 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Samples of PM2.5 particulates were collected from indoor air of 36 cafés, 14 cafés in which only water pipe (WS) was used, eight in which only cigarette was smoked (CC), six in which both waterpipe and cigarette were smoked (WCC), and eight cafés in which no smoking occurred (SFC) in Tehran. After that, the concentration of lead (Pb), cadmium (Cd), nickel(Ni), and chromium (Cr) was examined by atomic absorption spectrophotometer (AAS) (GF 5000, Australia). The results indicated that the ƩMetal concentration (Mean ±SD) in WCC, WC, CC, and SFC cafés were 1118.5±50.42, 663.64±40.79, 425.57±17.55, and 79.02±5.13 ng/m3, respectively. The mean bioaccessibility of Pb, Cr, Ni, and Cd was obtained as 39.7, 31.4, 7.35, and 74.6%, respectively. The results of risk assessment indicated that exposure to heavy metals in the indoor air of smoking cafés of Tehran is considered high.
1. Introduction
Waterpipe smoking has a history of several centuries and has been commonplace in East Mediterranean, Middle east, some South African and some Asian countries including Iran. In recent years, tobacco smoking by teenagers and the youth has increased considerably, such that public health authorities have introduced waterpipe smoking as a global epidemic [Citation1-4]. Tobacco smoking annually claims around 6 million lives worldwide [Citation5]. The health effects of tobacco smoking arise from the interaction of human body and the large number of toxins present in tobacco smoke, which happens through frequent inhalations [Citation5,Citation6]. Tobacco smokers are exposed to mainstream tobacco smoke (the smoke inhaled into their body in response to puffing waterpipe or cigarette). On the other hand, non-smokers are exposed to second-hand and third-hand smokes. Second-hand smoke includes the cigarette smoke exhaled by the smoker in addition to the smoke entering the air between one puff until the next puff in response to tobacco burning. This type of smoke is the main environmental tobacco smoke (ETS), which has smaller particles and higher toxicity compared to the mainstream smoke [Citation7]. Third-hand smoke includes the smoke remaining after stubbing cigarette in the air alongside the sticky remnants of tobacco smoke, which remain on different surfaces such as clothes, furniture and wall, which can change into freeform and be inhaled further [Citation8]. The bad news is that such chemicals are not removed even after ensuring ventilation during or after tobacco smoking. In this case, the toxins that exist on different surfaces and are scattered in closed spaces such as the house or workplace through the exhalation of smokers can be a risk factor for developing different diseases including asthma, cardiovascular disease and other health problems [Citation5].
Tobacco smoking inside waterpipe cafés creates risk both for the smokers and for the staff of cafés, the public and the non-smokers exposed to environmental tobacco smoke (ETS) [Citation9]. As mentioned earlier, ETS is a source of different particular chemicals and toxic gases inside these cafés [Citation10]. Therefore, knowing how exposure to particulate matters (PM) resulting from tobacco smoke inside these cafés affects the health of exposed individuals is important. Exposure to ETS is harmful for the public especially for children and increases the risk of developing serious respiratory problems such as increase in the number and intensity of asthmatic attacks as well as the upper respiratory tract infections [Citation11]. In addition, tobacco smoke is a known carcinogenic agent for humans, and inhaling second and even third-hand smoke increases the risk of developing lung cancer as well as cardiovascular disease even in non-smokers [Citation12]. Therefore, assessment of individual exposure to dangerous particulate and gaseous pollutants inside waterpipe cafés is crucial.
Over 7000 organic and inorganic chemicals from different classes are emitted during tobacco smoking both in gaseous and particular phases into the indoor air of smoking cafés [Citation13]. Among the genera in the phase of particles, many studies have focused on heavy metals [Citation14–17]. Most of the heavy metals observed in tobacco smoke are a result of tobacco leaf burning. Tobacco is a fast-growing plant, which as with other natural trees absorbs heavy metals present in the soil into its tissues [Citation18]. Further, this plant is sensitive and thus is predisposed to develop different diseases. Therefore, many farmers have to use different chemical fertilizers, pesticides, and herbicides to protect this plant against pests. These pesticides, in turn, have usually heavy metals inside their composition, and eventually enter different parts of the tobacco plant [Citation19]. Therefore, the waterpipe smoker is exposed to large amounts of these hazardous pollutants. Different metals including arsenic, lead, cadmium, manganese, strontium, antimony and zinc have been found in tobacco smoke [Citation20]. Fromme et al. [Citation21] reported that a considerable concentration of carcinogenic metals such as lead (11.2 ng/m3), cadmium (0.38 ng/m3) and thallium (1.14 ng/m3) has been observed in the indoor air of smoking cafés. The high level of these pollutants in a closed space such as waterpipe cafés results in development of a low-quality air inside these cafés, thereby putting different groups of people at serious risks such as carbon monoxide poisoning, low birth rate of healthy babies by pregnant women, cardiovascular disease, bronchitis, asthma, lung cancer and other respiratory diseases [Citation22,Citation23]. These people include smokers, non-smokers, customers and the staff. The bioaccessibility of heavy metals in respiratory and digestive systems significantly affected the health risk evaluation of PMs, since these soluble portions are more likely to be capable of inducing toxicity [Citation24]. However, most of these studies used the total concentration of heavy metals, rather than their bioaccessible fractions to model the health risks of PMs. As a result, the health risk of heavy metals in PMs would be somewhat overestimated in this case. Meanwhile, the bioaccessibility of heavy metals in PMs from different areas might be discrepant due to their different chemic [Citation25,Citation26]. Thus, in our study, the bioaccessibility of selected metals was determined and the risk of exposure to heavy metals for individuals exposed to tobacco smoke inside these cafés was assessed based on bioaccessibility.
