Lin Z. Secondary mineral phases of metallic lead in soils of shooting ranges from Orebro county. Sweden Environ Geol. 1996;27:370–375.
- Cao X, Ma LQ, Chen M, et al. Lead transformation and distribution in the soils of shooting ranges in Florida, USA. Sci Total Environ. 2003;307:179–189.
- Hui CA. Lead distribution throughout soil, flora and an invertebrate at a wetland skeet range. J Toxicol Environ Health A. 2002;65:1093–1107.
- VanBon J, Boersema JJ. Sources, effects and management of metallic lead pollution: the contribution of hunting, shooting and angling. Contam Soil. 1988;3:269–271.
- Astrup T, Boddum JK, Christensen TH. Lead distribution and mobility in a soil embankment used as a bullet stop at a shooting range. J Soil Contam. 1999;8:653–665.
- Bruell R, Nikolaidis NP, Long RP. Evaluation of remedial alternatives of lead from shooting range soil, Environ. Eng Sci. 1999;16:403–414.
- Cao X, Ma LQ, Hardison D Jr, et al. Weathering of lead bullets and their environmental effects at outdoor shooting ranges. J Environ Qual. 2003;32:526–534.
- Dinake P, Kelebemang R, Sehube N. A comprehensive approach to speciation of lead and its contamination of firing range soils: A review. Soil Sediment Contam. 2019;2:431–459.
- Etim EU, Onianwa PC. Lead contamination of soil in the vicinity of a military shooting range in Ibadan, Nigeria. Toxicol Environ Chem. 2012;94:895–905.
- Sehube N, Kelebemang R, Totolo O, et al. Lead pollution of shooting range soils. S Afr J Chem. 2017;70:21–28.
- Eisler R. Lead hazards to fish, wildlife and invertebrates : A synoptic review. Contaminant Hazard Reviews Report 14. Biological Report. 1988;85:1–94.
- Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5:47–58.
- US Environmental Protection Agency (USEPA). Best management practices for lead at outdoor shooting ranges. EPA-902-B-01-001. 2005.
- Papanikolaou NC, Hatzidaki EG, Belivanis S. Lead toxicity update. A brief review. Med Sci Monit. 2005;11:329–336.
- Bannon DI, Drexler JW, Fent GM, et al. Evaluation of small arms range soils for metal contamination and lead bioavailability. Environ Sci Technol. 2009;43:9071–9076.
- Chen M, Daroub SH. Characterization of lead in soils of a rifle/pistol shooting range in central Florida. Soil Sediment Contam. 2002;7:1–17.
- Dinake P, Kelebemang R, Sehube N, et al. Quantitative assessment of environmental risk from lead pollution of shooting range soils. Chem Speciat Bioavailab. 2018;30:76–85.
- Bricka RM, Rivera YB, Deliman PN Vertical migration potential of metal contaminants at small arms firing ranges, camp edwards military reservation, MA. US Army Corps of Engineers. Technical Report IRRP-98-3. 1998.p.1–91.
- Tardy BA, Bricka RM, Larson SL Chemical stabilization of lead in small arms firing range soils–environmental quality and technology program. U.S. Army Corps of Engineers, ERDC/EL TR-03-20. Vicksburg, Mississippi; 2003. p.1–67.
- Chen M, Ma LQ, Harris WG. Distribution of Pb and as in soils at a shooting facility in central Florida. Soil Crop Sci Soc Florida Proc. 2001;60:15–20.
- Mariussen E, Johnsen IV, Stromseng AE. Application of sorbents in different soil types from small arms shooting ranges for immobilization of lead (Pb), copper (Cu), zinc (Zn), and antimony (Sb). J Soil Sediment. 2018;18:1558–1568.
- Etim EU. Lead removal from contaminated shooting range soil using acetic acid potassium chloride washing solutions and electrochemical reduction. J Health Pollut. 2017;7:22–34.
- Islam MN, Nguyen XP, Jung HY, et al. Chemical speciation and quantitative evaluation of heavy metal pollution hazards in two army shooting range backstop soils. Bull Environ Contam Toxicol. 2016;96:179–185.
