?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Flocculants agglomerate suspended microalgae cells, while cost and toxicity have led to the increased use of bio-flocculants. In this experiment, Chlorella pyrenoidosa was gathered by utilizing bio-flocculants from discarded clam shells. At pH 8, 0.2 mg/mL of bio-flocculant clam shell, 0.1 mg/mL of cationic inducer, and 240 rpm of mixing achieved 91.87 % flocculation efficiency and 458.1 mg of recovered biomass. Calcium ions in bio-flocculants are the main contributor to Chlorella pyrenoidosa flocculation, employing charge neutralization and sweeping as flocculation mechanisms. Zn2+ salt boosts flocculation by neutralizing the functional group's negative charge. The R2 values of 0.8969 and 0.8894 for harvesting efficiency and recovered biomass reflect the model's predictive power. XRD exhibited faint, indistinct peaks with considerable noise, indicating that the chitosan bioflocculant did not have a crystalline structure and that Chlorella had become fibrillar. Response Surface Methodology approach, which promotes flocculation, improves water reuse and microalgae harvesting.
1. Introduction
Recent analyses of the changes needed in the global energy system to combat climate change have determined that bioenergy will play a significant role in global renewable energy during the next decade [Citation1]. In order for biomass to be used as a feedstock for bioenergy and other bio-based products, its availability and production costs are crucial. There have been considerable attempts to assess the overall quantity of accessible biomass and its average production cost [Citation2,Citation3]. It is necessary to produce goods based on renewable natural resources in order to solve the difficulties generated by population increase and rising human requirements throughout time [Citation4].
Microalgae are one of the most autotrophic micro-organisms of plant life and are renowned for their capacity to quickly create and accumulate important products, including carbohydrates, proteins, fatty acids, and lipids [Citation5,Citation6]. Additionally, they are used as biofilters to remove contaminants from wastewater and as fertilizers for animal feed. Microalgae may also be used to produce biofuels such as methane [Citation7], biodiesel [Citation8], and biohydrogen [Citation9]. Due to these benefits, microalgae are regarded as one of the most promising renewable bioenergy sources [Citation10].
Chlorella is one of the most important commercialized microalgae species that may be used for food, animal feed, bio-energy, and other by-products [Citation11–13]. Chlorella has the potential to reproduce rapidly in growing circumstances, is simple to cultivate, produces oxygen via photosynthesis, and has a high protein content with amino acids as the primary component [Citation14]. There are several microalgae harvesting methods, including centrifugation, sonication, filtration, air floatation, coagulation, and flocculation. Among them, flocculation stands out due to its simplicity and relative low cost [Citation15,Citation16].
Clam shells (Perna viridis) are belong to the class bivalvia and the phylum Mollusca. The primary ingredient of the shell is calcium carbonate, which is either calcite or calcium carbonate. Waste clam shells are common in Indonesia, and only 30% weight (meat plus shells) was flesh [Citation17]. Clam shells include 66.70% Calcium Carbonate, 7.88% SiO2, 22.28% MgO, dan 1.25% Al2O3 [Citation18]. Due to their high calcium carbonate concentration, mussel shells may be used to purify water. Calcium carbonate in shellfish is able to purify water and can even lower iron, manganese, and other metal concentrations [Citation19].
Due to the tiny size of microalgae (a few mm) and their low concentration in the medium (500 ml), this constitutes a serious difficulty. Microalgae is presently collected by using centrifugation in commercial systems, however this approach uses a great deal of energy and is thus not cost-effective [Citation4]. Using metal coagulants like alum and ferric chloride, microalgae may be flocculated with ease. However, this needs a substantial quantity of coagulant and resulting in metal contamination of the collected biomass [Citation20]. Among them, flocculation is regarded the preferred harvesting method since it is very inexpensive and easy to implement. Due to the inclusion of chemicals or organic substances, flocculation enabled microalgae to form a mass and aggregate [Citation21]. Flocculation is an efficient procedure that permits the quick treatment of large numbers of microalgae cells [Citation22].
Hadiyanto et al. [Citation23] did research on the flocculation of Chlorella pyrenoidosa using eggshell as a flocculant, while Abdul Razack et al. [Citation24] conducted a study on the harvesting of C. vulgaris utilizing Strychnos potatorum as a flocculant. In contrast, Surendhiran & Vijay [Citation25] included ZnCl2 into the flocculation process of Nannochloropsis oculata). The research of Guldhe et al. [Citation26],, which harvested Ankistrodesmus falcatus using chitosan. There is also a research by Xu et al. [Citation27], that uses Chitosan powder extracted from crab shells as a coagulant for the harvesting of Chlorella sorokiniana.
In this study, microalgae had been harvested by the flocculation technique with Perna viridis bio-flocculants. Using response surface modeling (RSM) and center composite design (CCD) techniques, the impact of cationic inducer dosage, pH, mixing rate, and bio-flocculant on deposition time and flocculation efficiency will be assessed and adjusted. The RSM was used to identify the optimal flocculation conditions. RSM is a statistical method for constructing factorial experiments in order to generate mathematical models that enable the analysis of the influence of numerous variables on the intended response and the determination of the optimal conditions while minimizing the number of trials. Using the RSM-CCD approach to optimize bio-flocculants in the green shell microalgae harvesting process has never been done previously, hence we consider this a scientific novelty. The goals of the study were to improve the flocculation process by identifying optimal operating conditions and to promote for the use of clam shell as a sustainable bio-flocculant in the harvesting of Chlorella pyrenoidosa. Flocculation efficiency and biomass recovery were investigated and optimized in relation to pH, cationic inducer, and flocculant concentration using response surface methodology.
