ABSTRACT
Commissioned and de-commissioned shooting ranges continue to pose an environmental and human health risk due to the accumulation of toxic Pb emanating from spent munitions. The phytotoxic effects of Pb accumulation in plants include inhibition of root growth and lowering of plant metabolism. The uptake of Pb by plants is directly affected by factors such as plant species and physicochemical properties of the soil. However, scientists and researchers have leveraged on the ability of some plant species to accumulate and tolerate Pb toxicity and applied them in the control and management of Pb pollution of shooting range soils. This technique is called phytoremediation. The objectives of this review are: (i) to assess the prevalence of toxic Pb metal in plant species growing in and nearby shooting ranges, (ii) to establish the soil-plant mechanistic pathway for Pb (iii) discuss the effectiveness of phytoremediation technology towards shooting range soil amendment.
1. Introduction
Vegetation and crops in agricultural fields in the vicinity of shooting ranges are at risk of pollution from Pb emanating from the use of Pb-containing munitions [Citation1–3]. The extended residence time of Pb in soils due to its insoluble mineralogical products and lack of deterioration from microbial activity means that the bioavailability and bioaccessibility of this toxic heavy metal can exist in the soil for a very long time [Citation1]. In addition, the half life of Pb in the soil is approximated to be in the range of 740 to 5900 years [Citation4]. The background concentrations of Pb in soils lie within the range of 10 to 30 mg/kg [Citation4]. The distribution and accumulation of Pb in soils arising from anthropogenic activities such as shooting practices is well documented [Citation5–7]. Total Pb concentrations of up to 1 × 104 μg/g have been reported in shooting range soils [Citation8]. Furthermore, large quantities of used Pb containing projectiles amounting to 1 × 108 spent Pb shots per hectare have been recovered in shooting range soils [Citation8]. In most studies, total Pb concentrations in shooting range soils were found to be exceedingly higher than the set regulatory limits. For example, in Norway, total Pb concentration of 33,000 mg/kg was established in shooting range soil [Citation9]. This shooting range experienced Pb loading of 330 times the set World Health Organization maximum limit of 100 mg/kg. Similarly, the United States Environmental Protection Agency's maximum contaminant limit (MCL) of 400 mg/kg was surpassed 83 times [Citation9]. The use of Pb in industrial and household products is highly controlled and in some cases prohibited due to the detrimental effects from exposure to Pb [Citation10]. Human and animal exposure to Pb through contact with polluted soils or consumption of contaminated plant products such as fruits and vegetables can lead to severe health problems and death [Citation11,Citation12]. Blood Pb concentrations of more than 10 μg/dl have been reported in 42.4% of shooters in South Africa [Citation12].
The bioaccumulation of Pb in plants and crops cultivated near shooting ranges increases the chance of Pb migration through the food chain [Citation13,Citation14]. Total Pb concentration of 1390–1450 ppm/kg has been reported in Vetiver grass tissue in the USA [Citation15]. Similarly, total Pb concentrations of up to 70 mg/kg (dry weight) have been determined in plant leaves [Citation16]. In other studies plant roots have experienced Pb concentrations of the range 1347.2 to 3825.7 mg/kg (DW) in a shooting range soil contaminated with over 5998 mg/kg of Pb [Citation4]. The accumulation of Pb in the different plant tissues is also determined by whether the tissue is an above-ground biomass or below-ground biomass [Citation17]. Additionally, it is important to distinguish the pathway through which Pb reached plant tissues, whether it was through surface deposition on the above-surface portions of the plants or via root absorption [Citation18]. Most of the studies carried out to investigate bioaccumulation of plants growing near shooting ranges have discovered that these plants absorbed total Pb concentrations much higher than the maximum permissible limit of 2 mg/kg (in plants) set by WHO [Citation19]. The World Health Organization and the Food and Agriculture Organisation (FAO) have also set maximum permissible Pb concentration of 0.3 mg/kg (DW) in edible vegetables [Citation4]. Most of the studies that have been carried out have not satisfied this limit either. The detrimental effects of Pb absorption by plants are well documented. The accumulation of Pb in plants has been reported to decrease dry weight and photosynthesis process [Citation20]. In the same way, elevated concentrations of Pb in plants have been found to inhibit root growth, lower water absorption and plant metabolism. These observations suggest the severity of this problem to negatively affect the quality of agricultural output and the eventual migration of Pb through the food chain [Citation19].
Quantification of pollution risk from Pb towards plants has been carried out using various risk assessment methods and formulae. Pollution risk assessment indices and factors such as translocation factor (TF), biological concentration factor (BCF), biological accumulation factor (BAF) and hazard quotient (HQ) have all been used to establish the degree of Pb contamination in Plants [Citation19]. The soil physicochemical properties have a bearing on the rate of Pb uptake by plants. Soil pH plays a significant role in the solubility and bioavailability of Pb in shooting range soils. Plants growing in acidic soils tend to absorb more Pb compared to those growing in alkaline soils [Citation6]. This is due to the fact that low soil pH solubilizes Pb minerals and makes Pb bioavailable to plants [Citation6]. In addition, plants growing in sandy soils that are polluted with Pb tend to accumulate higher concentrations of Pb compared to those growing in clay soils. Pb is more bioavailable in sandy soils than in clay soils [Citation6]. On the other hand, high content of organic matter in shooting range soils has been reported to transform Pb into stable Pb-organo complexes. Elevated content of organic matter in the soil produces more carbon dioxide (CO2) resulting in formation of stable Pb compounds that are less bioavailable [Citation6]. In a related study, Darling et al. (2003), investigated Pb mobilisation in 17 shooting range soils with pH ≤ 6 and discovered that Pb dissolution was favoured in these soils and the solubility was even more in shooting ranges where the soils had low clays and organic matter [Citation21]. Over and above, the type of plant is of great influence on the amount of Pb that it can absorb. There are plants that are very effective phytoextractants and phytoaccumulants of heavy metals in the soil and these kinds of plants have been exploited by scientists and researchers in soil reclamation and remediation efforts [Citation22,Citation23]. The control and management of Pb contamination of shooting range soils by plants take place through processes called phytoremediation and phytostabilisation. These processes are able to reduce the mobility and leaching of Pb in shooting range soils by immobilizing and stabilizing it and thereby minimizing exposure to biota [Citation24]. Furthermore, phytoremediation technique has been found to be cost effective and environmentally friendly since it does not add new pollutants to the soil [Citation25]. The soil structure and composition are not disturbed when this technique is applied [Citation24]. In addition, phytoremediation occurs with the concomitant reduction of other processes such as soil erosion and decrease in dust caused by the wind and thereby reducing the deposition of Pb on above-ground plant parts [Citation18]. The objectives of this review include: (i) to discuss the pathways of Pb in shooting range soils to vegetation growing in shooting ranges; (ii) to investigate the characteristics of plants that make them excellent Pb phytoexctractants; (iii) to examine the effect of soil physicochemical properties on soil-plant Pb pathways; and (iv) to discuss in depth the economic and environmental benefits of phytoremediation strategies.
2. Prevalence of Pb in plants growing in and around shooting ranges
It is a known fact that Pb has no nutritional value in plants [Citation4]. The deposition of Pb in shooting range soils is not restricted only to the soil, plants and microorganisms that have direct and indirect contact with the polluted soils are at risk of absorbing and accumulating this toxic heavy metal in their tissues [Citation18]. In addition, Pb collected in plant tissues can reach human beings and animals through consumption of contaminated plant products [Citation13]. It is against this backdrop that scientists and researchers are continuously screening vegetation growing in shooting range soils and crops grown in agricultural fields near shooting ranges for possible contamination from Pb. A ton of evidence exists that confirms accumulation of Pb and its apparent toxicity towards vegetation found in and around shooting ranges [Citation1,Citation15,Citation16]. shows examples of studies carried out in the past 25 years that show that the uptake of Pb by plants growing in shooting ranges is a growing environmental concern.
Table 1. Recent studies carried out on Pb uptake by vegetation growing in and around shooting ranges
It can be deduced from that most studies around the world are now focussing on Pb pollution risk towards vegetation growing in and around shooting ranges. Most of the studies carried out in some countries have established that concentrations of Pb in plant tissues are higher than the countries’ established regulatory and guidance limits. In one of the first studies carried out in Finland where total Pb concentrations in shooting range soil exceeded background soil concentration of 240 mg/kg by more than 200 fold, some edible fruits such as lingonberries accumulated Pb concentrations of up to 0.3 mg/kg [Citation16]. The amount of Pb in these fruits rendered them inedible according to the Finnish food safety guideline of 0.1 mg/kg and the Food and Agriculture Organization (FAO) or World Health Organization (WHO) set limit of 0.3 mg/kg tolerated by a healthy human being [Citation29]. A positive correlation between total Pb concentration in soil, the Pb short fall zone and plant-available Pb was established in a study in England [Citation1]. The highest plant Pb uptake of up to 4102 mg/kg was experienced in soils that accumulated the highest number of Pb shots and pellets at 257 Pb pellets per soil core and highest total Pb concentration of 5000–10,620 mg/kg in the soil. Conversely, a decrease in the number of plants per square meter was experienced in soils with high total Pb concentration. In addition, plants that accumulated the highest amount of Pb (5000–10,620 mg/kg) displayed reduced stem diameters (0.6 mm) compared to plants with stem diameter of 2.88 mm and growing in soils with low Pb content (less than 500 mg/kg) [Citation1]. The most affected plant tissues in this study were the roots which accumulated up to 470 mg/kg and all the plant tissues contained Pb concentrations much greater than the set statutory limit of 20 mg/kg for Pb in edible plants and vegetables [Citation1]. The roots have been found to possess the ability to alter the soil characteristics that aid the roots to retain more Pb [Citation30]. In a similar study, Hui et al. (2002) have also shown that Pb concentration in plants correlated positively with the Pb shot and pellet densities [Citation6]. Plants growing in soil with the highest density of Pb pellets and shots reaching highs of 1,620 shot pellets/kg (dry soil) accumulated Pb concentrations of up to 18.1 mg/kg (soil Pb of 16,200 mg/kg). In contrast, plants growing in soils containing only 4 shots/kg (dry soil) and total Pb concentration of 75.1 mg/kg were able to absorb just 2.77 mg/kg in their tissues [Citation6]. Furthermore, a study in New Zealand has demonstrated a positive linear correlation between the quantity of Pb in the roots and leaves of all five plants studied and the amount of Pb discovered in the soil [Citation4]. The concentrations of Pb in the plant tissues were much greater than the WHO set critical limit of 0.3 mg/kg, reaching highs of 3825.7 mg/kg [Citation4].