- Johnsen IV, Mariussen E, Voie O. Assessment of intake of copper and lead by sheep grazing on a shooting range for small arms: a case study. Environ Sci Pollut Res. 2019;26:7337–7346.
- Pedersen KB, Jensen PE, Ottosen LM, et al. The relative influence of electrokinetic remediation design on the removal of As, Cu, Pb and Sb from shooting range soils. Eng Geol. 2018;238:52–61.
- Vandebroek E, Haufroid V, Smolders E, et al. Occupational exposure to metals in shooting ranges: A biomonitoring study. Saf Health Work. 2018;10:87–94.
- Mariussen E, Johnsen IV, Stromseng AE. Distribution and mobility of lead (Pb), copper (Cu), zinc (Zn), and antimony (Sb) from ammunition residues on shooting ranges for small arms located on mires. Environ Sci Pollut Res Int. 2017;24:10182–10196.
- Mariussen E, Heier LS, Teien HC, et al. Accumulation of lead (Pb) in brown trout (Salmo trutta) from a lake downstream a former shooting range. Ecotoxicol Environ Saf. 2017;135:327–336.
- Okkenhaug G, Smebye AB, Pabst T, et al. Shooting range contamination: mobility and transport of lead (Pb), copper (Cu) and antimony (Sb) in contaminated peatland. J Soils Sed. 2017;18:3310–3323.
- Rodríguez-Seijo A, Cachada A, Gavina A, et al. Lead and PAHs contamination of an old shooting range: A case study with a holistic approach. Sci Total Environ. 2017;575:367–377.
- Etim EU. Distribution of soil-bound lead arising from rainfall-runoff events at impact berm of a military shooting range. J Environ Prot. 2016;7:623–634.
- Rodríguez-Seijo A, Alfaya MC, Andrade ML, et al. Copper, chromium, nickel, lead and zinc levels and pollution degree in firing range soils. Land Degrad Dev. 2016;27:172–1730.
- Selonen S, Setala H. Soil processes and tree growth at shooting ranges in a boreal forest reflect contamination history and lead-induced changes in soil food webs. Sci Total Environ. 2015;518–519:320–327.
- Li Y, Zhu Y, Zhao S, et al. The weathering and transformation process of lead in China’s shooting ranges. Environ Sci Processes Impacts. 2015;17:1620–1633.
- Luo W, Verweij RA, van Gestel CAM. Toxicity of Pb contaminated soils to the oribatid mite platynothrus peltifer. Ecotoxicology. 2015;24:985–990.
- Perroy RL, Belby CS, Mertens CJ. Mapping and modeling three dimensional lead contamination in the wetland sediments of a former trap-shooting range. Sci Total Environ. 2014;487:72–81.
- Liu Y, Fang Z, Xie C, et al. Analysis of existing speciation and evaluation of heavy metals pollution of soil in a shooting range. Nature Environ Pollut Technol. 2014;13:449–456.
- Luo W, Verweij RA, van Gestel CAM. Contribution of soil properties of shooting fields to lead biovailability and toxicity to Enchytraeus crypticus. Soil Biol Biochem. 2014;76:235–241.
- Lafond S, Blais JF, Martel R, et al. Chemical leaching of antimony and other metals from small arms shooting range soil. Water Air Soil Pollut. 2013;224:1371–1385.
- Kim D-H, Hwang B-R, Moon D-H, et al. Environmental assessment on a soil washing process of a Pb-contaminated shooting range site: a case study. Environ Sci Pollut Res. 2013;20:8417–8424.
- Ash C, Tejnecky V, Sebek O, et al. Fractionation and distribution of risk elements in soil profiles at a Czech shooting range. Plant Soil Environ. 2013;59:121–129.
- Laporte-Saumure M, Martel R, Mercier G. Pore water quality in the upper part of the vadose zone under an operating Canadian small arms firing range backstop berm. Soil Sediment Contam. 2012;21:739–755.
- Selonen S, Liiri M, Strommer R, et al. The fate of lead at abandoned and active shooting ranges in a boreal pine forest. Environ Toxicol Chem. 2012;31:2771–2779.