2. Materials and methods
2.1 Culture preparation of Chlorella pyrenoidosa
Chlorella pyrenoidosa microalgae were obtained from Ugoplankton, Jepara, Central Java, Indonesia. In the UPT C-BIORE Laboratory at the Diponegoro University in Semarang, Indonesia, microalgae were cultivated, tested, and the findings analyzed. The cultivation was conducted in an aquarium with an aerator (BS-410, Amara, Shanghai, China). The cultures were put in an incubator that was lit by a Philips 8 W tube lamp with a light intensity of 1500 lux (light/darkness ratio of 24:0 h). To maintain the growth of Chlorella pyrenoidosa, nutrients are added every two days in the form of a combination of 0.05 g/L TSP, 0.05 g/L Urea, and 0.2 g/L NaHCO3. Optical Density was measured using a Spectrophotometer with a wavelength of 680 nm (Spectroquant Prove 100, Merck KGaA, Darmstadt, Germany).
2.2 Synthesis of bio-flocculant
The technique of making bioflocculants from Perna viridis shells was adopted from Suparmaniam et al., as seen below [Citation28]. Perna viridis shells were cleaned thoroughly with distilled water. Perna viridis shell is then heated at a temperature of 105°C for 30 minutes to evaporate the water content. After that, the shell is crushed into a fine powder. This dry fine powder is then sieved through a sieve with a mesh size 200. Finally, Perna viridis powder was dissolved in 0.1 N HCl solution (Sigma Aldrich, St. Louise, USA) and stirred with a magnetic stirrer for 3 min.
2.3 Bio-floculant experiment
Experiments on flocculation were conducted during the stationary growth phase of microalgae. The optimization research used 400 mL of Chlorella pyrenoidosa. By measuring flocculation effectiveness and biomass, the impacts of bio-flocculation factors such as pH, bio-flocculant concentration, cationic inducer, and mixing rate were evaluated [Citation29]. The 1 mg/mL stock solution was made by dissolving 100 mg of clam shell bioflocculant in 10 mL of 0.1 M HCl. Using a magnetic stirrer, the solution was swirled for 10 minutes until the powder was entirely dissolved. The solution is then diluted with deionized water to 100 mL. This is repeated for each variable, and the resulting solution is ready to be added to 400 mL Chlorella pyrenoidosa medium with the addition of 1 M HCl or 1 M NaOH starting at pH 6 to 10 and swirled with a magnetic stirrer at 200, 220, 240, 260, 280 rpm for 15 minutes. The settling time of microalgae flocs was set to 15 minutes [Citation27]. The optical density of 680 nm (OD680) in a Spectrophotometer Spectroquant Prove 100 (Darmstadt, Germany) was used to calculate the concentration of microalgae biomass in the tube during one hour.
2.4 Flocculation efficiency and biomassanalysis
Using a spectrophotometer, the initial concentration of microalgal biomass was measured in quart cuvettes with an optical density of 680 nm (OD680). At the end of the bio-flocculation time, the optical density of the supernatant was measured at 2/3 of the height of the pellucid culture. The flocculation efficiency was determined as followed equation (1) [Citation22,Citation30]:
where ODi was OD680 value of sample and ODf was OD680 value of control. Through an Ultra Micro Fiber, 500 mL of microalgae cell suspension was filtered. The biomass content of microalgae cultures was then determined gravimetrically by drying the samples in plates at 60°C for 24 hours until constant weight. The weight of the final plate was used to measure the dry biomass of microalgae.
2.5 Response surface methodology
Response Surface Methodology (RSM) was used to test the effect of four independent factors. To study four flocculation parameters, a central composite design (CCD) of the experiments was designed. Optimized utilizing a four-level factorial design and a small central composite design [Citation31]. RSM is renowned for evaluating the interaction between the important variables of an experiment and optimizing them [Citation32]. The four independent variables were examined at five levels (−2, −1, 0, +1, +2) using 30 experimental runs and six central points that were repeated.
Using Design-Expert software, experimental design and multiple regression analyses of experimental data were conducted (version 10, Stat-Ease Inc., MN, USA). Using ANOVA, the experimental results were statistically assessed. The bio-flocculation activity-representing response variable [Y] was fitted using the following second-order polynomial equation (2):
Where Y is the predicted response, β0 was the constant, X1 – X4 were the input variables. β1 – β4 were the linear coefficients, β12 – β44 were the second order interactive coefficients and β11 – β44 were the quadratic coeicients. The actual values of the different code levels consisting of pH [X1], Chitosan concentration [X2], Cationic Inducer [X3], and Mixing Rate [X4] are shown in and the effect of flocculation efficiency is represented as Y.
Table 1. Coded value based on the factor at a time experiment for 4 variables employed in the study.
2.6 SEM, XRD, and FTIR analysis
Utilizing a scanning electron microscope, the cell surface of Chlorella sp. control and Chlorella obtained using clam shells as bioflocculant was analyzed. Microalgae flocs were studied under a light microscope before to and after flocculation. Using SEM and Energy Dispersive X-ray spectroscopy (EDX) to assess the material’s inorganic constituents [Citation33]. The study was conducted at room temperature using A Jeol (model JSM-6510 LA, Tokyo, Japan) at 2000x magnification. X-ray diffraction analysis was carried out with a Siemens D500 X-ray diffractometer with Cu Kα radiation. Fourier Transform Infrared (FTIR) is a common technique for analyzing the surface colonization of microorganisms [Citation34]. FTIR equipment of the Perkin Elmer Type Frontier (United States) was used to capture 4000–400 cm−1 spectrum. Changes in polymer functional groups were evaluated through FTIR analysis. FTIR analysis was performed on samples of control microalgae or microalgae that had not been flocculated and compared with samples of flocculated Chlorella utilizing dried clam shells or biomass.