The uptake of Pb by vegetation has been found to be one of the routes through which Pb moved through the food chain. Studies have been carried out that indicate that areas highly polluted with Pb pose risk to uptake of Pb by animals grazing in those areas [Citation13]. In a study by Robinson et al. (2008), Pb concentration of up to 4,640 mg/kg was recovered in the leaves of plants and this concentration was much greater than the 30 mg/kg indicated to be toxic to livestock [Citation18]. As a result, strict majors need to be taken in order to control and manage the mobility, bioavailability and bioaccessibility of Pb in shooting range soils.
3. Soil-plant Pb mechanistic pathways
Plants absorb Pb from the soil through a passive ion exchange process that takes place at an accelerated rate up to a point where ion exchange sites in the spaces not occupied by the roots are well equilibrated with the soil liquid mixture [Citation31]. There are various routes through which Pb accumulated in shooting range soils reach plant tissues [Citation1,Citation16]. After absorption by the roots, Pb is translocated into shoots through the xylem [Citation32]. It has also been established that various classes of proteins are also responsible for the translocation of Pb from the roots into the shoot [Citation32]. Proteins belonging to the CPx-type ATPases protein class have been linked to the transport of toxic heavy metals such as Pb using ATP across cell membranes [Citation33]. A large quantity of Pb is harvested from the soil through plant roots and translocated to the shoots, branches and leaves [Citation4,Citation34]. A study by Rooney et al. (1999) established higher concentration of Pb in the plants roots measuring up to 3825 mg/kg compared to 100 mg/kg in stem tissues [Citation4]. In a related study, Cao et al. (2003) recovered 750 mg/kg of Pb in plants roots compared to 420 mg/kg in the shoots [Citation5]. In another study by Fayiga et al. (2016), the roots of grasses found in three shooting ranges in Florida, USA, collected Pb concentrations of the range 1,893–5,021 mg/kg compared to the shoots that accumulated 252–880 mg/kg of Pb [Citation35]. Furthermore, in a shooting range in Spain, Pb concentrations of 33.30–1,107.42 mg/kg have been determined in the roots of A. capillaris species compared to 10.90–135.23 mg/kg found in the roots [Citation36]. Pb concentrations absorbed by plant roots and shoots were much higher than those observed in the control sites of 9.82 mg/kg in the roots and 6.43 mg/kg in the shoots [Citation36]. A more pronounced Pb content has been reported in tuberous plants such as carrots, sweet potatoes, cassava, yam and dahlias [Citation34].
The accumulation of Pb in the plant roots is made possible through the binding of Pb to ion-exchangeable sites on the cell wall and formation of Pb precipitates such as Pb-carbonates and Pb-oxides outside the cells [Citation37]. The transport of Pb from the soil into the plant roots takes place across the root-cell plasma membrane through voltage gated plasma membrane cation channels such as Ca-channels [Citation38]. Significant amount of Pb is normally found in the surface and sub-surface soil layers and a reduction in Pb concentration is observed with increasing soil depths [Citation37]. The amount of Pb absorbed by plants through the roots is largely dependent on Pb concentration in soil at the depths reached by the plant roots [Citation6]. After absorption by the roots, Pb is largely restricted to the roots because of strong Pb binding to the carboxyl groups of galacturonic acid and glucuronic acid in the cell walls of the root cells [Citation39]. The consequence of this strong binding is the restricted migration of Pb via apoplast [Citation39]. Various plant root factors directly affect the absorption of Pb from the soil by the roots. These factors include root surface area, root exudates, degree of transpiration and mycorrhization process [Citation37]. A general observation is that monocotyledons accumulate less amounts of Pb in their roots compared to dicotyledons [Citation38]. Pb translocated to other parts of plant from the roots is normally of lower amounts because of Pb precipitation and immobilisation in the cell walls of plant roots [Citation20]. Pb translocation to the shoot takes place via the root apoplast and across the cortex, collecting near the endodermis that functions as a semi-barrier to the transport of Pb from the plant roots to shoots [Citation40]. This factor contributes to higher Pb concentrations observed in plant roots than shoots [Citation41]. The casparian strips of the root endodermis are the main obstacles for Pb migration across the endodermis into the plant middle cylindrical tissue [Citation42]. The predominant pathway of Pb transport from the root to shoot is via the apoplast at lower Pb concentration. However, as the concentration of Pb increases in the roots, the restriction in mobility function of the plasmalemma is destroyed leading to large quantities of Pb entering the cells [Citation43]. This causes damage to the cell and interrupts the efficacy of the plasmalemma to act as a barrier towards Pb transport from root to shoot and intercepts the discriminatory porosity of the plasmalemma and tonoplast [Citation43].
In most cases, a decrease in the concentration of Pb in plant tissues away from the roots is observed, with relatively lowest Pb concentrations in plant tissues furthest away from the plant roots. The main reason for this observation is the increased restriction of Pb in plant root cell walls than in other organs of the plant. This is caused by strong binding of Pb in lignified root tissues than non-lignified plant tissues such as the shoots, branches and leaves [Citation43]. Contrastingly, there are cases where the translocation rate of Pb from the plant roots into the shoots may be high resulting in higher total Pb concentrations in the shoots than in the roots. This observation was made by Magaji et al. (2018) in which two of the eight plant species studied accumulated higher concentrations of Pb in the plants shoots compared to the roots [Citation44]. Pb concentrations of 12.30 and 11.01 mg/kg were discovered in the shoots of A. zygia and V. paradoxa plants respectively, compared to 8.71 mg/kg (A. zygia) and 9.02 (V. paradoxa) mg/kg found in the roots [Citation44]. However, in some cases, total concentration of Pb in plant leaves may be relatively high due to Pb deposited on the leaves surface emanating from dust collecting in the waxy cuticles of the leaves [Citation4]. The ability of plant leaves to absorb Pb depends on the morphology of the leaves [Citation37]. Furthermore, the age of the leaves can determine their ability to absorb and accumulate Pb [Citation45]. Aging leaves tend to accumulate high concentrations of Pb compared to younger leaves [Citation45]. Robinson et al. (2008) have reported concentrations in the range 50–4640 mg/kg in the leaves of 10 plant species growing in shooting range soils with Pb loading of 14,000–156,000 mg/kg [Citation18]. Pb concentrations in some of the plant leaves were reported to be 10 to 50 times higher than the set maximum toxicity level of 30 mg/kg for consumption by livestock and substantially higher than the European Union established maximum level of 0.2 mg/kg in cereal grains [Citation18,Citation46]. In a study by Selonen et al. (2012), Pb concentrations of 0.97–30 mg/kg were reported in the grass leaves growing in Pb polluted shooting range soils in Finland and providing a pathway through the food chain by being available to herbivores grazing the contaminated grass [Citation47]. Robinson et al. (2008) made an important observation, whereby Pb uptake increased acutely when total Pb concentrations in the soil reached a specific threshold of 60,000 mg/kg [Citation18]. This has an implication on the rate of Pb transport through the food chain as the rate of Pb uptake by plants increased beyond this concentration and thereby making Pb more available to herbivores. The degree and rate of Pb uptake from the soil by plants depends on factors such as plant species and the physical and chemical properties of the soil [Citation31]. In general, the concentration of Pb in different plant tissues decreases in the order: roots>leaves>stem>seeds [Citation37]. The abilities of these different plant organs to absorb Pb from the soil have been applied towards shooting range soil remediation and reclamation strategies [Citation36,Citation48].
The accumulation of Pb in above ground plant biomass can exacerbate the migration of Pb through the food chain when herbivores feed on contaminated plant materials [Citation13]. In a study by Johnsen et al. (2019), Pb concentrations of up to 5 mg/kg were determined in the faeces of sheep after consuming grasses contaminated with Pb reaching highs of 29 mg/kg [Citation13]. The livers obtained from over 32 slaughtered sheep were found to contain Pb concentration in the range 0.19–0.3 mg/kg and it is fortunate that this Pb concentration was not greater than the Pb concentration of 0–3 mg/kg (dw) regarded to be standard in the sheep livers [Citation13]. Therefore, under favourable Pb soil-plant mechanistic pathways, the rate of Pb uptake by plant would be higher resulting in higher concentrations of Pb in above ground plant organs and increased migration through the food chain.