- Evangelou MWH, Hockmann K, Pokharel R, et al. Accumulation of Sb, Pb, Cu, Zn and Cd by various plants species on two different relocated military shooting range soils. J Environ Manag. 2012;108:102–107.
- Fayiga AO, Saha U, Cao X, et al. Chemical and physical characterization of lead in three shooting range soils in Florida. Chem Speciat Bioavailab. 2011;23:148–154.
- Laporte-Saumure M, Martel R, Mercier G. Characterization and metal availability of copper, lead, antimony and zinc contamination at four Canadian small arms firing ranges. Environ Technol. 2011;32:767–781.
- Conesa HM, Wieser M, Studer B, et al. Effects of vegetation and fertilizer on metal and Sb plant uptake in a calcareous shooting range soil. Ecol Eng. 2011;37:654–658.
- Laporte-Saumure M, Martel R, Mercier G. Evaluation of physicochemical methods for treatment of Cu, Pb, Sb, and Zn in Canadian small arm firing ranges backstop soils. Water Air Soil Pollut. 2010;213:171–189.
- Yin X, Gao B, Ma LQ, et al. Colloid-facilitated Pb transport in two shooting-range soils in Florida. J Hazard Mater. 2010;177:620–625.
- Clausen J, Korte N. The distribution of metals in soils and pore water at three U.S. military training facilities. Soil Sediment Contam. 2009;18:546–563.
- Hartikainen H, Kerko E. Lead in various chemical pools in soil depth profiles on two shooting ranges of different age. Boreal Environ Res (Suppl A). 2009;14:61–69.
- Rauckyte T, Zak S, Pawlak Z, et al. Lead leachability from shooting range soils. Ecol Chem Eng A. 2009;16:419–426.
- Robinson BH, Bischofberger S, Stoll A, et al. Plant uptake of trace elements on a Swiss military shooting range: uptake pathways and land management implications. Environ Pollut. 2008;153:668–676.
- Cao X, Dermatas D. Evaluating the applicability of regulatory leaching tests for assessing lead leachability in contaminated shooting range soils. Environ Monit Assess. 2008;139:1–13.
- Interstate Technology and Regulatory Council (ITRC). Characterization and remediation of soils at closed small arms firing ranges. Interstate Technology and Regulatory Council, Small Arms Firing Range Team. Blacksburg, VA; 2003. p.1–2. Available from: www.itrcweb.org/SMART-1.pdf
- Kelebemang R, Dinake P, Sehube N, et al. Speciation and mobility of lead in shooting range soils. Chem Speciat Bioavailab. 2017;29:143–152.
- Rooney CP, McLaren RG, Cresswell RJ. Distribution and phytoavailability of lead in a soil contaminated with lead shot. Water Air Soil Pollut. 1999;116:535–548.
- Sanderson P, Naidu R, Bolan N, et al. Effect of soil type on distribution and bioaccessibility of metal contaminants in shooting range soils. Sci Total Environ. 2012;438:452–462.
- Jorgensen SS, Willems M. The fate of lead in soils: the transformation of lead pellets in shooting range soils. Ambio. 1987;6:11–15.
- Ma LQ, Hardison D Jr, Harris WD, et al. Effects of soil property and soil amendment on weathering of abraded metallic Pb in shooting ranges. Water Air Soil Pollut. 2007;178:297–307.
- Bricka M Soil treatments to limit lead mobility’. Fourth National Shooting Range Symposium; Policy Track: Environmental Issues; Vicksburg, Mississippi; 2000. p.23–125.
- Fijalkowski K, Kacprzak M, Grobelak A, et al. the influence of selected soil parameters on the mobility of heavy metals in soils. Inz I Ochrona Srodowiska. 2012;5:81–92.
- Knechtenhofer LA, Xifra IO, Scheinost AC, et al. Fate of heavy metal in a strongly acidic shooting-range: small scale metal distribution and its relation to a preferential water flow. J Plant Nutr Soil Sci. 2003;2003(166):84–92.