3. Result and discussion
3.1. Mechanism of bioflocculation
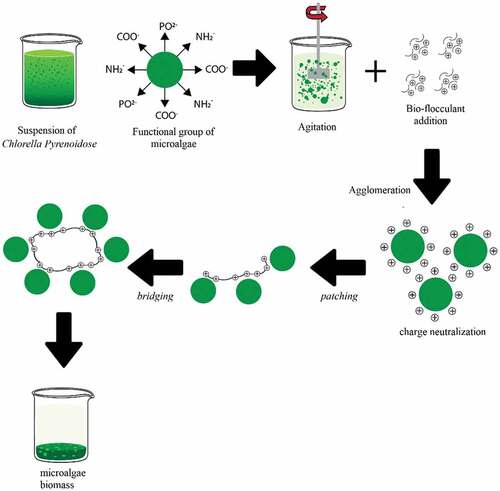
As shown in , it is crucial to examine the mechanism of microalgae bioflocculation by bioflocculants derived from clam shells. Under neutral pH settings, microalgal cells are negatively charged owing to the presence of active protons bonded to their surface, such as amine, phosphoric, hydroxyl, phosphodieter, and carboxyl functional groups [Citation28]. The pH of the microalgae culture is then adjusted to meet the predetermined conditions. Afterward, HCl is ionized into H+ and Cl−, while CaCO3 is ionized into Ca2+ and CO32-. Due to the low solubility of calcium carbonate in water, it should be emphasized that not all CaCO3 gets ionized to form calcium ions. Ca2+ then attaches to the negatively charged microalgae, resulting in a neutralizing reaction. It is difficult to show stoichiometry since the interaction between Ca2+ and microalgae is a physical binding generated by the difference in charge. Ca2+ binding to microalgae is enhanced by the presence of a cationic inducer, which also makes the environment more stable. When stirring is performed, the Ca2+ that binds microalgae aggregates and closes together via the process of patching and bridging, causing microalgal flocs to form. At the time of flocculation with clam shells, excess CaCO3 in decomposes into CaCl2 through the following breakdown reaction equation (3) and (4):
3.2. Effect of pH on bioflocculation
The pH is one of the factors that affects the flocculation efficiency of Chlorella pyrenoidosa. This was confirmed by the ANOVA results, which provided a P-value < 0.01 suggesting that the pH component was important for flocculation efficiency. This is based on the findings of prior research. According to previous research, autoflocculation occurs at high pH owing to differences in protonation and structural changes within the floc [Citation35]. Statistical investigation demonstrated that the influence of pH on flocculation efficiency is extremely strong and substantial, and that flocculation efficiency increases as pH rises.
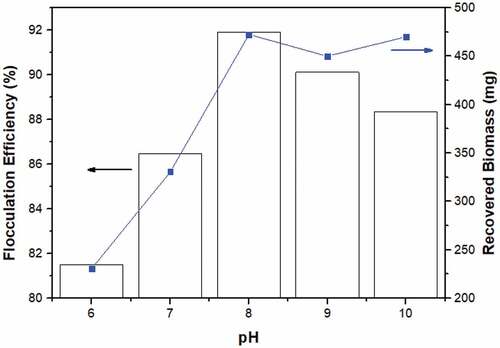
In acidic environments, the active sites on the microalgal cell wall were likely to be protonated and deprotonated as the pH rose [Citation36]. Calcium-rich mollusk skeletons may absorb and disrupt the negative charge on microalgae cells, resulting in flocculation. In the presence of a fixed dose of 0.2 mg/mL of clam shell bioflocculants, pH 8 was determined to be the optimal pH for harvesting 400 mg of microalgae culture, achieving a flocculation efficiency of around 91.89% as shown in . This may be a result of the increased electrostatic interaction between negatively charged deprotonated microalgae cells and positively charged bioflocculant particles at pH 8. However, any pH value over 8 resulted in a reduction in flocculation effectiveness.
It is known that pH variations affect the flocculant structure. Changes in pH may alter the surface charge of microalgal cells, leading them to become protonated when pH decreases and deprotonated when pH rises. Surface charge differences influence particle interactions and flocculation effectiveness. The cell surface may become negatively charged as a consequence of acid dissociation at higher pH values, i.e. pH greater than 6.0 [Citation37]. At extreme pH (very low and high pH), flocculation was likely impaired due to repulsive forces between particles with identical charges. In other words, microalgae cells and bioflocculant particles may have identical charges at that pH, resulting in repulsion [Citation37]. In addition, at extreme pH, partial dissolution of calcium-rich bioflocculant may release cationic and anionic ions into aqueous solution, which may subsequently adsorb onto the surface of bioflocculant, causing same-species hindrance [Citation38]. However, it has been noted that some microalgae species prefer to auto-flocculate at high pH owing to the precipitation of metal ions in the culture medium [Citation32]. Several studies have shown that auto-flocculation increases microalgae recovery by up to 90%, however the procedure is time-consuming and often requires the addition of calcium and magnesium ions [Citation39]. However, if the pH is too high, the eggshell load becomes negative, resulting in repulsion and a reduction in flocculation effectiveness [Citation27]. Moreover, Vandamme et al. [Citation15] found that repulsive forces might diminish particle interaction, hence disrupting the flocculation process.
3.2. Effect of bioflocculant concentration
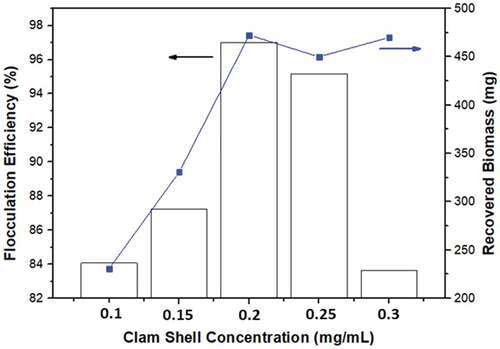
The optimal dosage of clam shells may increase flocculation because adsorption and charge neutralization are the most probable mechanisms involved in coagulation as shown in . The calcium carbonate (CaCO3) content of clam shell is 97% [Citation27,Citation40]. Extraction of bioflocculant from these shells by combining shell powder with an aqueous solution resulted in the partial breakdown of calcium carbonate and the release of a number of ions [Citation41]. Due to their high CaO content, clam shells have significant bioflocculant potential. Because Ca2+ ions may induce negative ions to form flocs that disperse throughout the microalgae solution.