In Tehran, there are many smoking cafés in which different types of tobacco including fruit flavored, traditional and cigarette are offered to customers. However, unfortunately, no study has been conducted about the level of heavy metals in the indoor air of these cafés. Therefore, in the present study, for the first time the concentration of PM2.5-bounded heavy metals has been examined in the indoor air of waterpipe and cigarette cafés of Tehran. This study was designed and implemented with the following objectives 1) investigating the concentration of PM2.5-bounded heavy metals in the indoor air of waterpipe cafés with both fruit-flavored and traditional tobacco; 2) investigating the concentration of PM2.5-bounded heavy metals in the indoor air of cigarette cafés and comparing it with waterpipe cafés; 3) investigating the effect of construction characteristics and its different factors on the concentration of heavy metals in the indoor air of cafés; 4) determining the bioaccessibility of selected metals; and 5) assessing the risk of exposure to heavy metals for individuals exposed to tobacco smoke inside these cafés.
2. Materials and methods
2.1. Design of the study and selecting the sampling sites
In the present study, the quality of the indoor air of tobacco cafés in Tehran, capital of Iran, was examined. For this purpose, 36 cafés were chosen in Tehran, and the concentration of PM2.5-bounded heavy metals in their indoor air was investigated from December 2017 to March 2018. Out of this number, 14 were places in which only waterpipe was smoked (WC), eight of them were places in which only cigarette was smoked (CC), six were cafés in which both cigarette and waterpipe were smoked (WCC), and eventually eight cafés were those in which no tobacco was smoked, i.e. they were smoking-free cafés as the control group (SFC). In SFC cafés, some sources such as airborne dusts, heating and cooling equipment, cooking equipment, wall painting and wooden structures can be considered as heavy metal-releasing sources. Note that some of the cafés were located in the basement while some others were situated in the ground floor. Before beginning the sampling, first the necessary explanations for persuading the owners and managers of the selected cafés were given to acquire the permission of sampling from their indoor air. Once they were persuaded and after receiving written informed consent form, sampling operation was initiated. For each of the cafés, background information including the area of the places, mode of ventilation including natural (window opening), watercooler, air-conditioning, rate of ventilation, the number of doors and windows, the number of ventilators, number of active hookah heads, type of tobacco (fruit-flavored or traditional tobacco) and other information were collected using a predesigned checklist. The information associated with each of the cafés is provided in Table S1. Sampling from the PM2.5 particles was performed during busy working days of the day (17–21). From each of the sampling stations, samples were taken twice (once on one day during the weekdays such as Monday, and another time at the weekend such as Thursday or Friday; due to the large number of waterpipe smokers).
2.2. Sampling procedure
Note that the suction and sampling equipment for particles were calibrated before starting the sampling. Taking samples from PM2.5 particles in the indoor air of the 36 cafés of interest was performed using FRM OMNITM Air Sampler device at a flow rate of 5 L/min. The filter used in the device was a PTFE filter with 47 mm diameter and pore size of 0.2 μm to collect the PM2.5. Sampling equipment was installed at a height of 1.5 meters from the ground and the air suction was carried out for 2 hours at a flow rate of 5 liters per minute. Note Sampling was performed at peak hours of the population, usually between 6 and 8 p.m.
Before the sampling, the Teflon filters were conditioned at 20°C and humidity of 50% for 48 hours. They were then weighed using a laboratory scale (Sartorius AG, Goettingen, Germany) with sensitivity of 1 µg. To determine the concentration of PM2.5 particles, the sampled filters were re-weighed after the sampling and based on the difference between the initial and secondary weight
PM2.5 = the concentration of particulate matters with an aerodynamic diameter less than or equal to 2.5 micron
Wf = filter weight at the end of the sampling (g)
Wi = filter weight before beginning the sampling (g)
V = total passed air volume during the sampling in terms of the standard air volume (m3)
2.3. Samples digestion and their chemical analysis
To specify the concentration of heavy metals in PM2.5, the loaded filters were acid-digested at 175°C for 5 h in a high-pressure Teflon digestion bomb with 5 ml HNO3 (69%), 2.5 ml HClO4 (70%) and 0.3 ml HF (48%). The final solution of digestion was first concentrated and then diluted with 50 ml of deionized water, and then passed through polycarbonate filters with 0.4-micron pore size. Eventually, the digested samples were analyzed by atomic absorption spectrophotometer (AAS) (GF 5000, Australia) to determine the concentrations of Pb, Cd, Cr and Ni. For quality control and quality assurance, the devices were calibrated on a daily basis by calibration standards. A method blank, a spiked blank, a matrix spike and replication of samples were performed for each batch of the samples. The recovery value for the four metals in the spiked blank samples was obtained within the range of 74.3–112% with standard deviation of less than 10%, and for the four metals in the matrix spike samples, it was obtained within the range of 83.5–114.2% with standard deviation of around 10.7%. Very trace amounts were observed for some metals in the method blank samples. These values were properly deducted from the values read in the samples. The limits of quantification (LOQs) of the method for Pb, Cd, Cr and Ni were obtained as 0.033, 0.02, 0.032 and 0.025 µg/g, respectively.
To evaluate the oral bioaccessibility of heavy metals, physiologically based extraction test (PBET) was performed through simulating to chemical conditions of the human digestive system. To perform this test, the method employed by Moreda-Piñeiro [Citation27] as well as by Kang et al. [Citation28] was adopted with some modifications. The entire digestion process in this test was performed under absolute darkness conditions and simulated as human stomach. Briefly, one-fourth (1/4) of the filters was caught and added to propylene tube 10 ml (100 ml polypropylene tubes) containing 5 mL of gastric solution (containing 2 g/L of pepsin in 0.15 M NaCl acidified with HCl up to pH = 1.8). Then, under darkness conditions at 37°C, it was shaken for 2.5 h at 120 rpm, which was next centrifuged for 10 min at 3000 r/min. It was eventually filtered by Whatman filter paper 5 C and 0.45 µm syringe filter. The materials remaining in the reaction tube were re-added to the intestinal juice (containing 2 g/L pancreatin, 2 g/l amylase and 5 g/L bile salts in 0.15 M NaCl, pH = 6.8) and shaken under darkness condition at 37°C for 4 h at 100 rpm. Next, after dropwise addition of concentrated HCl, it was centrifuged for 5 min at 10000 r/min and eventually filtered by Whatman filter paper 5 C and 0.45 µm syringe filter. Finally, the resulting solutions were kept at 4°C until analysis of heavy metals by atomic absorption spectrophotometer (AAS, GF 5000, Australia).