- Rooney CP, McLaren RG, Condron LM. Control of lead solubility in soil contaminated with lead shot: effect of soil pH. Environ Pollut. 2007;149:149–157.
- Cerqueira B, Covelo EF, Andrade ML, et al. Retention and mobility of copper and lead in soils as influenced by soil horizon properties. Pedosphere. 2011;21:603–614.
- Muhlbachova G, Sagova-Mareckova M, Omelka M, et al. The influence of soil organic carbon on interactions between microbial parameters and metal concentrations at a long-term contaminated site. Sci Total Environ. 2015;502:218–223.
- Zheng G, Xu S, Liang M, et al. Transformations of organic carbon and its impact on lead weathering in shooting range soils. Environ Earth Sci. 2011;64:2241–2246.
- Dinake P, Kelebemang R, Sehube N, et al. Quantitative assessment of environmental risk from lead pollution of shooting range soils. Chem Speciat Bioavailab. 2018;30:76–85.
- Dermatas D, Menounou N, Dadachov M, et al. Lead leachability in firing range soils. Environ Eng Sci. 2006;23:88–101.
- Cao X, Dermatas D, Xu X, et al. Immobilization of lead in shooting range soils by means of cement, quicklime and phosphate amendments. Env Sci Pollut Res. 2008;15:120–127.
- Chrastny V, Komarek M, Hajek T. Lead contamination of an agricultural soil in the vicinity of a shooting range. Environ Monit Assess. 2010;162:37–46.
- Dermatas D, Shen G, Chrysochoou M, et al. Pb speciation versus TCLP release in army firing range soils. J Hazard Mater. 2006;136:34–46.
- Liu R, Gress J, Gao J, et al. Impacts of two best management practices on Pb weathering and leachability in shooting range soils. Environ Monit Assess. 2013;185:6477–6484.
- Sanderson P, Fangjie QF, Seshadri B, et al. Contamination, fate and management of metals in shooting range soils—a Review. Curr Pollut Rep. 2018;4:175–187.
- Khalid S, Shahid M, Khan N, et al. A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor. 2017;182:247–268.
- Chrysochoou M, Dermatas D, Grubb DG. Phosphate application to firing range soils for Pb immobilization: the unclear role of phosphate. J Hazard Mater. 2007;144:1–14.
- Sanderson P, Naidu R, Bolan N. Effectiveness of chemical amendments for stabilisation of lead and antimony in risk-based land management of soils of shooting ranges. Environ Sci Pollut Res. 2015;22:8942–8956.
- Shimizu S, Sato T, Katoh M. Formation of a lead insoluble phase using an immobilization material and its maximization in soil under unsaturated moisture conditions. J Soils Sediments. 2018;18:1052–1059.
- Thangavadivel K, Ranganathan S, Sanderson P, et al. Case study of testing heavy-particle concentrator-aided remediation of lead-contaminated rifle shooting range soil. Remediation. 2018;28:67–74.
- Barker AJ, Douglas TA, Ilgen AG, et al. Lead and antimony from bullet weathering in newly constructed target berms: chemical speciation, mobilization, and remediation strategies. Sci Total Environ. 2019;658:558–569.
- Tariq SR, Ashraf A. Comparative evaluation of phytoremediation of metal contaminated soil of firing range by four different plant species. Arab J Chem. 2016;9:806–814.
- Magaji Y, Ajibade GA, Yilwa VMY, et al. Concentration of heavy metals in the soil and translocation with phytoremediation potential by plant species in military shooting range. World Sci News. 2018;92:260–271.
- Ahmad M, Hashimoto Y, Moon DH, et al. Immobilization of lead in a Korean military shooting range soil using eggshell waste: an integrated mechanistic approach. J Hazard Mater. 2012;209–210:392–:401.
- Levonmaki M, Hartikainen H. Efficiency of liming in controlling the mobility of lead in shooting range soils as assessed by different experimental approaches. Sci Total Environ. 2007;388:1–7.
- Wawra A, Friesl-Hanl W, Puschenreiter M, et al. Degradation of polycyclic aromatic hydrocarbons in a mixed contaminated soil supported by phytostabilisation, organic and inorganic soil additive. Sci Total Environ. 2018;628–629:1287–1295.