The optimal concentration of bioflocculant is 0.2 mg/mL; if the concentration is too high, the bioflocculant does not form sediment but instead breaks the precipitate and creates turbidity in the solution. Due to the repulsion between positively charged particles of clam shells, this might result in deflocculation or stability of the particles [Citation37]. This validated the function of calcium ions as bioflocculant, as the coprecipitation of calcium ions and microalgae cells stimulated biofloc production at pH 8 and 100 mg flocculant clam shells.
3.3 Effect of cationic inducer
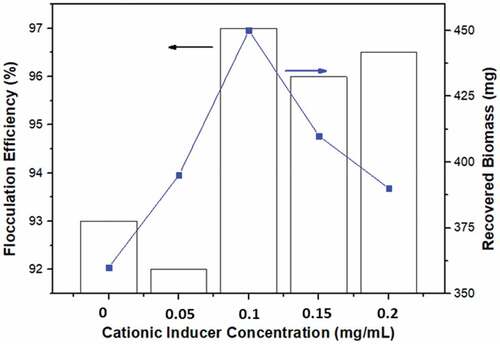
According to earlier investigations, ZnCl2 was utilized as a cationic inducer for Perna viridis flocculants [Citation25]. This technique, known as Divalent Cationic Bridging (DCB) by Sobeck & Higgins [Citation42], is used in wastewater treatment, which explains why floc properties are enhanced. As a link, the Zn2+ salt enhances the flocculation process by neutralizing the functional group’s residual negative charge [Citation32]. If the concentration of the cationic inducer is too high, the cell cohesion and flocculation efficiency will suffer. In this investigation, ZnCl2 was utilized as a cationic inducer, with 50 mg cationic inducer being optimum as shown in .
3.4 Effect of mixing rate
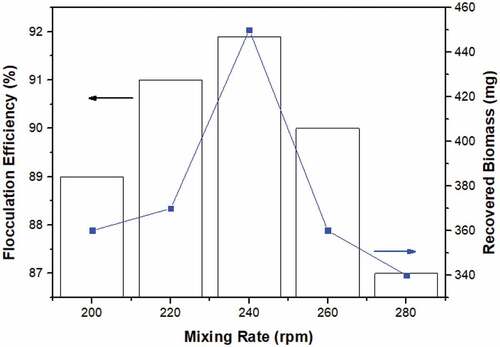
At a concentration of 0.2 mg/mL of clam shells, pH 8, and a concentration of 50 mg of cationic inducer, the stirring speed influenced the flocculation efficiency and biomass output (). 15 minutes of stirring at 240 revolutions per minute resulted in an optimal flocculation efficiency of 91.897%; if the mixing rate is too high, the flocculation efficiency will decline to 90% and then 87%, respectively. This phenomenon is a result of cell stability at high stirring rates [Citation43]. High-velocity mixing tends to break up floc, scatter fused cells, and reincorporate them into the medium.
3.5 Response surface analysis
Bioflocculation of Chlorella sp. was carried out using chitosan as a flocculant with optimization of the four variables used in the Central Composite Design (CCD) with 30 runs and 6 central points. The results of the prediction and experimental responses of each experiment are shown in . A positive sign indicates that the influence of the variable on flocculation is greater at higher concentrations, while the negative symbol shows that the influence of the variable on flocculation is greater at lower concentrations. Linear regression analysis was carried out using the second order polynomial equation according to the equation (5) and (6):
Table 2. Central composite design with observed responses pertaining to flocculation efficiency and recoverable biomass.
where Y flocculation efficiency and Y biomass are the response variables, and pH [X1], Chitosan [X2] concentration, Cationic Inducer [X3], and Mixing Rate [X4] are variables, and X12- X42 is the squared effect of variable. The derived regression equations (R2) are 0,8969 for harvesting efficiency and 0.8894 for collected biomass, indicating that the model is significant in terms of response prediction.
The coefficient is significant with a value of P < 0.05 and a magnitude greater than t. The coefficients on the response are marked with, X1, X2, X3, X4, X1X2, X1X3, X1X4, X2X3, X2X4, X3X4, X12, X22, X32, X42 and in this model the results obtained are significant [P 0,0001] . For flocculation efficiency, the ANOVA illustrated calculates F to be 9.32 and low P values, specifically P < 0.0001. reveals that the Linear vs Mean and Quadratic vs 2FI model was selected to describe the influence of the experimental variables (bioflocculant concentration, pH, cationic inducer concentration, and mixing rate) on the flocculation efficiency response. The P value for the Linear vs Mean model is less than 5%, hence it is advised to utilize it while optimizing the answer in question. However, the P value for the Quadratic vs 2FI model is 0.0001, indicating that the model has a smaller influence on explaining the anticipated result. However, it is suggested to use. shows that the biomass response yielded F = 8.61 and P < 0.0001 as indicated. demonstrates that the Linear vs Mean and 2FI vs Linear models were selected to describe the influence of experimental variables (bioflocculant concentration, pH, cationic inducer concentration, and mixing rate) on the flocculation efficiency response. The P value for the Linear vs Mean model is less than 5%, hence it is advised to utilize it while optimizing the questioned answer. In contrast, the P value for the 2FI vs Linear model is 0.2508, indicating that the model is very significant for explaining the questioned answer.
Table 3. ANOVA table for response surface function on flocculation efficiency.