After determining the concentration of metals, the bioaccessibility percentage of each of the metals was calculated by EquationEq. (2)(2)
(2) :
Where:
The numerator is the concentration of heavy metals inside the stomach and intestines as obtained by PBET, while the denominator is the total concentration of metals [Citation29].
2.4. Estimating the daily intakes of heavy metals and assessing health effects
There are three main routes for exposure of staff to heavy metals inside PM2.5 inside cafés: 1) through particles ingestion; 2) inhaling particles through mouth and nose and 3) intaking them through absorption of PM2.5-bounded heavy metals attached to the skin [Citation30]. The average daily exposure dose (ADD, mg·kg−1·day−1) of metals associated with indoor particles has been separately calculated for the three mentioned routes according to EquationEqs. (3(3)
(3) –Equation5
(5)
(5) ) and tabulated in Table S2. Note that the daily exposure dose through inhalation and skin contact was calculated based on the total concentration of heavy metals (acidic digestion), but in the route of ingestion, it was based on the oral bio-accessible concentrations [Citation31].
For Cr (as carcinogenic metal), the lifetime average daily doses (LADDs) were calculated, and its extent of carcinogenicity through the three mentioned routes was calculated by EquationEqs. (6(6)
(6) –Equation8
(8)
(8) ).
The hazard quotient (HQ) was used to calculate the non-carcinogenic effects of heavy metals associated with PM2.5 in the indoor air of the cafés. The hazard index (HI) refers to sum of HQs for different materials or different routes. Similarly, to estimate individual exposure to carcinogenic hazard throughout the life, cancer risk (CR) was calculated. The total cancer risk (TR) refers to the sum of CR resulting from exposure to the three routes. The potential carcinogenic and non-carcinogenic hazards resulting from the heavy metals were calculated by EquationEqs. (9(9)
(9) –Equation12
(12)
(12) ).
In these equations, Rfd represents the homologous reference dose and SF is the homologous slope factor. In this research, Rfd and SF values for metals were taken from the values presented by EPA [Citation32]. Hexavalent chromium (Cr(VI)) is far more toxic than trivalent chromium (Cr(III)). In this study, the toxicity of Cr(VI) was used to express the worst Cr status, where Rfd and SF of hexavalent Cr were assumed for the total Cr [Citation32,Citation33]. Rfd and SF values used in this study are presented in . If HI value is less than 1, no considerable non-carcinogenic effects threaten the individuals exposed to PM2.5 in the indoor air of cafés. However, values larger than 1 for HI suggest that there is the possibility of incidence of non-carcinogenic effects for exposed individuals, where the risks increase with elevation of HI value [Citation34]. Concerning carcinogenic metals, CR values between 1 × 10−4-1 × 10−6 represent acceptable or tolerable risk, higher than 1 × 10−4 is acceptable and values less than 1 × 10−6 have no carcinogenic effect [Citation35].
Table 1. Descriptive statistics of concentrations (ng.m−3) of Pb, Cd, Cr and Ni in indoor PM2.5 collected from WCC, WC, CC and SFC cafés
Table 2. Health risk from heavy metals in the studied indoor PM2.5 in WCC, WC, CC and SFC cafés
2.5. Statistical analyses
The data were analyzed by SPSS. Normality of data was evaluated using Kolmogorov–Smirnov (K-S) test. The significance of the difference between metal concentrations in the indoor air of smoking cafés and that of smoking-free cafés was examined using t-test. Level of significance of tests was considered as 0.05 and 0.01 (confidence level 95% and 99%). Path analysis was performed to determine the factors affecting the significance of the concentration of pollutants inside the waterpipe cafés using Amos 21.
3. Results and discussion
3.1. Heavy metal concentration in indoor air of smoking cafés
Descriptive statistics of the concentration obtained for the four metals of Pb, Cd, Cr and Ni in PM2.5 collected from the WCC, WC, CC and SFC are presented in . As can be seen, the metals were detected from all of the samples collected from these cafés. This suggests that smoking tobacco is an important source for emission of heavy metals in the indoor air of these cafés. It can also be suggested that PM2.5 is a source of aggregation of heavy metals in the indoor air [Citation36,Citation37]. The ƩMetal lied within the ranges of 1030–1235 with the mean of 1118 ng/m3 in WCC, 557–779 with the mean of 663 ng/m3 in WC, 374–469 with the mean of 425 ng/m3 in CC and 71.4–101.1 with the mean of 83.48 ng/m3 in SFC. The results of this study have shown high concentrations of heavy metals in the indoor air of these cafés, which can create considerable hazards for the public health. These results have been congruent with the findings of other studies elsewhere in the world which had reported elevated concentrations of PM, CO and other pollutants including polycyclic aromatic hydrocarbons, nitrogen oxide, black carbon, air nicotine and volatile air compounds inside cafés [Citation38,Citation39].