- Hashimoto Y, Matsufuru H, Sato T. Attenuation of lead leachability in shooting range soils using poultry waste amendments in combination with indigenous plant species. Chemosphere. 2008;73:643–649.
- Yin X, Saha UK, Ma LQ. Effectiveness of best management practices in reducing Pb-bullet weathering in a shooting range in Florida. J Hazard Mater. 2010;2010(179):895–900.
- Spuller C, Weigand H, Marb C. Trace metal stabilisation in a shooting range soil: mobility and phytotoxicity. J Hazard Mater. 2007;141:378–387.
- Rajapaksha AU, Ahmad M, Vithanage M, et al. The role of biochar, natural iron oxides, and nanomaterials as soil amendments for immobilizing metals in shooting range soil. Environ Geochem Health. 2015;37:931–942.
- Hashimoto Y, Taki T, Sato T. Sorption of dissolved lead from shooting range soils using hydroxyapatite amendments synthesized from industrial by-products as affected by varying pH conditions. J Environ Manag. 2009;90:1782–1789.
- Ogawa S, Katoh M, Sato T. Simultaneous lead and antimony immobilization in shooting range soil by a combined application of hydroxyapatite and ferrihydrite. Environ Technol. 2015;36:2647–2656.
- Ahmad M, Lee SS, Lim JE, et al. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere. 2014;95:433–441.
- Ahmad M, Ok YS, Rajapaksha AU, et al. Lead and copper immobilization in a shooting range soil using soybean stover- and pine needle-derived biochars: chemical, microbial and spectroscopic assessments. J Hazard Mater. 2016;301:179–186.
- Deem LM, Crow SE. Department of natural resources and environmental management; reference module in earth systems and environmental sciences, university of Hawaii Manoa, Honolulu, HI, United States. Elsevier Inc. 2017;p 1–4.
- Sanderson P, Naidu R, Bolan N. Application of a biodegradable chelate to enhance subsequent chemical stabilisation of Pb in shooting range soils. J Soils Sediments. 2017;17:1696–1705.
- Sanderson P, Naidu R, Bolan N, et al. Critical review on chemical stabilization of metal contaminants in shooting range soils. J Hazard Toxic Radioact Waste. 2012;16:258–272.
- Nriagu JO. Lead orthophosphates-IV formation and stability in the environment. Geochim Cosmochim Acta. 1974;38:887–898.
- Dermatas D, Chrysochoou M, Grubb DG, et al. Phosphate treatment of firing range soils: lead fixation or phosphorus release? J Environ Qual. 2008;37:47–56.
- Shi Z, Erickson LE. Mathematical model development and simulation of in situ stabilization in lead-contaminated soils. J Hazard Mater. 2001;87:99–116.
- Sanderson P, Naidu R, Bolan N, et al. Chemical stabilisation of lead in shooting range soils with phosphate and magnesium oxide: synchrotron investigation. J Hazard Mater. 2015;299:395–403.
- Ogawa S, Sato T, Katoh M. Formation of a lead-insoluble phase, pyromorphite, by hydroxyapatite during lead migration through the water-unsaturated soils of different lead mobilities. Environ Sci Pollut Res. 2018;25:7662–7671.
- Sanderson P, Naidu R, Bolan N. The effect of environmental conditions and soil physicochemistry on phosphate stabilisation of Pb in shooting range soils. J Environ Manag. 2016;170:123–130.
- Hashimoto Y, Sato T. Removal of aqueous lead by poorly-crystalline hydroxyapatites. Chemosphere. 2007;69:1775–1782.
- Hashimoto Y, Yoshida T, Sato T Phosphorus-induced lead immobilization in a shooting range soil using poorly-crystalline hydroxyapatite and incinerated poultry waste. ASA-CSSA-SSSA International Annual Meetings, NewOrleans, USA, 2007.
- Chen S, Xu M, Ma Y, et al. Evaluation of different phosphate amendments on availability of metals in contaminated soil. Ecotox Environ Safe. 2007;67:278–285.