Table 4. ANOVA table for response surface function on biomass.
show the effect of Perna viridis clam shells and pH on the flocculation process when Perna viridis is employed as a bio-flocculant. The graph demonstrates that Perna viridis, pH 8, and flocculants at a concentration of 0.2 mg/mL culture resulted in a flocculation efficiency of around 91%. This implies that the concentration supplied is optimum for floc formation. As seen in , a 0.1 mg/mL cationic inducer at pH 8 had a significant effect. In this environment, the greatest value was reported at a cationic inducer dosage of 0.1 mg/mL and a pH of 8. The flocculation process is significantly affected by the solution’s mixing velocity and pH (). Due to the improved cell stability at high speeds, the stirring speed has a significant influence on charge neutralization and sweep coagulation. The highest point in was reached with the addition of 0.2 mg/mL Perna viridis and 0.1 mg/mL cationic inducer due to the rapid flocculation of microalgae by ZnCl2, a cationic inducer with a significant positive charge that attracts and flocculates microalgal cells. depicts the connection between stirring speed and flocculant dose when 0.15 mg/mL Perna viridis is applied with 220 rpm stirring. Due to the insufficient Perna viridis concentration, the flocculation efficiency was insufficient. In , the connection between stirring speed and cationic inducer was adjusted too low, resulting in ineffective flocculation. Due to insufficient cationic inducer dose and insufficient stirring speed, the procedure was inefficient. As a result, a link was established between four variables: cationic inducer, pH, bio-flocculants, and stirring speed, all of which had a significant impact on the time required to reveal these four plots: cationic inducer, pH, bio-flocculants, and stirring speed. As seen in , a culture with pH 8 and bio-flocculants at a concentration of 0.2 mg/mL produced a biomass of 458.1 mg. As shown in , the peak was reached with a cationic inducer at a concentration of 0.1 mg/mL and a pH of 8, while the smallest gap was obtained with a cationic inducer at a concentration of 75 mg/L and a pH of 9. This was the result of an insufficient dosage of the cationic inducer, which resulted in less-than-desirable biomass. As demonstrated in , a stirring speed of 260 rpm and a pH of 9 resulted in a decrease in biomass to 81%, which was likely due to the higher cell stability created by excessive agitation. Using a 0.05 mg/mL cationic inducer with a 0.15 mg/mL Perna viridis yielded the lowest gap, as seen in . This happened because insufficient quantities of cationic inducer and flocculant were used. As a result, both the quantity and efficiency of biomass production were inefficient. The peak plot shown in was produced by swirling 0.2 mg/mL of clam shells at 240 rpm. This was the optimal dose, as it allowed Perna viridis to absorb all microalgal cells with sufficient agitation. As seen in , the biomass declined when the stirring speed was raised to its maximum. This was due to the fact that the stirring speed had a significant impact on charge neutralization and sweep coagulation, which occur as a consequence of cell stability.
Figure 6. 3D response surface and contour plots illustrating the interaction effects of a) Perna viridis-pH, b) cationic inducer-pH, c) mixing rate-pH, d) cationic inducer-Perna viridis, e) mixing rate- Perna viridis, and f) mixing rate-cationic inducer on the flocculation efficiency of Chlorella sp. Flocculation.
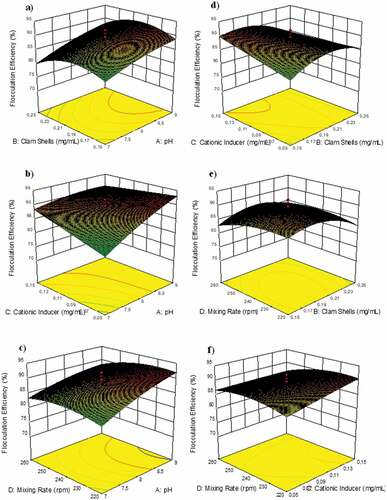
Figure 7. 3D response surface and contour plots illustrating the interaction effects of a) Perna viridis-pH, b) cationic inducer-pH, c) mixing rate-pH, d) cationic inducer-Perna viridis, e) mixing rate- Perna viridis, and f) mixing rate-cationic inducer on the biomass of Chlorella sp. flocculation.
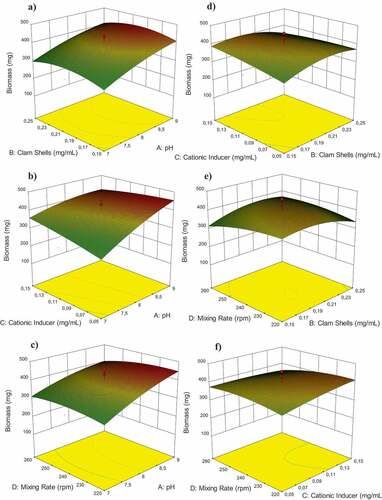
The form of the contour plot reveals the significance of the interaction between the elements. Using three-dimensional surfaces and contour plots, it is easy to study the reciprocal interaction and optimization of the tested variables. By analyzing response surface plots and contour representations of the observed interaction effects, the following optimal values for the examined variables were determined: pH 8, Perna viridis concentration of 0.2 mg/mL, cationic inducer concentration of 0.1 mg/mL, and stirring speed of 240 rpm. The model was tested in triplicate under ideal conditions, with a flocculation efficiency of 91.87% and a biomass recovery value of 458.1 mg. As a consequence of our research, we have determined that bio-flocculation using Perna viridis shells is an efficient technique for microalgal cell collection. The optimization results were then compared to those of various prior studies that harvested Chlorella through flocculation. Prochazkova et al. [Citation44], for instance, used 1.3 g/L Diethylaminoethyl/Polyethylenimine as a flocculant to collect Chlorella vulgaris with an efficiency more than 90%, a flocculation duration of 30 minutes, and a pH range of 2–10.