The statistical analysis also indicated that the concentration of ƩMetal in the different café was in the order of WCC>WC>CC>SFC. As can be observed in this order, the concentration of heavy metals has been higher in the indoor air of waterpipe cafés than in their cigarette counterparts. Smoking topography research has reported that smoking one cigarette involves 10–12, 50-ml puffs, while smoking one session of waterpipe lasting 45–60 min can include 100 around 500-ml puffs. Therefore, larger amounts of tobacco smoke are emitted in each puff into the indoor air of cafés [Citation41,Citation42]. In addition, cooling by water, flavorings and sweeteners of tobacco in waterpipe cause more and deeper puffs [Citation43][Citation43]. This extent of inhalation in a 45–60 min session of smoking waterpipe is very alarming because waterpipe smoke contains large amounts of combustion products including heavy metals, PAHs, BTEX, formaldehyde, etc. In an experimental study conducted by Thomas Eissenberg et al. [Citation44], it was found that the volume of produced smoke and the amounts of the pollutants including carbon oxide, tar and nicotine were larger from waterpipe than from cigarettes, which has been congruent with the findings of the present study.
Generally, the pattern of distribution of metals in the study cafés has been as follows:
Pb>Ni>Cr>Cd
As can be observed, the maximum concentration among the studied heavy metals in the indoor air of cafés is related to lead. The concentration of this metal lied within the following ranges: 734–887 with the mean of 797 ng/m3 in WCC, 444–589 with the mean of 514 ng/m3 in WC, 323–382 with the mean of 346 ng/m3 in CC and 55.01–74.5 with the mean of 62.88 ng/m3 in SF. Lead is a highly toxic metal and can exert serious effects on the brain, nervous system and bone marrow cells [Citation45]. In a study by Omari et al. to determine the concentration of heavy metals present in cigarette smoked in the brands sold in Kenya, it was reported that lead had the maximum concentration among the heavy metals [Citation46]. The mean total concentration of nickel in the indoor air of the WCC, WC, CC and NSC cafés was 174.41 ± 6.17, 79.39 ± 10.13, 41.56 ± 2.14 and 10.98 ± 1.48 ng/m3, respectively. Tobacco plant absorbs nickel from the soil of and accumulates it in its leaves, which in response to burning, it enters the indoor air of cafés as particulate matters [Citation47]. There is little information about the dynamics and extent of precipitation of nickel metal particles in the human respiratory tract and its subsequent stages such as absorption, distribution and excretion from the body [Citation48]. In the studies conducted by Nada et al. [Citation49] in Egypt and Perez-Bernal et al. [Citation50] in Spain, large amounts of nickel were reported in the cigarette samples, which is in line with the findings of the present research. The mean concentration of Cr metal in the indoor air of WCC, WC, CC and NSC cafés was 101.33 ± 4.72, 44.92 ± 4.25, 26.12 ± 1.71 and 6.35 ± 2.24 ng/m3, respectively. In previous studies, observations regarding existence of Cr in the smoke and ash of tobacco have also been reported [Citation51]. Accumulation of Cr in lung tissue is associated with a history of smoking, and it has been confirmed that Cr reaches the lungs in some forms [Citation52]. It has been reported that Cr concentration is significantly higher across all of the five lobes of the lungs of smokers than those of non-smokers [Citation53]. Therefore, Cr in the indoor air of the studied cafés is a public health concern which should be addressed. The mean concentration of cadmium metal in the WCC, WC, CC and NSC cafés was 43.75 ± 4.02, 24.42 ± 3.98, 11.01 ± 1.05 and 3.21 ± 0.61 ng/m3, respectively. According to International Agency for Research on Cancer (IARC), cadmium has been classified as group 1 carcinogen (definite carcinogen for humans) [Citation54], which is highly toxic for the kidneys, bone, nervous system, respiratory system and bloodstream [Citation55]. Elevation of blood cadmium is a story associated with increased peripheral artery disease [Citation56]. Previous studies have also reported the relationship between urinary cadmium and increased peripheral artery disease [Citation57], relationship between exposure to cadmium, cigarette smoking and pancreatic cancer [Citation56] and the relationship between cadmium exposure, smoking and diabetes [Citation57]. The biological half-life of cadmium is 13.6–23.5 years, and thus due to its long biological half-life, it can have bioaccumulation in different parts of the body of smokers [Citation58]. In another study, the relationship between history of smoking and cadmium accumulation in the lung tissue has been reported [Citation52].
3.2. Factors influencing metal levels
Among the factors affecting emission of pollutants in the indoor air of cafés, active waterpipe heads, type of tobacco and the floor where the café was located were examined. As can be seen in , the concentration of heavy metals inside the cafés was higher during the weekend sessions than during the week-day sessions. Specifically, the mean±SD of lead concentration in the indoor air of WCC, WC, CC and SFC cafés was 772.16 ± 40.21, 488.07 ± 27.05, 325.37 ± 24.87 and 58.62 ± 6.89 ng/m3 respectively during the weekday sessions. These concentrations at weekends in these cafés reached 821.83 ± 50.45, 541.75 ± 30.34, 368.25 ± 24.57 and 67.12 ± 8.15ng/m3, respectively. During the week-day sessions, the mean±SD of cadmium concentration in the WCC, WC, CC and SFC cafés was 36.50 ± 3.24, 21.71 ± 3.08, 9.10 ± 0.89 and 3.10 ± 0.38 ng/m3 respectively. In the weekend session, the values were 51.04 ± 5.74, 27.14 ± 4.89, 13.05 ± 1.33 and 3.25 ± 0.77ng/m3, respectively. During the week-day sessions, the mean±SD of Cr concentration in the WCC, WC, CC and SFC cafés was 93.85 ± 3.61, 40.51 ± 4.26, 23.50 ± 2.05 and 6.13 ± 0.26ng/m3, respectively. In the weekend sessions, the values were 108.83 ± 5.96, 49.37 ± 5.19, 28.74 ± 1.68 and 6.62 ± 0.57ng/m3, respectively. During the week-day sessions, the mean±SD of nickel concentration in the WCC, WC, CC and SFC cafés was 165.83 ± 6.87, 73.57 ± 9.66, 35.25 ± 2.77 and 10.06 ± 1.08ng/m3, respectively. Eventually, the values for the weekend were 187.00 ± 6.84, 85.21 ± 10.74, 47.87 ± 3.41 and 11.92 ± 2.88ng/m3, respectively (). Path analysis was used to investigate the effect of different properties and factors in the emission of pollutants inside the studied cafés. The results of this analysis showed that from among the factors selected in the study, ‘active waterpipe heads’ had the maximum impact in releasing of metals inside the cafés. With this analysis, the modulus standardized effect sizes (MSES) for the active waterpipe heads number was obtained as 0.49. In the weekend vacations, people have more time and come to these cafés more often to spend their time. Large amounts of heavy metals in the weekend sessions can be attributed to the higher number of active waterpipe heads in the weekend sessions.