4. Pb toxicity and tolerance in plants
Studies have shown that Pb is not an essential element in plants and does not have any nutritional value [Citation4]. However, this element has been found to occur naturally in plants, with some plants containing background concentrations of 2.1–2.5 mg/kg (DW) [Citation49]. Total Pb concentrations of 100–500 mg/kg in the soil have been reported to be toxic to plants [Citation49]. In addition, Pb concentrations of 30–300 mg/kg in plant tissues are regarded to be toxic and can lead to harmful effects such as decrease in plant dry weight, photosynthesis, root growth and a diminishing ability by the plant roots to absorb water from the soil [Citation3,Citation50]. In Finland, the growth of the pine tree was significantly reduced in an active shooting range compared to the trees growing in an abandoned shooting range [Citation51]. The stunted growth of the pine trees was believed to have been caused by the damaged roots and root connecting mycorrhizal fungi [Citation51]. The growth of the pine tree was observed after few years since cessation of shooting activities at the shooting range, an indication of reduced exposure to toxic Pb [Citation51]. The pine plants even grew taller than those found at the control site [Citation51].
Root growth is hindered by Pb accumulation in plant roots due to Pb-induced impedance of cell division in the tips of the plant roots [Citation52]. In a study by Lago-Vila et al. (2019), inhibition of root elongation was observed in three plant species growing in Pb polluted soils obtained from three shooting ranges [Citation53]. A decrease in plant root elongation from 6.66 cm to a range of 4.07–5.47 cm was observed for the sinapis alba plant species growing in three contaminated soils obtained from a Pb polluted trap shooting range (TSR). Likewise, a decrease in root elongation from 6.66 cm to the range of 3.75–4.87 cm was also observed for the same plant species growing in Pb polluted soils of a small arms firing range (SFR) [Citation53]. Root growth inhibition was also observed in the other two plant species, Lactuca sativa (1.05–42.87% root growth inhibition) and Festuca ovina (6.80–34.98% root growth inhibition) used in the same study [Citation53]. In other studies, high Pb concentrations in plant roots were found to damage the microtubules of the mitotic spindle resulting in blockage of the pro-metaphase cells caused by the induced c-mitoses [Citation54]. Other detrimental effects of Pb exposure in plants include stunted plant growth and chlorosis [Citation55]. The toxicity of Pb towards plant physiological processes is summarized in .
Table 2. Effect of Pb accumulation on plant physiological processes [Citation55]
A study in Australia observed reduction in the growth of the lettuce (Latuca sativa) shoot biomass grown for eight weeks in three different Pb polluted shooting range soils [Citation56]. The lettuce plants were able to accumulate 500–3,710 mg/kg DW of Pb and the total Pb concentrations at the four shooting ranges studied were in the range 2,330–12,167 mg/kg [Citation56].
The accumulation of Pb in plants can activate the enzyme activity or can inhibit it [Citation37]. An inhibition of most physiological processes such as hormonal functions, electron transport, membrane structure and water absorption is observed. Accumulation of Pb in plant tissues lowers the water absorption ability of the plant by destroying the cell turgidity and the flexibility of the cell walls and thereby reducing the capacity of the cells to store water [Citation57]. Stomatal closure due to increased and uncontrollable concentrations of abscisic acid (ABA) has been reported in plants with elevated concentrations of Pb [Citation58]. Pb is classified as a soft metal that has high affinity for soft donor ligands [Citation37]. Enzymes containing the thiol group (–SH) in their structure are at risk of inhibition of their activities due to complexation of Pb with thiol group of the enzyme [Citation59]. These thiol groups are usually located in the active site of the enzyme and are responsible for the enzyme functions. The thiol groups are also important stabilizers of the enzyme tertiary structure [Citation37]. Furthermore, Pb ions accumulating in plant tissues can block the carboxyl groups (–COOH) found in enzymes and thereby inhibiting the enzyme activity [Citation37]. The deposition of Pb in plant roots negatively affects their branching pattern [Citation37]. Degradation of protein molecules in plant tissues has been observed with accompanying remarkable modifications to the composition of triglyceride macromolecules [Citation60]. In plant leaves, Pb toxicity has been linked to reduced rate of chlorophyll synthesis due to its impedance of the plant uptake of nutritional elements such as magnesium and iron [Citation61]. Pb loading in plants has been reported to reduce the rate of photosynthesis due to degradation of the chloroplast, chlorophyll, carotenoids and plastoquinone [Citation3,Citation62]. Elevated concentration of Pb in plants also affects the photosynthesis chemical process through shortage of supply of carbon dioxide (CO2) caused by Pb-induced closure of the stomata [Citation62]. The complexation of Pb with protein molecules bearing the soft nitrogen (N–) and sulfur (S–) donor atoms in the chlorophyll leads to destruction of photosynthesis tools [Citation63]. The inhibition of electron transport has also been reported in the donor and acceptor sites of PSI, PSII and cytochrome b6f complex enzymes [Citation64]. Moreover, Pb has been found to occupy the place of Ca, Cl− and Mn in the oxygen-emitting extraneous polypeptide of PSII leading to the degradation of the oxygen-producing complex [Citation65]. Disruption of the respiration processes and lowering of the adenosine 5`-triphosphate (ATP) have been observed with increasing concentration of Pb in plants [Citation66]. The suppression of these processes has been linked to the disconnection of the oxidative phosphorylation [Citation66,Citation67]. The pronounced effect of Pb on the nutritional sufficiency of plants has been associated with the obstruction of entry of the cations K, Ca, Mg, Zn, Cu and Fe and nitrate ions from the soil solution into the plant roots [Citation49]. Pb is able to achieve this by altering the size of the active sites on the root surface for entry of essential elements and through Pb-induced changes in the activities and structure of enzymes found in the root membrane [Citation68]. The absorption of nitrate from the soil is also significantly lowered by Pb toxicity due to the inhibition of the activity of the nitrate reductase enzyme. This has also been linked with the disruption of the nitrogen metabolic processes [Citation69]. Elevated levels of reactive oxygen species (ROS), such as superoxide ion (O2−), hydroxyl free radicals (.OH) and hydrogen peroxide (H2O2) have been reported in plant tissues due to the harmful effects of Pb [Citation41]. Increased concentrations of these oxygen species lead to unbalanced redox reactions inside plant cells and thereby causing oxidative stress in young and developing plant organs [Citation41]. The deposition of reactive oxygen species such as hydrogen peroxide can induce oxidative deterioration of polyunsaturated fatty acids in plant tissue membrane, resulting in oxidative stress to the plant [Citation70].
Even though the toxicity of Pb towards plants is conspicuous, some plants are able to tolerate the accumulation of high levels of Pb in their tissues [Citation36,Citation44]. The translocation of Pb from the roots into above ground biomass is usually followed by sequestration and detoxification of Pb in the vacuoles of plants [Citation32]. The transportation of Pb into plant vacuole takes place via various transporter gene families including the ATP-binding cassette transporters (ABC), cation diffusion facilitator (CDF), heavy-metal ATPase (HMA) and natural resistance-associated macrophage protein (NRAMP) [Citation32]. Such plants are able to survive in Pb contaminated shooting ranges and without displaying any toxic effects from exposure to Pb. As a result, these types of plants have found use in the control and management of pollution from Pb in shooting ranges through a process called phytoremediation [Citation22,Citation25]. Plants that tolerate Pb are able to do that in two ways; (i) the ‘excluder’ technique and (ii) the accumulator technique [Citation71]. In the excluder technique, the total concentration of toxic Pb is kept at an unchanging low level up to the point of critical soil concentration when toxicity emerges and unhindered Pb transport takes place [Citation71]. The excluder plants are able to get rid of Pb through discharge of Pb precipitating chemical species such as oxalate that keeps Pb in a less toxic precipitate form inside plant tissues [Citation71]. The toxicity of Pb towards plant tissues can also be excluded by binding the Pb to carboxylate groups (–COOH) of uronic acid which prohibits its uptake by the roots [Citation71]. In a study by Robinson et al. (2008), the Equisetum arvense species displayed the excluder characteristics in which Pb concentration in plant tissues was kept unchanging at low levels of less than 100 mg/kg for soil Pb concentrations of up to 60,000 mg/kg [Citation18]. Pb can also be prohibited from reaching plant tissue through root avoidance of highly contaminated points within the soil core [Citation18]. On the other hand, the accumulator technique involves the active concentration of Pb inside plant tissues covering a full spectrum of the soil concentration which is associated with highly peculiar plant physiology [Citation71]. Plants that employ the accumulator technique are able to compensate for the accumulation of Pb in plant tissues through production of chemicals that lessen the toxic effects of Pb. These plants are able to produce antioxidant defence chemicals, elevate levels of polyamine and amino acids and drastic changes in hormonal balance [Citation71]. In a study by Lago-Vila et al. (2019), the Lactuca sativa L. species demonstrated tolerance of Pb toxicity due to the presence of high amounts of organic matter that complexed Pb and minimized its toxicity [Citation53]. Pb toxicity tolerance by Lactuca sativa L. species had seen an increase in the germination index (Gindex) of these plants in six shooting ranges polluted with 161.0–10,873 mg/kg of Pb [Citation53].