Hadiyanto et al. [Citation4] used a dose of 59.98 mg/120 ml eggshell as a flocculant for Chlorella pyrenoidosa with a flocculation efficiency of 90.78%. Kumar [Citation45] collected Chlorella sp. with 96.68% efficiency using 50 mg/L cationic locust bean gum biopolymer as a flocculant. Rakesh et al. [Citation46] collected Chlorella sp. using a total of 120 mg/L maize starch with an efficiency of 80%. In previous research, 90% microalgae recovery was achieved using auto-flocculation, but the procedure is time-consuming and often requires the addition of calcium and magnesium ions [Citation39]. The comparison above reveals that the optimized operating conditions are equivalent to or even superior than those of prior experiments. The kind and concentration of microalgae also influence the flocculation process from the microalgae’s vantage point. The higher the microalgae concentration, the greater the required flocculant dosage. In relation to flocculant interaction, each kind of microalgae has unique features.
3.6 Morphological analysis
depicts a SEM image of Chlorella sp. that had not been treated with Perna viridis shells, and depicts a Perna viridis as a clam shells agglomeration with microalgal cells and SEM images following flocculation at pH 8, 100 mg/mL flocculant concentration, 50 mg/mL cationic inducer concentration, and 240 rpm stirring speed. Existing research on microalgae harvesting using bioflocculants recovered from shell waste is sparse. These calcium-rich shell wastes have a high cationic charge density with diverse functional groups including carboxyl, sulfate, phosphate, and amino groups designed to adsorb and disrupt negatively-charged microalgae cells [Citation47].
The high CaO content of Perna viridis shells makes them a promising candidate for use as a bioflocculant. Ca2+ ions are capable of forming flocs for negatively charged ions, which bind to negatively charged microorganisms such as microalgae. When negatively charged algal cells coexist with positively charged Perna viridis shells in a solution, electrostatic repulsion between the cells lessens. At large quantities of flocculant, cationic polymers cover microalgal cells, leaving insufficient vacant sites and resulting in a net positive charge [Citation37]. In addition, this positive charge attracts the negatively charged cells in its surroundings, resulting in the formation of a floc. The morphology of microalgae changes when chitosan is applied as a bio-flocculant. The surface morphology of Perna viridis shells is varied. Perna viridis shells have been used to encapsulate Chlorella sp. cells, transforming their smooth surface into a fibrous one [Citation48–51]. Carbon from the bio-flocculant decreased the carbon content of Chlorella following flocculation, as seen in .
Table 5. Energy Dispersive X-ray spectroscopy (EDX) analysis results for the chemical constituents of Chlorella sp flocculation.
3.7 XRD analysis
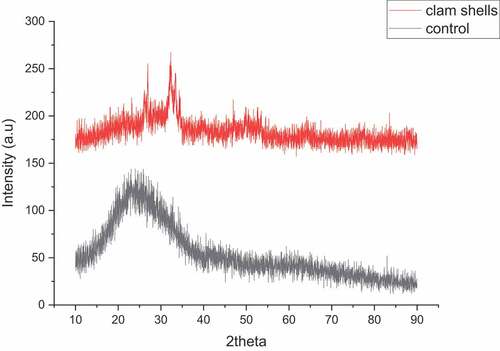
Using XRD, the crystallinity of dry bioflocculants isolated from clam shells was determined. In nature, calcium carbonate exists as three anhydrous crystalline polymorphs (calcite, aragonite, and vaterite), two hydrated crystalline polymorphs (calcium carbonate monohydrate (CaCO3.H2O) and calcium carbonate hexahydrate (CaCO3.6H2O), and one amorphous polymorph [Citation47]. However, these results indicate that the XRD spectra of the flocculants do not match those of calcium carbonate, which is the most thermodynamically stable component in the shell. In clam shell bioflocculants, wide peaks with low intensity and inability to be distinguished in the presence of high noise levels suggested that non-crystalline structure predominated. Amorphous calcium carbonate is a hydrated, irregular, and unstable crystalline precursor of calcium carbonate seen in a variety of animals, including adult sea urchin larvae and mollusc bivalves larvae. It is generally known that commercial and powdered calcium carbonate have hydrophobic properties and a high water absorption capacity [Citation52]. As seen in , the sample collects moisture from the air and upon exposure, attesting to the amorphous nature of calcium carbonate included in the bioflocculant.
3.8 FTIR analysis
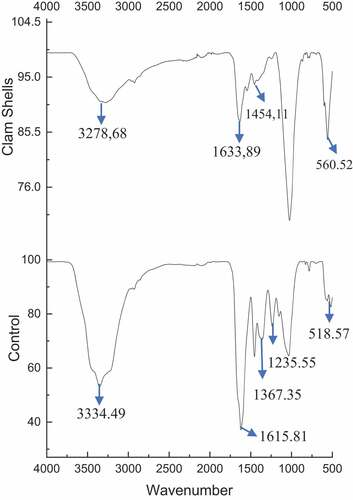
Using FTIR, the identification of functional groups and position of these groups in control microalgae, bio-flocculants, and flocs formed by addition of bio-flocculants extracted from clam shell were studied to determine the likelihood of biomolecular group retention or any chemical changes that occurred after the flocculation process. displays the FTIR spectrum of a sample of raw microalgae. The absorption spectra seen at 3334.49 cm−1 corresponds to -OH stretching. The existence of C = C explains the stretching that happened at the wide band peak of 2110 cm−1. The primary amide and carbonyl (C–O) groups in proteins have respective wavelengths of 1615.81 and 1633.89 cm−1, which are attributable to the C = O stretching vibration and a combination of -NH bending and C-N stretching vibrations in amide complexes. COO-carboxylate bond binding and (NCH3)3 lipid bending occur at a wavelength of 1367.35 cm−1 [Citation53–55]. On the other hand, 1454.11 cm−1 is distinguished by intense vibrations induced by the bending of CH2, the intensity of which then rises. The nucleic acid spectrum was discovered at 1235.55 cm−1. Residual absorption values of 560.52 cm−1 and 518.57 cm−1 revealed the existence of potent alkyl halides [Citation56].