In terms of the type of tobacco, out of 14 waterpipe cafés, fruit-flavored and traditional tobaccos were smoked in 8 and 6 of them, respectively. The mean concentration of metals was significantly higher in waterpipe cafés serving with flavored tobacco (). The mean±SD of the concentration of Pb, Cd, Ni and Cr in the waterpipe cafés with fruit-flavored tobacco was 538.83 ± 24.48, 27.55 ± 2.79, 86.44 ± 5.81 and 48.66 ± 2.92 ng/m3, respectively. These values in waterpipe cafés with traditional tobacco were 471.81 ± 14.49, 18.79 ± 0.87, 66.69 ± 3.64 and 38.20 ± 1.92 ng/m3 respectively. The tobacco used in preparing waterpipe is possibly the main source of generation of air pollutants. In comparison to cigarette smoke, different types and values of pollutants are produced in response to smoking waterpipe. More exposure to high molecular weight PAHs and benzene but less exposure to acrolein, propylene oxide, acrylonitrile, butadiene-1,3, nitrosamines, ethylene oxide and low molecular weight PAHs have been reported in waterpipe cafés in comparison to cigarette cafés [Citation59]. Different production rates of air pollutants by different tobaccos have been observed in previous studies [Citation44,Citation60,Citation61]. According to the results of path analysis, the ‘tobacco type’ with MSES = 0.33 has been the second influential factor in emitting the pollutants selected in this study. Specifically, in WCs in which fruit-flavored tobacco was used, the extent of production of metals was significantly higher than in WCs in which traditional tobacco was served. The higher concentration of heavy metals in fruit-flavored cafés might be attributed to the time necessary for smoking waterpipe with different types of tobacco. Waterpipes containing flavored tobacco take at least 4 times longer to smoke than their traditional counterparts. This may be owing to the soft and tasty smoke of flavored tobacco along with the tendency of young customers to spend more time on smoking this type of waterpipe [Citation61].
Figure 1. Average concentration of lead in indoor air of smoking cafés according to the ‘tobacco type’ and ‘the floor level’
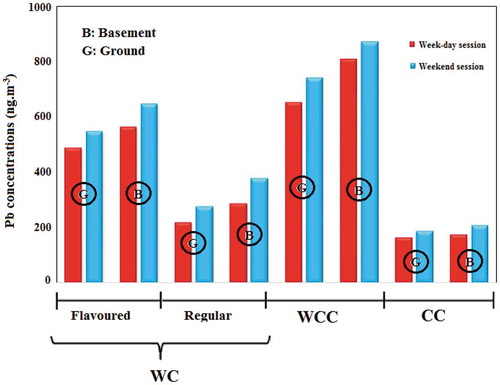
Figure 2. Average concentration of cadmium in indoor air of smoking cafés according to the ‘tobacco type’ and ‘the floor level’
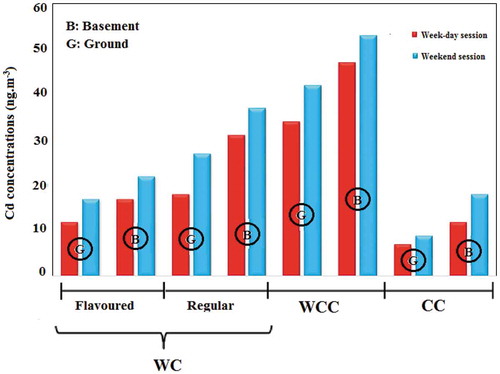
Figure 3. Average concentration of nickel in indoor air of smoking cafés according to the ‘tobacco type’ and ‘the floor level’
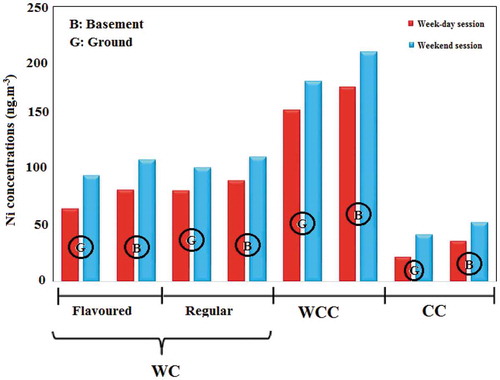
Figure 4. Average concentration of chromium in indoor air of smoking cafés according to the ‘tobacco type’ and ‘the floor level’
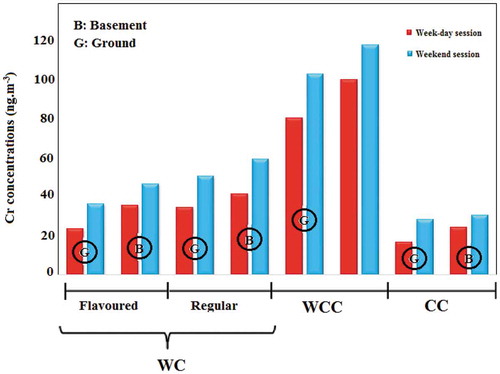
In addition, flavored tobaccos have large amounts of organic chemicals, aroma, essences and flavoring additives, which are added during the manufacturing process to this type of tobaccos. The high heavy metal concentration in these cafés can be attributed to these chemical compounds [Citation62]. Similar results have been reported by previous studies in production of PM, CO and benzene, toluene, ethyl benzene and xylene (BTEX) [Citation40, Citation63, Citation64]. Further, the concentration of pollutants was significantly higher in cafés located in the basement than those situated in the ground-floor (). According to the results of path analysis, MSES = 0.19 was obtained for the café location floor. Basements are usually confined places which have walls with no holes with very limited natural ventilation. Since ventilation is an influential factor in treating the air inside places and cafés, the cafés located in the basement expectedly suffered larger amounts of pollutants.