Other plants are able to deal with elevated levels of toxic Pb through a detoxification strategy that involves transformation of toxic Pb into a less toxic complex through binding of Pb to chemical species in plant tissues and converting it into less toxic Pb-complexes [Citation37]. The detoxification process can take the form of isolating the Pb and its chemicals in the cell vacuoles so that it does not reach plant tissues. In addition, the toxicity of Pb can be subdued through binding Pb with glutathione antioxidant and amino acids. In a study by Magaji et al. (2018), Pb tolerance by eight plant species was observed in which some plant species accumulated up to 12.30 mg/kg in the shoot [Citation44].
5. Quantification of Pb pollution risk towards plants
The toxicity of Pb and hence its pollution risk towards receptors such as plants can be assessed using various pollution risk assessment indices and factors such as translocation factor (TF), biological concentration factor (BCF), biological accumulation factor (BAF), hazard quotient (HQ), germination index (GI), root growth inhibition (GI) and bioaccessibility as shown in [Citation25,Citation36,Citation50,Citation72,Citation73]. The hazard quotient (HQ) is used to estimate ecological risk of Pb towards receptors such as plants growing in Pb polluted shooting range soils [Citation73]. It is defined as the ratio of exposure concentrations to a toxicological benchmark [Citation74]. Hazard quotient of greater than one indicates possible toxicity risk and its pronounced effects and hence further assessment of the plant is required () [Citation73].
Table 3. Risk assessment of Pb towards plants
Bioaccessibility studies have been carried out towards establishing and estimating the bioavailability of Pb to plants [Citation73]. Bioaccessibility analysis helps allay the assumption that the amount of Pb accumulated in shooting range soils will all be absorbed by plants growing in the polluted soils. As a result a more realistic estimate of Pb uptake by plants is ascertained and its concomitant toxicity risk [Citation73]. The uptake and accumulation of Pb in plants can also be expressed through the bioconcentration factor (BCF) [Citation75]. Bioconcentration factor describes the quotient of plant tissue Pb concentration to soil total Pb concentration [Citation75]. BCF ˂ 1 implies low bioavailability of Pb in the plants.
The translocation of Pb from the roots to different plant tissues such as the shoots can be estimated using the translocation factor (TF) [Citation36]. Translocation factor is defined as the quotient of total Pb concentration (mg/kg) in shoots to that in the roots [Citation36]. A translocation factor greater than one (TF > 1) denotes efficient translocation of Pb from the root to the shoot. Furthermore, (TF > 1) translate to high chances of Pb being available to animals that feed on the polluted plant and thereby increasing migration of Pb in the food chain (). In addition to using translocation factor (TF), Seijo et al. (2016) also applied bioconcentration factor (BCF) and biological absorption coefficient (BAC) to evaluate Pb translocation in plants growing in polluted shooting range soils [Citation36]. Plants with BAC > 1 and BCF > 1 tend to be good extractants and phytostabilizers of Pb respectively (). Plants that are excellent the phytostabilizers make them good candidates for phytoremediation of polluted shooting range soils through immobilization of Pb in the roots and reduces migration of Pb through the food chain.
The detrimental effects of Pb on plants can also be assessed by studying the growth of the roots for a particular plant growing in a Pb polluted shooting range soil compared to the unpolluted control soils. Pb toxicity can therefore be quantified through determination of the germination index (Gindex) and root elongation index (RI) [Citation53]. Germination index describes the product of seed germination (%) and root elongation (mm) of plants in shooting range soils relative to the product of seed germination (%) and root elongation (mm) of the same plant species growing in control soils [Citation76]. Germination index (Gindex) of 90–110% indicates no Pb toxicity, Gindex ˂ 90% indicates inhibition effect to the germination and root elongation of plants whereas Gindex > 110% refers to plants with a stimulation effect. Two shooting ranges situated in Monforte de Lemos, Spain, were found to contain Pb deposition of 161–10,873 mg/kg. Toxicity of Pb towards three plant species growing in the two shooting ranges was studied. The findings indicated Gindex ˂ 90% (62% and 82%) for the S. alba plant species sampled from two sites on the trap shooting range (TSR1 and TSR2), demonstrating inhibition of germination and plant growth due to Pb toxicity as shown in [Citation76].
The hyperaccumulative properties of plants towards Pb have been exploited by scientists and researchers in the phytoremediation efforts towards the control and mitigation of Pb pollution of shooting range soils [Citation23,Citation25,Citation36,Citation44,Citation78]. Phytoremediation strategies have been regarded as green techniques since they do not produce or add any toxins to the environment. Moreover, such soil amendment applications are non-intrusive since they cause little to no destruction to the ecological make-up of the shooting range soils providing little upset to biota.
6. Factors affecting the uptake and translocation of Pb in plants
The bioavailability and bioaccessibility of Pb in shooting range soils depends, to a large extent, on the physicochemical properties of the soil and the plant species itself [Citation36,Citation50]. The soil physicochemical properties have a significant impact on the weathering and speciation of Pb in the soil [Citation79,Citation80]. The uptake of Pb by plants is influenced largely by soil physical and chemical properties such as soil pH, moisture, cation exchange capacity, organic matter content and soil texture [Citation75]. In addition, the plant species, root zone and root structure also do have significant impact on the rate of Pb uptake and its translocation in plants [Citation81].
6.1. Plant species
The uptake of Pb from the soil is to a large extent influenced by the plant species [Citation81]. Plants that have the capacity to uptake large quantities of Pb from the soil are referred to as metallophytes or hyperaccumulators [Citation32]. The hyperaccumulators do not store the absorbed toxic Pb metal in their roots, but rather translocate it to above ground plant parts such as shoots and leaves at concentrations of 100–1000 times higher than in non-hyperaccumulator plants [Citation32]. In addition, the uptake of this high concentration of Pb does not present any toxic results in plants. Hyperaccumulators possess three characteristics that distinguish them from their non-hyperaccumulator counterparts. These include; (i) greater ability for heavy metal uptake such as Pb, (ii) root-to-above ground biomass translocation of heavy metal, and (iii) sequestration and detoxification of heavy metal as shown in [Citation32,Citation82]. Moreover, the amount of Pb accumulated may differ between varieties of the same plant species that have been exposed to the same concentration of Pb [Citation25]. This can give insight into Pb-specific hyper-accumulators that can be applied towards control and management of Pb pollution in shooting range soils [Citation25].
Figure 1. Processes of heavy metal distribution and tolerance in a)non-hyperaccumulator and b) hyperaccumulator plants. (1) indicates heavy metal uptake by the plant roots, (2) heavy metal sequestration in root vacuoles (3) root-to-shoot heavy metal translocation and (4) heavy metal binding to the cell walls and sequestration in vacuoles. The bold arrows indicate a stronger process while the thin arrows show a less strong process
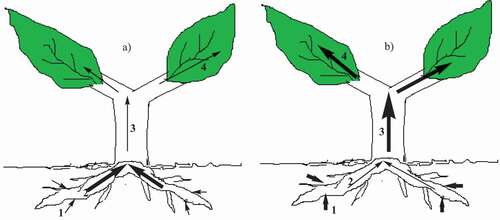
Three different plant species growing in Switzerland were investigated in a pot experiment towards uptake of Pb in shooting range soil contaminated with about 500 mg/kg of Pb [Citation75]. It was established that three plant species; Plantago lanceolata, Lolium perenne, and Triticum aestivum displayed significant Pb uptake in their roots and shoots. T. aestivum was able to absorb the highest concentration of Pb (~200 mg/kg) in the roots compared to L. perenne (~130 mg/kg) and P. lanceolata (~110 mg/kg). The translocation of Pb from the roots to the shoots also varied with the plant species. P. lanceolata experienced the highest translocation of Pb to the shoot which saw its shoot accumulating ~15 mg/kg of Pb compared to ~10 mg/kg and ~5 mg/kg in the respective shoots of L. perenne and T. aestivum [Citation75]. In addition, the study by Conesa et al. (2011) was also able to establish that all the three plant species investigated had bioconcentration factors below one, a general indication of low root-to-shoot Pb transfer [Citation75]. This implies that the possible use of these three plant species in phytoremediation of Pb polluted shooting range soils would not pose a significant risk of Pb transfer into the food chain. In a related study carried out in the same country, Switzerland, the shoots of the following plant species; Chenopodium album, Grasses, Trifolium spp., Persicaria lapathifolia palida and Persicaria lapathifolia lapathifolia were harvested from the shooting range sites at which soil samples were collected [Citation50]. This study further investigated the impact of the soil characteristics such as the acidic soils versus calcareous soils on the uptake of Pb by the plants. The C. album species accumulated the highest concentration of Pb (~60 mg/kg) in the shoot followed by Trifolium spp. (~22 mg/kg), Grasses (~18 mg/kg) and Persicaria lapathifolia palida absorbed the lowest concentration of Pb (~10 mg/kg) in its shoot. It is worth noting that the uptake of Pb took place under acidic soil conditions. In contrast, the uptake of Pb by the plant species growing in calcareous soils was lower compared to acidic soils. Under calcareous soils, C. album accumulated the least amount of Pb (~2 mg/kg) compared to the other three plant species studied. On the other hand, Trifolium spp., Persicaria lapathifolia palida and Grasses growing in calcareous soils accumulated 3, 4 and 6 mg/kg of Pb [Citation50]. This study was able to demonstrate the effectiveness of the acidic soils towards the dissolution of Pb minerals and making it available for uptake by plants compared to the calcareous soils with its pH in the alkaline (pH ~ 8.5) region. The elevated pH of the calcareous soil may have exacerbated the partitioning of Pb on Fe and Mn hydroxides and thereby restricting its mobility and bioavailability. Furthermore, the transformation of Pb into less soluble Pb-carbonates in the presence of high content of calcium carbonate may have made Pb less available for uptake by plants in the calcareous soil.
