Abstract
Objectives: Adhesion and marginal adaptation of a claimed bioactive restorative material (ACTIVA BioACTIVE Restorative) to human teeth were compared with those of a resin-modified glass ionomer cement (Fuji II LC) and a control resin composite (Ceram X Mono).
Material and Methods: Shear bond strength and marginal adaptation to enamel and dentine were assessed after no pretreatment of the hard tissues or after etching with phosphoric acid (ACTIVA BioACTIVE Restorative and Ceram X Mono) or polyacrylic acid (Fuji II LC). For ACTIVA BioACTIVE Restorative, the effect of applying a self-etch adhesive (Xeno Select, Dentsply Sirona) was also investigated. Data were analyzed using non-parametric tests (α = 0.05).
Results: Bond strength and marginal adaptation in enamel and dentine were significantly different among the investigated materials (p<.05). Due to loss of restorations, it was not possible to measure bond strength of ACTIVA BioACTIVE Restorative if no pretreatment was performed or if dentine was etched; however, use of the self-etch adhesive resulted in similar bond strength as Ceram X Mono. Etching improved adhesion of Fuji II LC to enamel and dentine. Regarding marginal adaptation, ACTIVA BioACTIVE Restorative showed the highest wall-to-wall contraction to enamel in all pretreatment groups and the overall highest wall-to-wall contraction to dentine after etching. Due to loss of restorations, no marginal assessment was possible on cavities with margins in dentine when no pretreatment was used. The use of a self-etch adhesive with ACTIVA BioACTIVE Restorative resulted in similar adaptation to dentine compared to the other materials.
Conclusion: The self-adhesive property of ACTIVA BioACTIVE Restorative is nonexistent.
Introduction
Despite the many advantages and recent improvements within resin composites for dental applications, a drive for the development of alternative, smart restorative materials is observed [Citation1]. The recent trend shows restorative resin composites that are user-friendly while either maintaining sufficient mechanical properties or with claimed bioactivity.
A relatively recent development is ACTIVA BioACTIVE Restorative (Pulpdent Corporation, Watertown, MA), a resin-modified glass ionomer cement (RMGIC) which the manufacturer states to be enhanced with ‘rubberized’ resin. Earlier work has investigated some properties of interest of this material: flexural strength was shown to be similar to that of flowable and bulk-fill resin composites [Citation2,Citation3] and significantly greater than that of other RMGIC [Citation2]. High fracture toughness was observed compared to compomers and RMGIC [Citation4]. Wear was reported to be similar to that of a resin composite [Citation5], although the material’s compromised hardness suggests the importance of using an abrasive-resistant resin composite as coverage [Citation3]. The promising mechanical behavior somewhat diverted research from other relevant properties, such as the claimed bioactivity and self-adhesion.
Considering the fact that ACTIVA BioACTIVE Restorative is a RMGIC, self-adhesion to the hard dental tissues would be anticipated. Nevertheless, a very high initial failure rate of restorations made with ACTIVA BioACTIVE Restorative was recently reported in a 1-year clinical follow-up of posterior restorations in adults [Citation6]. The main reasons for failure described in this randomized study were a high incidence of loss of restorations, post-operative sensitivity and secondary caries [Citation6]. It is therefore important to investigate this material’s quality of bond and ability to provide good cavity seal, both factors of relevance for the material’s performance [Citation7]. Whereas an almost intact interface was registered for ACTIVA BioACTIVE when the restoration was not challenged [Citation8], conference abstracts and reports have hinted that marginal adaptation of ACTIVA BioACTIVE Restorative could be problematic [Citation9,Citation10]. Studies reported overall higher microleakage of cavities restored with ACTIVA BioACTIVE Restorative if no previous etching was performed nor an adhesive applied, compared to cavities restored with resin composite [Citation11,Citation12]. The laboratory and clinical findings indicate that the self-adhesive ability of ACTIVA BioACTIVE Restorative is not fully elucidated. The aim of this study was to investigate, in vitro, the adhesion, marginal adaptation and volumetric contraction of the claimed bioactive restorative material (ACTIVA BioACTIVE Restorative) in comparison to a RMGIC and a resin composite.