3.3. Bioaccessibility and health risk assessment
The results obtained from PBET test are presented in . As can be seen, the mean bioaccessibility of Pb, Cr, Ni and Cd was obtained as 39.7%, 31.4%, 7.35% and 74.6%, respectively. PBET results can represent how much of the ingested PM2.5-bounded heavy metals are dissolved in stomach and intestines. These findings have been in line with the results of other studies suggesting that the bioaccessibility of different metals is largely different [Citation34] . The difference in the bioaccessibility percentage of metals can be attributed to the fact that different forms of heavy metals may occur in the stomach and digestive system [Citation65]. The results of bioaccessibility of metals were used to determine the daily exposure dose through ingestion and assessing the risk caused by the uptake of heavy metals in this pathway.
HQ values and the carcinogenicity hazard of heavy metals are presented in , while shows the HI values. Among the carcinogenic metals, only Cr was analyzed and its carcinogenicity hazard through the three routes of exposure including ingestion, inhalation and skin contact was assessed. As observed in , the carcinogenicity hazard values of this metal for the staff of WCC cafés through ingestion, inhalation and skin contact have been 2.9 × 10−3, 7.4 × 10−3 and 1.3 × 10−5, respectively. For WC staff, these values were 1.2 × 10−4, 7.1 × 10−4 and 8.5 × 10−6. These values for the CC cafés were 5.6 × 10−5, 1.3 × 10−4 and 8.1 × 10−7, respectively. Eventually, for SFC staff, these values were 2.7 × 10−6, 1.3 × 10−5 and 3.3 × 10−10, respectively. As can be seen, the sum of carcinogenicity hazard in WCC, WC and CC has been higher than 1*104, which is unacceptable limit. However, in SFC cafés, the value has been between 1*104 and 1*106, lying within the acceptable range [Citation35]. Concerning the non-carcinogenicity hazard, it is observed that the HI calculated for WCC, WC, CC and SFC has been 12.98, 6.57, 3.93 and 0.217, respectively. As can be seen, the non-carcinogenicity risk in waterpipe and cigarette cafés has extremely exceeded the safe limit recommended by EPA (HI = 1). However, in SFCs, it is below this recommended limit [Citation31, Citation35] . These results suggest that smoking tobacco has been the main cause of emission of metals in the indoor air of waterpipe and cigarette cafés, significantly jeopardizing the health of staff, customers and the public.
Table 3. Hazard Index from PM2.5-heavy metal exposure in indoor air of WCC, WC, CC and SFC cafés
3.4. Uncertainty and limitations associated with the risk assessment
Several studies have established an exposure–response relationship between the level/duration of exposure to heavy metals and the occurrence of heavy metal poisoning [Citation64]. However, the quantitative relationship between intake and risk is still not confirmed due to uncertainties associated with the prevalence of heavy metal poisoning and inhalation of heavy metals contaminated-air. For examples, the amount of the heavy metal consumed by the individual is an important factor in the risk estimate. In our estimation, we assume that heavy metal in indoor air of waterpipe/cigarette cafés is the only source of daily heavy metal intake. However, several studies have proved that heavy metals in drinking water, vegetables, cereals, root crops, soil, etc., can pose significant health risk. Hence, our assumptions could lead to uncertainties of heavy metals exposure and underestimation of the risk. Therefore, future studies on risk assessment should consider taking more data from each exposure pathways to reduce the uncertainty in the risk estimate.
Conclusion
Although the present study was the first to deal with the concentration, bioaccessibility and risk assessment caused by exposure to heavy metals in the indoor air of waterpipe, cigarette and smoking-free cafés in Tehran, it had some limitations. Despite these, it offered valuable results indicating that the concentration of heavy metals is considerably high in the indoor air of smoking cafés in Tehran, such that it can create serious hazards for the health of both the staff and customers. The results of the study also indicated that those who consume waterpipes with fruit-flavored tobacco are exposed to larger amounts of hazardous heavy metals. Therefore, the risk of developing cancer and non-cancer chronic diseases is higher in these individuals. In addition, the cafés located in the basement accumulate more amounts of pollutants inside them and therefore pose more serious risks to the health of customers because of poor ventilation system or even its absence. This study also examined the bioaccessibility of the metals. The results found that the bioaccessibility of different metals had significant differences with other, which can be due to the fact that different forms of heavy metals may occur in the stomach and digestive system. Further, risk assessment caused by exposure to heavy metals in the indoor air of these cafés showed that the carcinogenicity and non-carcinogenicity risk values inside WCC, WC and CC cafés have exceeded the recommended value by EPA. Therefore, it is essential that further and better studies and monitoring be regulated on these environments and proper controlling policies should be adopted for this public health threat.
Acknowledgments
This research work was financially supported by Tobacco Control Research Center (TCRC) (Grant No. TCRC/IATA1396-10), we gratefully acknowledge them.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Shankar A, Yuan J-M, Koh W-P, et al. Morbidity and mortality in relation to smoking among women and men of Chinese ethnicity: the Singapore Chinese health study. Eur J Cancer. 2008;44:100–109.
- Asfar T, Ward KD, Eissenberg T, et al. Comparison of patterns of use, beliefs, and attitudes related to waterpipe between beginning and established smokers. BMC Public Health. 2005;5:19.