- Yoon JK, Cao X, Ma LQ. Application methods affect phosphorus-induced lead immobilization from a contaminated soil. J Environ Qual. 2007;36:373–378.
- Conesa HM, Wieser M, Gasser M, et al. Effects of three amendments on extractability and fractionation of Pb, Cu, Ni and Sb in two shooting range soils. J Hazard Mater. 2010;181:845–850.
- Kilgour DW, Moseley RB, Barnett MO, et al. Potential negative consequences of adding phosphorus-based fertilizers to immobilize lead in soil. J Environ Qual. 2008;37:1733–1740.
- Moon DH, Cheong KH, Khim J, et al. Stabilization of Pb2+ and Cu2+ contaminated firing range soil using calcined oyster shells and waste cow bones. Chemosphere. 2013;91:1349–1354.
- Kumarathilaka P, Ahmad M, Herath I, et al. Influence of bioenergy waste biochar on proton- and ligand-promoted release of Pb and Cu in a shooting range soil. Sci Total Environ. 2018;625:547–554.
- Ahmad M, Rajapaksha AU, Lim JE, et al. Biochar as a sorbent for contaminant managementin soil and water: a review. Bioresour Technol. 2014;99:19–33.
- Uchimiya M, Bannon DI, Wartelle LH. Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J Agric Food Chem. 2012;60:1798–1809.
- Uchimiya M, Klasson KT, Wartelle LH, et al. Influence of soil properties on heavy metal sequestration by biochar amendment: 1. Copper sorptionisotherms and the release of cations. Chemosphere. 2011;82:1431–1437.
- Qiu Y, Zheng Z, Zhou Z, et al. Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour Technol. 2009;100:5348–5351.
- Moon DH, Park JW, Cheong KH, et al. Stabilization of lead and copper contaminated firing range soil using calcined oyster shells and fly ash. Environ Geochem Health. 2013;35:705–714.
- Sanderson P, Naidu R, Bolan N. Ecotoxicity of chemically stabilised metal(loid)s in shooting range soils. Ecotoxicol Environ Saf. 2014;100:201–208.
- Seshadri B, Bolan NS, Choppala G, et al. Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere. 2017;184:197–206.
- Andersson S, Nilsson SI. Influence of pH and temperature on microbial activity, substrate availability of soil-solution bacteria and leaching of dissolved organic carbon in a mor humus. Soil Biol Biochem. 2001;33:1181–1191.
- Derome J. Detoxification and amelioration of heavy-metal contaminated forest soils by means of liming and fertilisation. Environ Pollut. 2000;107:79–88.
- Basta NT, Pantone DJ, Tabatabai MA. Path analysis of heavy metal adsorption by soil. Agron J. 1993;85:1054–1057.
- Turpeinen R, Salminen J, Kairesalo T. Mobility and bioavailability of lead in contaminated boreal forest Soil. Environ Sci Technol. 2000;34:5152–5156.
- Sporting Arms and Ammunition Manufacturers’ Institute, SAAMI. 1996. Lead mobility at shooting ranges. Project No. 13081.01, Flintlock Ridge Office Center, 11 Mile Hill Road, Newtown, CT 06470. p. 22–24.
- Fayiga AO. Remediation of inorganic and organic contaminants in military ranges. Environ Chem. 2019;16:81–91.
- Okkenhaug G, Gebhardt KAG, Amstaetter K, et al. Antimony (Sb) and lead (Pb) in contaminated shooting range soils: sb and Pb mobility and immobilization by iron based sorbents, a field study. J Hazard Mater. 2016;307:336–343.
- Yoo JC, Shin YJ, Kim EJ, et al. Extraction mechanism of lead from shooting range soil by ferric salts. Process Saf Environ. 2016;103:174–182.
- Gu B, Schmitt J, Chen Z, et al. Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environ Sci Technol. 1994;25:38–46.
- Okkenhaug G, Amstatter K, Bue HL, et al. Antimony (Sb) contaminated shooting range soil: sb mobility and immobilization by soil amendments. Environ Sci Technol. 2013;47:6431−6439.