In a study by Tariq and Ashraf (2016), the uptake of Pb by four different plant species; Brassica campestris, Helianthus annuus, Pisum sativum and Zea mays growing in shooting range soil with high Pb loading (1,331 mg/kg) were compared [Citation25]. Out of the four plant species studied, P. sativum was able to absorb over 96.23% of Pb from the shooting range soil, an indication of high Pb removal efficiency. In addition, these plant species exhibited the highest bioconcentration factor (BCF), a confirmation that it is a hyper-accumulator. On the other hand, Z. maize displayed the second highest extraction efficiency towards Pb with a phytoextraction capacity of 66.36% [Citation25]. The H. annus and B. campestris were the least effective towards Pb uptake from the soil achieving Pb removal efficiency of 48.86% and 33.85% respectively. This study showed the varying capabilities of the four hyper-accumulators towards the uptake of Pb from polluted shooting range soils.
More studies have been carried out in recent years in order to determine the applicability of phytoremediation as a substitute for the control and management of Pb pollution in shooting range soils [Citation23]. This form of shooting range pollution management strategy has been found to be cost effective and environmentally friendly compared to other techniques such as chemical stabilization and soil removal [Citation83–85].
6.2. Effect of soil pH
The uptake of Pb by plants from the soil takes place through the Langmuir process that is largely affected by pH [Citation86]. The pH has been found to play a significant role in proton production by the roots leading to acidification of the rhizosphere and thus favouring Pb dissolution [Citation32]. The absorption of Pb in the soil by plants has been found to increase with increasing pH in the range 3.0–8.5 [Citation86]. The low pH inhibits precipitation of Pb in the plant cell walls and its retention and thereby facilitates its translocation to the shoots [Citation87]. In a study by Robinson et al. (2008), elevated soil pH of 6.9 and high organic carbon of up to 7.3% lowered the phytotoxicity of Pb towards plants leading to revegetation of the shooting range [Citation18]. The high pH reduces the weathering, transformation and dissolution of Pb and thereby restricting its availability for uptake by plant roots [Citation79]. In addition, Pb chemical species such as hydrocerussite [Pb3(CO3)2(OH)2] and cerussite (PbCO3) are stable at elevated pH making Pb not available for absorption by plants roots [Citation5]. Evangelou et al. (2012) determined high concentrations of Pb in plants growing in acidic soils, which were 1.7 times higher than the Pb concentrations in calcareous soil [Citation50]. The high pH of the calcareous soils decreased the mobility and availability of Pb through its adsorption in the Fe and Mn oxides and hydroxides that were formed at elevated pH levels. Furthermore, the calcareous soil contains high concentrations of CaCO3 that is able to precipitate Pb and transform it into the less soluble Pb-carbonates resulting in reduced availability of Pb for plant uptake [Citation50]. The findings by Evangelou et al. (2012) were in agreement with the study carried out by Conesa et al. (2011) in which Plantago lanceolate L. plant species accumulated same range of Pb concentrations under similar calcareous soil conditions of the shooting range [Citation50,Citation75]. The impact of pH on Pb uptake by plants has seen the adjustment of soil pH with chemicals such as lime to pH range of 6.5 to 7.0 in order to minimize Pb absorption by plants [Citation22].
Pb toxicity in plants has been shown to be directly related to plant available Pb fraction in the soil due to favourable conditions of pH, electrical conductivity, composition of soil solution and mineralogical composition of the soil [Citation56]. Formation of chelates between ligands such as histidine or citrates and Pb is pH controlled and this establishes an equilibrium between the chelators and hydrated Pb cations moving along the transpiration path and the immobile Pb binding sites in the plant cell wall surrounding the xylem vessels [Citation88].
Changes in the soil pH to more alkaline levels due to addition of soil amendments such as lime and MgO promoted the formation of insoluble Pb-hydroxides, leading to reduction in exchangeable Pb in the studied shooting range soils [Citation56]. The formation of hydr(oxide) precipitates is responsible for the immobilisation of Pb and reduction of its plant uptake from the soil. In a study by Magaji et al. (2018), a weakly alkaline soil pH (7.2) coupled with electrical conductivity of 8.11 μS/cm favoured plant Pb uptake by eight plant species growing in a Pb polluted shooting range soils [Citation44]. Translocation factors (TF) of up to 1.76 were determined for the studied plant species. The acidic pH of the trap shooting range (TSR) soil found in Spain provided suitable conditions for the dissolution, transformation, mobility and bioavailability of Pb resulting in enhanced plant Pb uptake [Citation53]. The uptake of Pb by seedlings of three different plant species, Sinapis alba L, Lactuca sativa L and Festuca ovina L, growing in acidic shooting range soils lowered their germination index an indication of manifestation of Pb toxicity. The plants also experienced inhibition of root growth caused by Pb phytotoxicity from Pb uptake under favourable conditions of acidic soils [Citation53].
6.3. Effect of soil cation exchange capacity
In addition to the soil pH, cation exchange capacity (CEC) has also been found to play a crucial role in the mobility, bioavailability and eventual uptake of Pb by plants from shooting range soil [Citation5]. The soil CEC describes the number of exchangeable cations that can be taken up by a specified mass of soil and therefore determines the binding ability of such soil [Citation89]. It is influenced to a large extent by the concentration of negative charges on soil colloidal surfaces and the comparative density of positive charges arising from metal species in soil solution [Citation90,Citation91]. In some cases, the negative charges on soil colloidal surfaces may be controlled by the pH of the soil solution while in some situations cationic substitution of Si4+ by Al3+ would have occurred in clay minerals based on their similar shapes [Citation91]. As a result, the negative charges on the soil colloidal surfaces have to be cancelled out by a corresponding equal number of cationic species from the soil solution. This process is called cation exchange and it is reversible due to the formation of weak electrostatic bonds between the cations and the negatively charged soil colloidal surfaces [Citation90]. The attached cations can therefore be replaced by other loosely adsorbed cations and this process is largely dependent on the cation charge and it is negatively affected by the hydration of the ionic radius [Citation91]
Furthermore, elevated soil pH may inhibit Pb uptake by plants due to increased adsorption of Pb within the soil cation exchange sites [Citation89]. In consequence, soils with high CEC experience enhanced binding capacity towards Pb resulting in its reduced mobility and availability for plant uptake [Citation92]. The binding of metal cations by soils rich in clay minerals decreases in the order Cu2+ > Cd2+ > Fe2+ > Pb2+ > Ni2+ > Co2+ > Mn2+ > Zn2+ [Citation93]. In a study by Conesa et al. 2011, Pb uptake by three plant species was significant due to prevailing favourable soil physicochemical properties such as the high soil CEC of 10.3 cmol/kg [Citation75]. High cation exchange capacity of the soil provides for an enhanced exchange of Pb ions sorped into the soil fraction and release of these Pb ions into soil solution and their ultimate uptake by plants [Citation75]. This exchange of Pb ions between the soil exchange sites and the soil solution accelerates the dissolution of Pb into the soil solution and its absorption by plant roots. It is worth noting that soil CEC alone cannot be a major determinant of the effectiveness of Pb uptake by plants since other soil properties such as pH, texture and moisture content should be at play. As indicated in a study by Conesa et al. (2011), the shooting range soils were found to possess other favourable physicochemical properties such as high clay and silt fractions of 45% and 52% respectively [Citation75]. Such soils have demonstrated enhanced Pb uptake by plant roots. Rodriguez-Seijo et al. (2016), discovered high translocation factors of 0.43 and 0.66 for soils with respective high CEC of 6.03 and 9.51 cmol/kg. Soils that experienced low CEC values translocated less concentrations of Pb from their roots to shoots such as soil with CEC of 2.65 cmol/kg and corresponding translocation factor of 0.13 [Citation36].
6.4. Effect of soil organic matter
Soil organic matter refers to the fraction of the soil that comprises the remains of plants and animals that have been returned to the soil and are at various states of decomposition [Citation24,Citation94]. This decomposition process of the once living organism results in the formation of a dark coloured and porous material called humus [Citation94]. The rate of decomposition of dead organism remains is influenced by various factors such as the quantity of animal and plant residues in the soil and the physicochemical properties of the soil such as soil pH and moisture [Citation24].
Organic matter is important to the soil in that it serves as a nutrient reservoir and helps improve the soil structure, reduce erosion [Citation94]. It also plays a crucial role in the evolution of the soil separates, strengthens infiltration rate and water-holding capacity of the soil [Citation95]. The resultant increase in the water-holding capacity of the soil due to elevated levels of organic matter is caused mainly by the concomitant increase in the quantity of micropores and macropores in the soil that are formed from the agglomeration of soil particles [Citation94]. A study by Hudson et al. (1994) has shown that water-holding capacity in the soil can increase by 3.7% for a corresponding increase of 1% in the soil organic matter [Citation96]. Elevated levels of organic matter in the soil have the tendency to increase soil pore volume leading to an increase in the adhesive and cohesive forces inside the soil and an accompanying expansion in the water-holding capacity of the soil [Citation94,Citation97].