- Prasad JB, Dhar M. Tobacco use in India and its states: burden of smoking and smokeless forms of tobacco (2015–25) and its predictors. J Cancer Policy. 2017;14:21–26.
- Simansalam S, Hadijah Shamsudin S, Mohamed MHN. Malaysian pharmacy students’ intention to provide smoking cessation counseling. Curr Pharm Teach Learn. 2017;9:918–924.
- Drago G, Perrino C, Canepari S, et al. Relationship between domestic smoking and metals and rare earth elements concentration in indoor PM 2.5. Environ Res. 2018;165:71–80.
- Eissenberg T. Now is the time for effective regulation regarding tobacco smoking using a waterpipe (Hookah). J Adolesc Health. 2019;64:685–686.
- Öberg M, Jaakkola MS, Woodward A, et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146.
- Winickoff JP, Friebely J, Tanski SE, et al. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics. 2009;123:e74–e79.
- Gurung G, Bradley J, Delgado-Saborit JM. Effects of shisha smoking on carbon monoxide and PM2. 5 concentrations in the indoor and outdoor microenvironment of shisha premises. SciTotal Environ. 2016;548:340–346.
- Sleiman M, Gundel LA, Pankow JF, et al., 2010. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proceedings of the National Academy of Sciences 107, 6576–6581.
- Simoni M, Carrozzi L, Baldacci S, et al. The Po River Delta (north Italy) indoor epidemiological study: effects of pollutant exposure on acute respiratory symptoms and respiratory function in adults. Arch Environ Health. 2002;57:130–136.
- ARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, International Agency for Research on Cancer. Tobacco smoke and involuntary smoking. Iarc; 2004.
- Rodgman A, Perfetti TA. The chemical components of tobacco and tobacco smoke. CRC press; 2016.
- Lugon-Moulin N, Zhang M, Gadani F, et al. Critical review of the science and options for reducing cadmium in tobacco (Nicotiana tabacum L.) and other plants. Adv Agron. 2004;83:112–181.
- Arora M, Weuve J, Schwartz J, et al. Association of environmental cadmium exposure with periodontal disease in US adults. Environ Health Perspect. 2009;117:739.
- Safari Y, Karimaei M, Sharafi K, et al. Persistent sample circulation microextraction combined with graphite furnace atomic absorption spectroscopy for trace determination of heavy metals in fish species marketed in Kermanshah, Iran, and human health risk assessment. J Sci Food Agric. 2018;98:2915–2924.
- Verma S, Yadav S, Singh I. Trace metal concentration in different Indian tobacco products and related health implications. Food Chem Toxicol. 2010;48:2291–2297.
- Pappas R, Polzin G, Watson C, et al. Cadmium, lead, and thallium in smoke particulate from counterfeit cigarettes compared to authentic US brands. Food Chem Toxicol. 2007;45:202–209.
- Sebiawu GE, Mensah NJ, Ayiah-Mensah F. Analysis of heavy metals content of tobacco and cigarettes sold in Wa Municipality of Upper West Region, Ghana. Analysis. 2014;25.
- Swami K, Judd CD, Orsini J. Trace metals analysis of legal and counterfeit cigarette tobacco samples using inductively coupled plasma mass spectrometry and cold vapor atomic absorption spectrometry. Spectrosc Lett. 2009;42:479–490.
- Fromme H, Dietrich S, Heitmann D, et al. Indoor air contamination during a waterpipe (narghile) smoking session. Food Chem Toxicol. 2009;47:1636–1641.
- Akl EA, Gaddam S, Gunukula SK, et al. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol. 2010;39:834–857.
- Arfaeinia H, Moradi M, Sharafi K, et al. Evaluation of public health impacts related to urban air pollution in Shiraz and Bushehr, Iran. Int J Pharm Technol. 2015;7:9811–9824.
- Bi X, Li Z, Sun G, et al. In vitro bioaccessibility of lead in surface dust and implications for human exposure: A comparative study between industrial area and urban district. J Hazard Mater. 2015;297:191–197.
- Haghnazari L, Mirzaei N, Arfaeinia H, et al. Speciation of As (ΙΙΙ)/As (V) and total inorganic arsenic in biological fluids using new mode of liquid-phase microextraction and electrothermal atomic absorption spectrometry. Biol Trace Elem Res. 2018;183:173–181.
- Walraven N, Bakker M, Van Os B, et al. Factors controlling the oral bioaccessibility of anthropogenic Pb in polluted soils. SciTotal Environ. 2015;506:149–163.
- Moreda-Piñeiro J, Moreda-Piñeiro A, Romarís-Hortas V, et al. In-vivo and in-vitro testing to assess the bioaccessibility and the bioavailability of arsenic, selenium and mercury species in food samples. Trends Analyt Chem. 2011;30:324–345.
- Kang Y, Man YB, Cheung KC, et al. Risk assessment of human exposure to bioaccessible phthalate esters via indoor dust around the Pearl River Delta. Environ Sci Technol. 2012;46:8422–8430.
- Kang Y, Cheung KC, Wong MH. Mutagenicity, genotoxicity and carcinogenic risk assessment of indoor dust from three major cities around the Pearl River Delta. Environ Int. 2011;37:637–643.
- Du Y, Gao B, Zhou H, et al. Health risk assessment of heavy metals in road dusts in urban parks of Beijing, China. Procedia Environ Sci. 2013;18:299–309.
- Huang H, Jiang Y, Xiaoyun X, et al. In vitro bioaccessibility and health risk assessment of heavy metals in atmospheric particulate matters from three different functional areas of Shanghai, China. SciTotal Environ. 2018;610:546–554.
- Cheng Z, Chen L-J, Li -H-H, et al. Characteristics and health risk assessment of heavy metals exposure via household dust from urban area in Chengdu, China. SciTotal Environ. 2018;619:621–629.