- Johnson CA, Moench H, Wersin P, et al. Solubility of antimony and other elements in samples taken from shooting ranges. J Environ Qual. 2005;34:248–254.
- Tandy S, Meier N, Schulin R. Use of soil amendments to immobilize antimony and lead in moderately contaminated shooting range soils. J Hazard Mater. 2017;324:617–625.
- Wang L, Yu K, Li J-S, et al. Low-carbon and low-alkalinity stabilization/solidification of high-Pb contaminated soil. Chem Eng J. 2018;351:418–427.
- Wang L, Cho D-W, Tsang DCW, et al. Green remediation of As and Pb contaminated soil using cement-free clay based stabilization/solidification. Environ Int. 2019;126:336–345.
- Fayiga AO, Saha UK. Effect of phosphate treatment on Pb leachability in contaminated shooting range soils. Soil Sed Contam. 2017;26:115–126.
- Liu L, Li W, Song W, et al. Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ. 2018;633:206–219.
- Stanforth R, Yap C-F, Nayar R. Effects of weathering on treatment of lead contaminated soils. J Environ Eng. 2005;131:38–48.
- Violante A, Cozzolino V, Perelomov L, et al. Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr. 2010;10:268–292.
- Scheckel KG, Ryan JA. Effects of aging and pH on dissolution kinetics and stability of chloropyromorphite. Environ Sci Technol. 2002;36:2198–2204.
- Dinake P, Maphane O, Sebogisi K, et al. Pollution status of shooting range soils from Cd, Cu, Mn, Ni and Zn found in ammunition. Cogent Environ Sci. 2018;4:1528701.
- Martinez CE, Jacobson AR, McBride MB. Lead phosphate minerals: solubility and dissolution by model and natural ligands. Environ Sci Technol. 2004;38:5584–5590.
- Raicevic S, Perovic V, Zouboulis AI. Theoretical assessment of phosphate amendments for stabilization of (Pb + Zn) in polluted soil. Waste Manage. 2009;29:1779–1784.
- Brantley SL. Kinetics of mineral dissolution: kinetics of water-rock interaction. New York: Springer; 2008. p. 151–210.
- Kumpiene J, Lagerkvist A, Maurice C. Stabilization of Pb- and Cu-contaminated soil using coal fly ash and peat. Environ Pollut. 2007;145:365–373.
- Sorvari J, Antikainen R, Pyy O. Environmental contamination at Finnish shooting ranges—the scope of the problem and management options. Sci Total Environ. 2006;366:21–31.
- Hockmann K, Tandy S, Studer B, et al. Plant uptake and availability of antimony, lead, copper and zinc in oxic and reduced shooting range soil. Environ Pollut. 2018;238:255–262.
- Rodriguez-Seijo A, Lago-Vila M, Andrade ML, et al. Pb pollution in soils from a trap shooting range and the phytoremediation ability of Agrostis capillaris L. Environ Sci Pollut Res. 2016;23:1312–1323.
- Lee KY, Kim KW. Heavy metal removal from shooting range soil by hybrid electrokinetics with bacteria and enhancing agents. Environ Sci Technol. 2010;44:9482–9487.
- Pedersen KB, Jensen PE, Ottosen LM, et al. Influence of electrode placement for mobilising and removing metals during electrodialytic remediation of metals from shooting range soil. Chemosphere. 2018;210:683–691.
- Islam MN, Park JH. Immobilization and reduction of bioavailability of lead in shooting range soil through hydrothermal treatment. J Environ Manag. 2017;191:172–178.
- AEIC.Remedial Investigations and Feasibility Studies. AEI consultants, Walnut Creek, CA. 2018. Available from: https://aeiconsultants.com/services/site-investigation-and-remediation/remedialinvestigations-and-feasibility-studies/
- US Environmental Protection Agency Superfund remedial investigation/feasibility study (site characterization). U.S. Environmental Protection Agency. Washington, DC PA; 2017. Available from: https://www.epa.gov/superfund/superfund-remedial-investigationfeasibility-study-site-characterization