Conclusion
Current study reveals the novel potential of bioflocculants extracted from clam shell for harvesting Chlorella pyrenoidosa at up to 91.98% and 458.1 mg of flocculation efficiencies and biomass recovery at pH 8, bioflocculant concentration of 0.2 mg/mL, ZnCl2 concentration of 0.1 mg/mL, and mixing rate of 240 rpm. Characterization research demonstrated that co-precipitation of calcium ions, which are plentiful in these shell-derived bioflocculants, is the most important factor in the efficient flocculation of microalgae cells. However, the Response Surface Methodology and ANOVA-derived regression equations (R2) for harvesting efficiency and biomass recovery are 0.8969 and 0.8894, respectively, demonstrating that the model is significant in terms of response prediction. The SEM and EDX investigations revealed that mollusk shells underwent morphological changes. Clam shells surface structure is highly varied. The smooth surface of Chlorella sp. was converted into a fibrillar structure, and XRD analysis revealed low-intensity, indistinct peaks with considerable noise, suggesting that the chitosan bioflocculant had a non-crystalline structure. The absorption spectra at 3334.49 cm−1, which corresponds to -OH stretching, the primary amide and carbonyl (C–O) groups in proteins with respective wavelengths of 1615.81 and 1616.89 cm−1, and the nucleic acid spectrum discovered at 1235.55 cm−1 are the most important peaks for determining the properties of clam bio-flocculant. Clam shells are inexpensive, non-toxic, and need minimum preparation. These results provide a cost-effective alternative to standard chemical flocculants for microalgae harvesting.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Van Vuuren DP, Bellevrat E, Kitous A, et al. Bio-Energy Use and Low Stabilization Scenarios. Energy J. 2010;31:193–222.
- DOE, USDA. Biomass as feedstock for a bioenergy and bioproducts industry : the technical feasibility of a billion-ton annual supply. Agriculture April. 2005.
- Gan J, Smith CT. Availability of logging residues and potential for electricity production and carbon displacement in the USA. Biomass Bioenergy. 2006;30(12):1011–1020.
- Hadiyanto H, Widayat W, Christwardana M, et al. The flocculation process of Chlorella sp. using chitosan as a bio-flocculant: optimization of operating conditions by response surface methodology. Curr Res Green Sustainable Chem. 2022;5:100291.
- Rawat I, Ranjith Kumar R, Mutanda T, et al. Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl Energy. 2013;103:444–467.
- Pulz O, Gross W. Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol. 2004;65(6):635–648.
- Spolaore P, Joannis-Cassan C, Duran E, et al. Commercial applications of microalgae. J Biosci Bioeng. 2006;101(2):87–96.
- Gavrilescu M, Chisti Y. Biotechnology - A sustainable alternative for chemical industry. Biotechnol Adv. 2005;23(7–8):471–499.
- Kapdan IK, Kargi F. Bio-hydrogen production from waste materials. Enzyme Microb Technol. 2006;38(5):569–582.
- Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25(3):294–306.
- Han W, Jin W, Ding W, et al. Effects of nutrient composition, lighting conditions, and metal ions on the growth and lipid yield of the high-lipid-yielding microalgae (Chlorella pyrenoidosa) cultivated in municipal wastewater. J Environ Chem Eng. 2021;9(6):106491.
- Ansari FA, Nasr M, Rawat I, et al. Artificial neural network and techno-economic estimation with algae-based tertiary wastewater treatment. Journal of Water Process Engineering. 2021;40:101761.
- Ansari FA, Nasr M, Guldhe A, et al. Techno-economic feasibility of algal aquaculture via fish and biodiesel production pathways: a commercial-scale application. SciTotal Environ. 2020;704:135259.
- Jiménez-Llanos J, Ramírez-Carmona M, Rendón-Castrillón L, et al. Sustainable biohydrogen production by Chlorella sp. microalgae: a review. Int J Hydrogen Energy. 2020;45(15):8310–8328.
- Vandamme D, Foubert I, Fraeye I, et al. Flocculation of Chlorella vulgaris induced by high pH: role of magnesium and calcium and practical implications. Bioresour Technol. 2012;105:114–119.
- Wu X, Ge X, Wang D, et al. Distinct coagulation mechanism and model between alum and high Al13-PACl. Colloids Surf A Physicochem Eng Asp. 2007;305(1–3):89–96.
- Sinardi PS, and Notodarmojo S. The chemical characteristics of chitosan extracted from green mussels shell (Mytilus virdis linneaus) and its potential application as a natural coagulant. In The Second International Conference on Sustainable Infrastructure and Built Environment, 19-20 November, Bandung, Indonesia, 2013;1:11–17.
- Siregar SM. Pemanfaatan kulit kerang dan resin epoksi terhadap karakteristik beton polimer. Universitas Sumatera Utara. 2009; June.
- Wulandari CD, Setyobudiarso H, Koteldae M. Utilization of chitosan clam bloodshells as a coagulant for processing electroplatting waste. J Phys. 2021;1869(1):012001. IOP Publishing.
- Shelef G, Sukenik A. Microalgae harvesting and processing : a literature review. Off Sci Tech Inform. 1984;6204677.
- Barros AI, Gonçalves AL, Simões M, et al. Harvesting techniques applied to microalgae: a review. Renew Sust Energ Rev. 2015;41:1489–1500.
- Oh HM, Lee SJ, Park MH, et al. Harvesting of Chlorella vulgaris using a bioflocculant from Paenibacillus sp. AM49 Biotechnol Letters. 2001;23(15):1229–1234.
- Hadiyanto H, Christwardana M, Widayat W, et al. Optimization of flocculation efficiency and settling time using chitosan and eggshell as bio-flocculant in Chlorella pyrenoidosa harvesting process. Environ Technol Innovation. 2021;24:1–27.
- Abdul Razack S, Duraiarasan S, Santhalin Shellomith AS, et al. Statistical optimization of harvesting Chlorella vulgaris using a novel bio-source. Strychnos Potatorum Biotechnol Reports. 2015;7:150–156.