- Huang M, Chen X, Zhao Y, et al. Arsenic speciation in total contents and bioaccessible fractions in atmospheric particles related to human intakes. Environ Pollut. 2014;188:37–44.
- EPA U. Risk assessment guidance for superfund: volume 3—process for conducting probabilistic risk assessment chapter l, Part A. Washington, IX; 2001.
- EPA A. Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A). EPA/540/1-89/002; 1989.
- Górka-Kostrubiec B. The magnetic properties of indoor dust fractions as markers of air pollution inside buildings. Build Environ. 2015;90:186–195.
- Olujimi O, Steiner O, Goessler W. Pollution indexing and health risk assessments of trace elements in indoor dusts from classrooms, living rooms and offices in Ogun State, Nigeria. J Afr Earth Sci. 2015;101:396–404.
- Hazrati S, Rostami R, Fazlzadeh M. BTEX in indoor air of waterpipe cafés: levels and factors influencing their concentrations. SciTotal Environ. 2015;524:347–353.
- Weitzman M, Yusufali AH, Bali F, et al. Effects of hookah smoking on indoor air quality in homes. Tob Control. 2017;26:586–591.
- WHO. Media centre. 2017. [cited 2017 Oct].. Available from: 〈http://www.who.int/mediacentre/factsheets/fs339/en/〉
- Maziak W, Rastam S, Ibrahim I, et al. CO exposure, puff topography, and subjective effects in waterpipe tobacco smokers. Nicotine Tob Res. 2009;11:806–811.
- de Sousa Viana GF, Garcia KS, Menezes-Filho JA. Assessment of carcinogenic heavy metal levels in Brazilian cigarettes. Environ Monit Assess. 2011;181:255–265.
- Association, A.L. An emerging deadly trend: waterpipe tobacco use. Washington: American Lung Association; 2007.
- Eissenberg T, Shihadeh A. Waterpipe tobacco and cigarette smoking: direct comparison of toxicant exposure. Am J Prev Med. 2009;37:518–523.
- Omari MO, Kibet JK, Cherutoi JK, et al. Heavy metal content in mainstream cigarette smoke of common cigarettes sold in Kenya, and their toxicological consequences. Int Res J Environ Sci. 2015;4:75–79.
- Pappas RS. Toxic elements in tobacco and in cigarette smoke: inflammation and sensitization. Metallomics. 2011;3:1181–1198.
- ATSDR. Draft toxicological ProWle for nickel. US Department of Health and Human,Atlanta, Georgia Services; 2003.
- Nada A, Abdel-Wahab M, Sroor A, et al. Heavy metals and rare earth elements source-sink in some Egyptian cigarettes as determined by neutron activation analysis. Appl Radiat Isot. 1999;51:131–136.
- Pérez-Bernal JL, Amigo JM, Fernández-Torres R, et al. Trace-metal distribution of cigarette ashes as marker of tobacco brands. Forensic Sci Int. 2011;204:119–125.
- Sógor C, Gáspár A, Posta J. Flame atomic absorption spectrometric determination of total chromium and Cr(VI) in cigarette ash and smoke using flow injection/hydraulic high-pressure sample introduction. Microchem J. 1998;58:251–255.
- Pääkkö P, Kokkonen P, Anttila S, et al. Cadmium and chromium as markers of smoking in human lung tissue. Environ Res. 1989;49:197–207.
- Tsuchiyama F, Hisanaga N, Shibata E, et al. Pulmonary metal distribution in urban dwellers. Int Arch Occup Environ Health. 1997;70:77–84.
- IARC. 2009. IARC monographs on the evaluation of carcinogenic risks to humans. Available from: http://monographsiarcfr/ENG/Classification/crthgr01php
- ATSDR. Toxicological profile for cadmium.Vol. 20, 2008. p. 43–189. http://wwwatsdrcdcgov/ToxProfiles/tp5-ppdf
- Navas-Acien A, Selvin E, Sharrett AR, et al. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201.
- Schwartz GG, Reis. IM. Is cadmium a cause of human pancreatic cancer? Cancer Epidemiol Prev Biomarkers. 2009;9:139–145.
- Schwartz GG, Il’yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26:468–470.
- Suwazono Y, Kido T, Nakagawa H, et al. Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers. 2009;14:77–81.
- Jacob P, Raddaha. AH, Dempsey D, et al. Comparison of nicotine and carcinogen exposure with water pipe and cigarette smoking. Cancer Epidemiol Prev Biomarkers. 2013;22:765–772.
- Baker RR, Proctor CJ. The origins and properties of environmental tobacco smoke. Environ Int. 1990;16:231–245.
- Fazlzadeh M, Rostami R, Hazrati S, et al. Concentrations of carbon monoxide in indoor and outdoor air of Ghalyun cafes. Atmos Pollut Res. 2015;6:550–555.
- Farley SM, Schroth KR, Grimshaw V, et al. Flavour chemicals in a sample of non-cigarette tobacco products without explicit flavour names sold in New York City in 2015. Tob Control. 2018;27:170–176.
- Gholamreza Heydari FT, Fazlzadeh M, Jafari AJ, et al. Levels and health risk assessments of particulate matters (PM2.5 and PM10) in indoor/outdoor air of waterpipe cafés in Tehran. Iran Environ Sci Pollut Res. 2019;1–11.
- Obiri S, Dodoo D, Essumang D, et al. Cancer and non-cancer risk assessment from exposure to arsenic, copper, and cadmium in borehole, tap, and surface water in the Obuasi municipality, Ghana. Human Ecol Risk Assess. 2010;16:651–665.
- Wang J, Li S, Cui X, et al. Bioaccessibility, sources and health risk assessment of trace metals in urban park dust in Nanjing, Southeast China. Ecotoxicol Environ Saf. 2016;128:161–170.