The decomposition process of the dead plant and animal materials leads to the liberation of various products such as CO2, H2O, energy and essential nutrients [Citation90]. In addition, the humus consists of the acids fulvic, hymatomelanic and humic which contain acidic functional groups and can therefore form organo-metal complexes with toxic heavy metals such as Pb and thereby controlling their solubility, mobility and bioavailability [Citation24]. In a study carried out by Ma et al. (2007), at a shooting range in Florida (USA), the binding of Pb in the sorption sites of the bio-chemicals found in the organic matter resulted in the formation of water-soluble organo-Pb complexes that made Pb more mobile and bioavailable for plant uptake [Citation98]. In a similar study by Rodriguez-Seijo et al. (2016), the high organic matter content (12.32%) in an old trap shooting range soil found in Spain played a significant role in the uptake of Pb (1,107 mg/kg) from the soil into the roots [Citation36]. In the same study, organic matter of 6.20% recorded at a different sampling site translated into only 694 mg/kg of Pb absorbed by the roots. It is also important to note that organic matter works in conjunction with other soil physicochemical properties to effect an efficient and effective Pb uptake by plants.
6.5. Effect of root structure
Plant characteristics such as root cross-sectional and surface area, root secretions, mycorrhization and transpiration rate have significant impact on the absorption rate and uptake of Pb [Citation37]. The solubility of Pb in the soil also has a marked influence in the absorption and uptake of Pb from the soil by plants [Citation99]. Pb that exists in the form of carbonate and phosphate precipitates in the soil is not readily available for uptake by plants. It is worth noting that Pb in the soil is categorized as Lewis acid and it is able to make strong covalent and ionic bonds with organic ligands and chemical species in soils and plants [Citation37]. The presence of microorganisms in the soil also affects Pb uptake and translocation by plants through processes such as bioaccumulation and biosorption [Citation100].
6.6. Effect of soil texture
Soil texture describes the relative fraction of particulate matter of various dimensions which may include sand, silt and clay that constitute the mineral component of the soil [Citation24,Citation94]. The corresponding particle sizes for sand, silt and clay are >50 µm, 2–50 µm and ˂ 2 µm respectively [Citation90]. Soil texture has a great influence on the moisture-holding capacity of the soil and sandy soils have been found to possess the lowest moisture-holding capacity compared to clay soils. Silt soils on the other hand have lower moisture-holding capacity compared to clay soils [Citation94]. Soil texture also plays a significant role in Pb availability in plants such that plant Pb uptake in fine sand fraction was more pronounced compared to other soil fractions that were mostly clay and course sand [Citation56]. The findings by Sanderson et al. (2014), were in agreement with those by Qian et al. (1996) in which the highest extractability of Pb was observed in the fine sand fraction [Citation56,Citation101]. It has been established that sandy soils make Pb more available to plants and thereby increasing their uptake by plants and subsequent translocation to above ground biomass [Citation6]. On the other hand, clay soils demonstrate strong binding affinity towards Pb and thereby immobilize Pb and make it less available for plant uptake [Citation90]. The mechanism through which clay soil fraction binds Pb is thought to occur through the adsorption of Pb via ion exchange and distinct sorption process [Citation102]. The mechanism for the specific adsorption of Pb has been established to involve the initial adsorption of the hydroxyl ions by the clay soil fraction followed by the electrostatic interaction between Pb and the adsorbed hydroxyl ions [Citation102]. This binding of Pb by clay soil fraction, as stated above, restricts the availability of Pb for plant uptake and translocation to above ground plant organs.
7. Phytoremediation approach
Scientists and researchers are continuously searching for eco-friendly techniques and methods for the control and management of Pb pollution of shooting range soils. The cost implications of such methods have also been a topical discussion for sustainable soil remediation and reclamation efforts. In recent years, phytoremediation has become an increasingly cost effective, efficient and environmentally friendly technology for the amendments of highly polluted soils [Citation22,Citation23,Citation36,Citation103]. Phytoremediation describes application of engineered green plants to remove, immobilize, contain and stabilize environmental pollutants such as trace and heavy metals, organic substances and radioactive compounds found in the soil [Citation104,Citation105]. This technique utilizes processes in the plant that may be chemical, biological or physical that assist in the uptake and translocation of pollutants through the plant resulting in improved quality of the soil [Citation104]. Plants are able to achieve these processes by employing such mechanisms as phytostabilization, phytoextraction, phytovolatilization and rhizofiltration [Citation104]. Phytostabilization involves the immobilization of Pb in the soil by plants through absorption and precipitation in the root zone and thereby limiting its mobility in the soil [Citation22,Citation106]. On the other hand, phytoextraction mechanism entails the uptake of Pb by plant roots and its translocation into above ground plant biomass [Citation106]. In contrast, phytovolatilization involves the uptake of Pb by plants and its loss as secondary Pb species through transpiration into the atmosphere. This process is usually more pronounced in growing plants that uptake water along with Pb species and their loss through the plant leaves via transpiration [Citation106]. Lastly, there are instances whereby the control of Pb pollution in soil may be mitigated through rhizofiltration in which Pb in soil solution surrounding the plants root zone is absorbed and sequestrated within the roots [Citation106].
There has been a surge, in recent years, in the number of studies that assessed the effectiveness of vegetation towards shooting range soils amendments and reclamation efforts [Citation22,Citation23,Citation36,Citation103]. Examples of studies where phytoremediation strategies have been effective include such studies as those carried out by Tariq and Ashraf (2016), Rodriguez-Seijo et al. (2016) and Sneddon et al. (2009). Tariq and Ashraf (2016) reported the phytoextraction ability of Pisum sativum that demonstrated Pb removal efficiency of 96.23% from shooting range soil polluted with over 1,331 mg/kg of Pb [Citation25,Citation36,Citation107]. In a study by Rodriguez-Seijo et al. (2016) in a shooting range in Spain, the phytoremediation effectiveness of Agrostis capillaris L. grass towards Pb immobilization in which 1,107 mg/kg of Pb was absorbed by the roots of the grass with about 135 mg/kg translocated into the shoots in a [Citation36]. In the United Kingdom, a study by Sneddon et al. (2009) established a concentration of 38 mg/kg in the shoots of L. Perenne growing in soils containing contaminated with 43.89–159.98 mg/kg of Pb emanating from ammunition [Citation107]. The advantage of phytoremediation technology to other soil remediation techniques such as chemical and physical amendments is that it is less disruptive to the ecosystem [Citation104]. There is no destruction of the soil structure and loss of habitat for living organisms compared to soil removal techniques [Citation22,Citation36]. Above all, this method is cost effective and does not introduce foreign chemicals into the environment compared to chemical amendments [Citation22]. In addition, to the control and management of pollutants in the soil, phytoremediation serves another purpose in that plants prevent soil erosion by holding the soil together with their roots and reduce the impact from runoff water from rainfall. Plant roots also produce into the soil chemicals that serve as a source of nutrients for the microbes found in the rhizosphere [Citation108]. As a result, the density of microbial communities is usually higher in the rhizosphere than in the soils furthest away from the plant roots. This describes the interdependence between the soil microbial populations and plants [Citation108]. The multifaceted benefits of phytoremediation has led to its recommendation by the United States Environmental Soil Protection Agency (USEPA) as one of the methods that can be employed for the control and management of Pb pollution in shooting range [Citation106].
8. Conclusion
Shooting ranges do not only pose pollution risk to the soils found in shooting range premises but to the vegetation growing in and nearby these shooting ranges as well. The uptake of Pb by plants takes place through various chemical and physical processes. The efficiency of Pb uptake by plants depends on many factors such as the plant species itself and the soil physicochemical properties. Lead pollution of shooting range soils has deleterious effects on plants due to the uptake of this toxic heavy metal by plants. Most of the shooting ranges are not fenced and therefore act as grazing fields for livestock and animals. This may result in the migration of Pb through the food chain. In addition, arable farming activities taking place nearby shooting ranges are also at risk of crop contamination from this deadly heavy metal. However, the uptake of Pb from the soil by plants has manifested into the application of plants towards control and management of Pb pollution in shooting range soils. This technique is called phytoremediation and it has been widely accepted by environmental protection agencies such as the USEPA as a technology to remediate Pb polluted soils. Phytoremediation technology makes use of plants that are hyper-accumulators and significant strides have been made in the application of this technology towards shooting range soils amendments and reclamation efforts. Investigation into possible pollution of both surface and underground water sources found near shooting ranges is a continuous process.
Acknowledgments
The authors would like to thank Botswana International University of Science and Technology (BIUST) for the resources used in the preparation of this review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mellor A, McCartney C. The effects of lead shot deposition on soils and crops at a clay pigeon shooting site in northern England. Soil Use Manage. 1994;10:124–129.
- Park J, Bae B. Uptake and transformation of RDX by perennial plants in Poaceae family (amur silver grass and reed canary grass) under hydroponic culture conditions. J Kor Soc Environ Eng. 2014;36:237–245.
- Scoriza RN, Correia MEF. Establishment of leguminous trees in the soil of a shooting range. Floresta E Ambient. 2019;26:e20170805.