- Surendhiran D, Vijay M. Exploration on bioflocculation of nannochloropsis oculata using response surface methodology for biodiesel production. Sci World J. 2014;2014:1–9.
- Guldhe A, Misra R, Singh P, et al. An innovative electrochemical process to alleviate the challenges for harvesting of small size microalgae by using non-sacrificial carbon electrodes. Algal Res. 2016;19:292–298.
- Xu Y, Purton S, Baganz F. Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour Technol. 2013;129:296–301.
- Suparmaniam U, Lam MK, Uemura Y, et al. Flocculation of Chlorella vulgaris by shell waste-derived bioflocculants for biodiesel production: process optimization, characterization and kinetic studies. SciTotal Environ. 2020;702:134995.
- Zhang H, Yang L, Zang X, et al. Effect of shear rate on floc characteristics and concentration factors for the harvesting of Chlorella vulgaris using coagulation-flocculation-sedimentation. SciTotal Environ. 2019;688:811–817.
- Papazi A, Makridis P, Divanach P. Harvesting Chlorella minutissima using cell coagulants. J Appl Phycol. 2010;22(3):349–355.
- Akış S, Özçimen D. Optimization of pH induced flocculation of marine and freshwater microalgae via central composite design. Biotechnol Prog. 2019;35(3):e2801.
- Zheng H, Gao Z, Yin J, et al. Harvesting of microalgae by flocculation with poly (γ-glutamic acid). Bioresour Technol. 2012;112:212–220.
- Li S, Wang P, Zhang C, et al. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. SciTotal Environ. 2020;714:136767.
- Hadiyanto H, Khoironi A, Dianratri I, et al. Interactions between polyethylene and polypropylene microplastics and Spirulina sp. microalgae in aquatic systems. Heliyon. 2021;7(8):e07676.
- Show KY, and Lee DJ Algal biomass harvesting. In: Pandey A, Lee DJ, Chisti Y, Soccol C, editors. Biofuels from algae. Elsevier; 2014. p. 85–110.
- Singh G, Patidar SK. Microalgae harvesting techniques: a review. J Environ Manage. 2018;217:499–508.
- Choi HJ. Effect of eggshells for the harvesting of microalgae species. Biotechnol Biotechnol Equipment. 2015;29(4):666–672.
- Zulfikar MA, Mariske ED, Djajanti SD. Adsorption of lignosulfonate compounds using powdered eggshell. Songklanakarin J Sci Technol. 2012;34(3):309–316.
- Japar AS, Takriff MS, Yasin NHM. Harvesting microalgal biomass and lipid extraction for potential biofuel production: a review. J Environ Chem Eng. 2017;5(1):555–563.
- Wu Q, Chen J, Clark M, et al. Adsorption of copper to different biogenic oyster shell structures. Appl Surf Sci. 2014;311:264–272.
- Podstawczyk D, Witek-Krowiak A, Chojnacka K, et al. Biosorption of malachite green by eggshells: mechanism identification and process optimization. Bioresour Technol. 2014;160:161–165.
- Sobeck DC, Higgins MJ. Examination of three theories for mechanisms of cation-induced bioflocculation. Water Res. 2002;36(3):527–538.
- Blockx J, Verfaillie A, Thielemans W, et al. Unravelling the mechanism of chitosan-driven flocculation of microalgae in seawater as a function of pH. ACS Sustain Chem Eng. 2018;6(9):11273–11279.
- Prochazkova G, Podolova N, Safarik I, et al. Physicochemical approach to freshwater microalgae harvesting with magnetic particles. Colloids Surf B Biointerfaces. 2013;112:213–218.
- Kumar MNVR. A review of chitin and chitosan applications. Science. 2000;46(1):1–27.
- Rakesh S, Tharunkumar J, Sri B. Sustainable cost-effective microalgae harvesting strategies for the production of biofuel and oleochemicals. Highlights Biosci. 2020July;3:1–8.
- Bozbaş SK, Boz Y. Low-cost biosorbent: anadara inaequivalvis shells for removal of Pb(II) and Cu(II) from aqueous solution. Process SafEnviron Prot. 2016;103:144–152.
- Banerjee C, Ghosh S, Sen G, et al. Study of algal biomass harvesting using cationic guar gum from the natural plant source as flocculant. Carbohydr Polym. 2013;92(1):675–681.
- Bolto B, Gregory J. Organic polyelectrolytes in water treatment. Water Res. 2007;41(11):2301–2324.
- Morales J, de la Noüe J, Picard G. Harvesting marine microalgae species by chitosan flocculation. Aquacult Eng. 1985;4(4):257–270.
- Salim S, Bosma R, Vermuë MH, et al. Harvesting of microalgae by bio-flocculation. J Appl Phycol. 2011;23(5):849–855.
- Bootklad M, Kaewtatip K. Biodegradation of thermoplastic starch/eggshell powder composites. Carbohydr Polym. 2013;97(2):315–320.
- Duygu DY. Fourier transform infrared (FTIR) spectroscopy for identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr J Biotechnol. 2012;11(16):3817–3824.
- Giordano M, Kansiz M, Heraud P, et al. FOURIER TRANSFORM INFRARED SPECTROSCOPY AS A NOVEL TOOL TO INVESTIGATE CHANGES IN INTRACELLULAR MACROMOLECULAR POOLS IN THE MARINE MICROALGA CHAETOCEROS MUELLERII (BACILLARIOPHYCEAE). J Phycol. 2001;37(2):271–279.
- Stuart BH. Infrared spectroscopy: fundamentals and applications. New Jersey, USA: John Wiey & Sons; 2004.
- Indhumathi P, Soundararajan M, Shabudeen PS, et al. Utilization, isolation and characterization of Chlorella vulgaris for carbon sequestration and waste water treatment by performing FTIR spectral studies. Asian J Microbiol Biotechnol Environ Sci. 2013;15:661–666.