- Rooney CP, McLaren RG, Cresswell RJ. Distribution and phytoavailability of lead in a soil contaminated with lead shot. Water Air Soil Pollut. 1999;116:535–548.
- Cao X, Ma LQ, Chen M, et al. Lead transformation and distribution in the soils of shooting ranges in Florida. USA Sci Total Environ. 2003;307:179–189.
- Hui CA. Lead distribution throughout soil, flora and an invertebrate at a wetland skeet range. J Toxicol Environ Health A. 2002;65:1093–1107.
- Astrup T, Boddum JK, Christensen TH. Lead distribution and mobility in a soil embankment used as a bullet stop at a shooting range. J Soil Contam. 1999;8:653–665.
- Stansley W, Widjeskog L, Roscoe DE. Lead contamination and mobility in surface water at trap and skeet ranges. B Environ Contam Tox. 1992;49:640–647.
- Mariussen E, Johnsen IV, Stromseng AE. Application of sorbents in different soil types from small arms shooting ranges for immobilization of lead (Pb), copper (Cu), zinc (Zn), and antimony (Sb). J Soil Sediment. 2018;18:1558–1568.
- US Environmental Protection Agency (USEPA). Best management practices for lead at outdoor shooting ranges. EPA-902-B-01-001. 2005, Papanikolaou NC, Hatzidaki EG, Belivanis S. Lead toxicity update. A brief review. Med Sci Monit. 2005;11:329–336.
- Eisler R. Lead hazards to fish, wildlife and invertebrates : a synoptic review. Contaminant Hazard Reviews, Report 14; Biological Report.1988;85:1–94.
- Mathee A, Jager P, Naidoo S, et al. Exposure to lead in South African shooting ranges. Environ Res. 2017;153:93–98.
- Johnsen IV, Mariussen E, Voie O. Assessment of intake of copper and lead by sheep grazing on a shooting range for small arms: a case study. Environ Sci Pollut Res. 2019;26:7337–7346.
- Fisher IJ, Deborah JP, Thomas VG. A review of lead poisoning from ammunition sources in terrestrial birds. Biol Conserv. 2006;131:421–432.
- Wilde EW, Brigmon RL, Dunn DL, et al. Phytoextraction of lead from firing range soil by Vetiver grass. Chemosphere. 2005;61:1451–1457.
- Mannenin S, Tanskanen N. Transfer of lead from shotgun pellets to humus and three plant species in a Finnish shooting range. Arch Environ Contam Toxicol. 1993;24:410–414.
- Lee IS, Kim OK, Chang YY, et al. Heavy metal concentrations and enzyme activities in soil from a contaminated Korean shooting range. J Biosci Bioeng. 2002;94:406–411.
- Robinson BH, Bischofberger S, Stoll A, et al. Plant uptake of trace elements on a Swiss military shooting range: uptake pathways and land management implications. Environ Pollut. 2008;153:668–676.
- Nazir R, Khan M, Masab M, et al. Accumulation of Heavy Metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water collected from Tanda Dam kohat. J Pharm Sci Res. 2015;7:89–97.
- Koeppe DE. The uptake, distribution and effect of cadmium and lead in plants, Stevens report. Stevens Inst Technol. 1977;7:197–206.
- Darling CTR, Thomas VG. The distribution of outdoor shooting ranges in Ontario and the potential for lead pollution of soil and water. Sci Total Environ. 2003;313:235–243.
- Sanderson P, Fangjie QF, Seshadri B, et al. Contamination, fate and management of metals in shooting range soils—a Review. Curr Pollut Rep. 2018;4:75–187.
- Bandara T, Vithanage M. Phytoremediation of shooting range soils. Ansari AA, Gill SS, Gill R, et al., Editors. Phytoremediation. Cham: Springer International Publishing; 2016. p. 469–488.
- Dinake P, Kelebemang R, Sehube N. A comprehensive approach to speciation of lead and its contamination of firing range soils: a review. Soil Sediment Contam. 2019;2:431–459.
- Tariq SR, Ashraf A. Comparative evaluation of phytoremediation of metal contaminated soil of firing range by four different plant species. Arab J Chem. 2016;9:806–814.
- Mozafar A, Ruh R, Klingel P, et al. Effect of Heavy metal contaminated shooting range soils on mycorrhizal colonization of roots and metal uptake by leek. Environ Monit Assess. 2002;79:177–191.
- Wan X-M, Tandy S, Hockmann K, et al. Changes in Sb speciation with waterlogging of shooting range soils and impacts on plant uptake. Environ Pollut. 2013;172:53–60.
- Busby RR, Barbato RA, Jung CM, et al. Photoperiod and soil munition constituent effects on phytoaccumulation and rhizosphere interactions in boreal vegetation. Water Air Soil Pollut. 2018;229:380.
- EC-REGULATION-1881/2006. Commission regulation (EC) No 1881/2006 – setting maximum levels of certain contaminants in foodstuff. Off J Eur Union. 2006;49:5–24.
- Cacador I, Vale C, Catarino F. The influence of plants on concentration and fractionation of Zn, Pb, and Cu in salt marsh sediments (Tagus Estuary, Portugal). J Aquat Ecosystem Health. 1996;5:193–198.
- DeShields BR, Meredith RW, Griffin D, et al. The use of field methods to evaluate the toxicity of lead to plants at a small arms firing range. In: DeLonay AJ, Greenber BM, editors. Environmental toxicology and risk assessment. Vol. 7. ASTM STP 1333. West Conshohocken, PA: American Society for Testing and Materials; 1998. p. 166–183.
- Singh S, Parihar P, Singh R, et al. Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics and ionomics. Front Plant Sci. 2015;6:1143.
- Williams LE, Pittman JK, Hall JL. Emerging mechanisms for heavy metal transport in plants. Biochim Biophys Acta. 2000;1465:104–126.
- Sorvari J. Environmental risks at Finnish shooting ranges—A case study. Hum Ecol Risk Assess. 2007;13:1111–1146.
- Fayiga AO, Uttam S. The effect of bullet removal and vegetation on mobility of Pb in shooting range soils. Chemosphere. 2016;160:252–257.
- Rodriguez-Seijo A, Lago-Vila M, Andrade ML, et al. Pb pollution in soils from a trap shooting range and the phytoremediation ability of Agrostis capillaris L. Environ Sci Pollut Res. 2016;23:1312–1323.
- Sharma P, Dubey RS. Lead toxicity in Plants. Braz J Plant Physiol. 2005;17:35–52.
- Huang JW, Chen J, Berti WR, et al. Phytoremediation of lead-contaminated soil: role of synthetic chelates in lead phytoextraction. Environ Sci Technol. 1997;31:800–805.
- Rudakova EV, Karakis KD, Sidorshima ET. The role of plant cell walls in the uptake and accumulation of of metal ions. Fiziol Biochim Kult Rast. 1988;20:3–12.
- Jones LHP, Clement CR, Hopper MJ. Lead uptake from solution by perennial ryegrass and its transport from roots to shoots. Plant Soil. 1973;38:403–414.
- Verma S, Dubey RS. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003;164:645–655.
- Seregin IV, Ivaniov VB. Histochemical investigation of cadmium and lead distribution in plants. Russ J Plant Physiol. 1997;44:915–921.
- Seregin IV, Shpigun LK, Ivaniov VB. Distribution and toxic effects of cadmium and lead on maize roots. Russ J Plant Physiol. 2004;51:5250–5533.
- Magaji Y, Ajibade GA, Yilwa VMY, et al. Concentration of heavy metals in the soil and translocation with phytoremediation potential by plant species in military shooting range. World Sci News. 2018;92:260–271.
- Godzik B. Heavy metal contents in plants from zinc dumps and reference area. Pol Bot Stud. 1993;5:113–132.
- European Communities Council. Commission Regulation 466/2001 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Commun. 2001;L77:1–13.
- Selonen S, Liiri M, Strommer R, et al. The fate of lead at abandoned and active shooting ranges in a boreal pine forest. Environ Toxicol Chem. 2012;31:2771–2779.
- Hashimoto Y, Matsufuru H, Sato T. Attenuation of lead leachability in shooting range soils using poultry waste amendments in combination with indigenous plant species. Chemosphere. 2008;73:643–649.
- Kabata-Pendias A, Pendias H. Trace elements in soils and plants, 2nd Edition. Boca Raton,Florida: CRC Press; Vol. 1992, p. 365.
- Evangelou MWH, Hockmann K, Pokharel R, et al. Accumulation of Sb, Pb, Cu, Zn and Cd by various plants species on two different relocated military shooting range soils. J Environ Manag. 2012;2012(108):102–107.
- Selonen S, Setala H. Soil processes and tree growth at shooting ranges in a boreal forest reflect contamination history and lead-induced changes in soil food webs. Sci Total Environ. 2015;518–519:320–327.
- Eun SO, Youn HS, Lee Y. Lead disturbs microtubule organization in the root meristem of Zea mays. Physiol Plant. 2000;110:357–365.
- Lago-Vila M, Rodríguez-Seijo A, Vega FA, et al. Phytotoxicity assays with hydroxyapatite nanoparticles lead the way to recover firing range soils. Sci Total Environ. 2019;690:1151–1161.
- Wierzbicka M. Resumption of mitotic activity in Allium cepa root tips during treatment with lead salts. Environ Exp Bot. 1994;34:173–180.
- Burton KW, Morgan E, Roig A. The influence of heavy metals on the growth of Sitka-spruce in South Wales forests. II green house experiments. Plant Soil. 1984;78:271–282.
- Sanderson P, Naidu R, Bolan N. Ecotoxicity of chemically stabilised metal(loid)s in shooting range soils. Ecotoxicol Environ Saf. 2014;100:201–208.
- Iqbal J, Mushtaq S. Effect of lead on germination, early seedling growth, soluble protein and acid phosphatase content in Zea mays. Pak J Sci Ind Res. 1987;30:853–856.
- Bazzaz FA, Rolfe GL, Windle P. Differing sensitivity of corn and soybean photosynthesis and transpiration to lead contamination. J Environ Qual. 1974;3:156–158.
- Van Assche F, Clijsters H. Effects of metal on enzyme activity in plants. Plant Cell Environ. 1990;13:195–206.
- Stefanov K, Seizova K, Popova I, et al. Effects of lead ions on the phospholipid composition in leaves of Zea mays and Phaseolus vulgaris. J Plant Physiol. 1995;147:243–246.
- Burzynski M. The influence of lead and cadmium on the absorption and distribution of potassium, calcium, magnesium and iron in cucumber seedlings. Acta Physiol Plant. 1987;9:229–238.
- Rebechini HM, Hanzely L. Lead-induced ultrastructural changes in chloroplasts of the hydrophyte Cerato-phyllum demersum. Z Pflanzenphysiol. 1974;73:377–386.
- Ahmad A, Tajmir-Riahi HA. Interaction of toxic metal ions Cd2+, Hg2+ and Pb with light-harvesting proteins of chloroplast thylakoid membranes. An FTIR spectroscopic study. J Inorg Biochem. 1993;50:235–243.
- Sersen F, Kralova K, Bumbalova A. Action of mercury on the photosynthetic apparatus of spinach chloroplasts. Photosynthetica. 1998;35:551–559.
- Rashid A, Bernier M, Pazdernick L, et al. Interaction of Zn2+ with the donor side of Photosystem II. Photosynth Res. 1991;30:123–130.
- Miller RJ, Biuell JE, Koeppe DE. The effect of cadmium on electron and energy transfer reactions in corn mitochondria. Physiol Plant. 1973;28:166–171.
- Tu Shu I, Brouillete JN. Metal ion inhibition of corn root plasma membrane ATPase. Photochemistry. 1987;26:65–69.
- Godbold DL, Kettner C. Lead influences root growth and mineral nutrition of Picea abies seedlings. J Plant Physiol. 1991;139:95–99.
- Burzynski M, Grabowski A. Influence of lead on nitrate uptake and reduction in cucumber seedlings. Acta Soc Bot Pol. 1984;53:77–86.
- Girroti AW. Photodynamic peroxidation in biological systems. Photochem Photobiol. 1990;51:497–509.
- Baker AJM. Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutr. 1981;3:643–654.
- Malik RN, Husain SZ, Nazir I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak J Bot. 2010;42:291–301.
- Bennett JR, Kaufman CA, Koch I, et al. Ecological risk assessment of lead contamination at rifle and pistol ranges using techniques to account for site characteristics. Sci Total Environ. 2007;374:91–101.
- Suter IIGW, Efroymson RA, Sample BE, et al. Ecological risk assessment for contaminated sites. Boca Raton: Lewis Publishers; 2000. p. 438.
- Conesa HM, Wieser M, Studer B, et al. Effects of vegetation and fertilizer on metal and Sb plant uptake in a calcareous shooting range soil. Ecol Eng. 2011;37:654–658.
- Agnieszka B, Tomasz C, Jerzy W. Chemical properties and toxicity of soils contaminated by mining activity. Ecotoxicology. 2014;23:1234–1244.
- Sanderson P, Naidu R, Bolan N. Effectiveness of chemical amendments for stabilisation of lead and antimony in risk-based land management of soils of shooting ranges. Environ Sci Pollut Res. 2013;22:8942–8956.
- Hockmann K, Tandy S, Studer B, et al. Plant uptake and availability of antimony, lead, copper and zinc in oxic and reduced shooting range soil. Environ Pollut. 2018;238:255–262.
- Sehube N, Kelebemang R, Totolo O, et al. Lead pollution of shooting range soils. S Afr J Chem. 2017;70:21–28.
- Kelebemang R, Dinake P, Sehube N, et al. Speciation and mobility of lead in shooting range soils. Chem Speciat Bioavailab. 2017;29:143–152.
- Yadav KK, Gupta N, Kumar A, et al. Mechanistic understanding and holistic approach of phytoremediation: a review on application and future prospect. Ecol Eng. 2018;120:274–298.
- Verbruggen N, Hermans C, Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009;181:759–776.
- Yin X, Saha UK, Ma LQ; Yin X, Saha UK, Ma LQ. Effectiveness of best management practices in reducing Pb-bullet weathering in a shooting range in Florida. J Hazard Mater. 2010;179:895–900.
- Seshadri B, Bolan NS, Choppala G, et al. Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere. 2017;184:197–206.
- Dinake P, Kelebemang R. Critical assessment of mechanistic pathways for chemical remediation techniques applied to Pb impacted soils at shooting ranges – a review. Environ Pollut Bioavailab. 2019;31:282–305.
- Lee S-Z, Chang L, Yang -H-H, et al. Absorption of characteristics of lead onto soils. J Haz Mat. 1998;63:37–49.
- Jarvis MD, Leung DWM. Chelated lead transport in Pinus radiate: an ultrastructural study. Environ Exp Bot. 2002;48:21–32.
- Clemens S, Palmgren MG, Kramer U. A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci. 2002;7:309–315.
- Nelson DW, Sommers LE Total carbon, organic carbon, and organic matter: in methods of soil analysis, Part 2 chemical and microbiological properties. 1982;9:p. 539–577. ASA, Madison, Wisconsin.
- Rieuwerts JS, Thornton I, Farago ME, et al. Factors influencing metal bioavailability in soils: preliminary investigations for the development of a critical loads approach for metals. Chem Speciat Bioavailab. 1998;10:61–75.
- Evans LJ. Chemistry of metal retention by soils. Environ Sci Technol. 1989;23:1046–1056.
- Rooney CP, McLaren RG, Condron LM. Control of lead solubility in soil contaminated with lead shot: effect of soil pH. Environ Pollut. 2007;149:149–157.
- Fijalkowski K, Kacprzak M, Grobelak A, et al. The influence of selected soil parameters on the mobility of heavy metals in soils. Inz I Ochrona Srodowiska. 2012;5:81–92.
- Nath TN. Soil texture and total organic matter content and its influences on soil water holding capacity of some selected tea growing soils in Sivasagar district of Assam, India. Int J Chem Sci. 2014;12:1419–1429.
- Kwiatkowska-Malina J. Functions of organic matter in polluted soils: the effect of organic amendments on phytoavailability of heavy metals. Appl Soil Ecol. 2018;123:542–545.
- Hudson BD. Soil organic matter and available water capacity. Soil Wat Con. 1994;49:189–194.
- Alexandra B, Jose B The importance of soil organic matter: key to drought-resistant soil and sustained food production. FAO Soil Bulletin 80, Food and Agriculture Organization of the United Nations 2005, pp 1–95.
- Ma LQ, Hardison JD, Harris WD, et al. Effects of soil property and soil amendment on weathering of abraded metallic Pb in shooting ranges. Water Air Soil Pollut. 2007;178:297–307.
- Blaylock MJ, Salt DE, Dushenkov S, et al. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ Sci Technol. 1997;31:860–865.
- Marschner P, Godbold DL, Jutschhe G. Dynamics of lead accumulation in mycorrhizal and non-mycorrhizal Norway spruce (Picea abies (L.) karst.). Plant Soil. 1996;178:239–245.
- Qian J, Shan X-Q, Wang Z-J, et al. Distribution and plant availability of heavy metals in different particle-size fractions of soil. Sci Total Environ. 1996;187:131–141.
- Farrah H, Pickering WF. Influence of clay-solute interactions on aqueous heavy metal ion levels. Water Air Soil Pollut. 1977;8:189–197.
- Migliorinia M, Pigino G, Bianchi N, et al. The effects of heavy metal contamination on the soil arthropod community of a shooting range. Environ Pollut. 2004;129:331–340.
- Tangahu BV, Abdullah SRS, Basri H, et al. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng. 2011;:939161:1–31.
- Hinchman RR, Negri MC, Gatliff EG Phytoremediation: using green plants to clean up contaminated soil, groundwater, and wastewater. Argonne National Laboratory Hinchman, Applied Natural Sciences, Inc, 1995. Pp. 1–13.
- United States Environmental Protection Agency, Use of field-scale phytotechnology, for chlorinated solvents, metals, explosives, and propellants, and pesticides. Phytotechnology mechanisms solid waste and emergency response (5102G), EPA 542-R- 05-002, 2005.
- Sneddon J, Clemente R, Riby P, et al. Source-pathway-receptor investigation of the fate of trace elements derived from shotgun pellets discharged in terrestrial ecosystems managed for game shooting. Environ Pollut. 2009;157:2663–2669.
- Sas-Nowosielska A, Galimska-Stypa R, Kucharski R, et al. Remediation aspect of microbial changes of plant rhizosphere in mercury contaminated soil. Environ Monit Assess. 2008;137:101–109.