Materials and methods
An overview of the study groups is shown in . Extracted human molars (n = 240) kept in 0.5% chloramine aqueous solution were embedded in epoxy resin. The molars were ground using sequential silicon carbide paper up to #1000 grit to expose a flat surface in enamel (n = 120) or dentine (n = 120). Half of the teeth were used to test for shear bond strength and the other half to assess marginal adaptation. The teeth used in this study were extracted for therapeutic reasons and collected from anonymous donors; therefore, approval from the national ethical committee was not required. This study was conducted in respect with the Declaration of Helsinki.
Table 1. Overview of the restorative materials and respective manufacturers as well as the pretreatment of enamel and dentine for the materials investigated in this study.
Shear bond strength
A restoration was placed on the surface of enamel or dentine by filling a split Teflon mould with a cylindrical opening (diameter 3.6 mm; height 2.5 mm), tightly clamped to the embedded tooth [Citation13], with one of the following materials: ACTIVA BioACTIVE Restorative, Fuji II LC (G.C. Europe N.V., Leuven, Belgium) or Ceram X Mono (Dentsply DeTrey GmbH, Konstanz, Germany). Clamping the tooth to the mould ensured that the restorative material did not extend outside the area limited by the mould’s orifice.
In restorations made with ACTIVA BioACTIVE Restorative and Fuji II LC, the enamel and dentine either: (a) did not receive any pretreatment or (b) were previously etched for 10 s (as per manufacturer’s instructions at the start of this study). In the latter case, enamel and dentine were etched with 38% phosphoric acid (Top Dent Etch Gel, DAB Dental A.B., Väsby, Sweden) prior to restoration with ACTIVA BioACTIVE Restorative and with 10% polyacrylic acid (Dentin Conditioner, G.C. Europe N.V., Leuven, Belgium) prior to restoration with Fuji II LC.
An additional group of specimens received the application of a universal adhesive using the self-etching technique (Xeno Select, Dentsply DeTrey, Konstanz, Germany) prior to restoration with ACTIVA BioACTIVE Restorative. The self-etch adhesive was applied twice to wet the tooth surface uniformly, gently agitated during 20 s and dried during 5 s for thorough evaporation of the solvent. The adhesive was light-cured for 10 s. The restorative material was inserted in the Teflon mould and subsequently light-cured for 40 s with an LED lamp at 1470 mW/cm2 (Elipar DeepCure-L, 3M Deutschland GmbH, Neuss, Germany). Power density of the lamp was monitored using a radiometer (Bluephase Meter II, Ivoclar Vivadent, Schaan, Liechtenstein).
At last, positive control specimens were prepared. Here, enamel and dentine were treated with the adhesive Xeno Select as previously described and restored with the resin composite Ceram X Mono.
Ten specimens were produced for each group. Immediately after light-curing, the specimens were separated from the mould and stored in phosphate-buffered saline solution at 37 °C. The specimens were then thermocycled for 6000 cycles between 5 °C and 55 °C water baths [Citation13] and subsequently stored in phosphate-buffered saline solution at 37 °C for 28 days before shear bond strength testing. Shear bond strength testing was performed in a universal testing machine (Instron, High Wycombe, UK) at a crosshead speed of 1 mm/min [Citation13].
Marginal assessment
Semi-spherical cavities with 3 mm diameter and 1.5 mm depth were prepared [Citation14] with margins either in enamel or dentine using a spherical diamond bur (no. 6801.314.029, Komet Dental, Gebr. Brasseler GmbH & Co. KG, Lemgo, Germany) and the exact cavity diameters were registered using a stereomicroscope under ×2.5 magnification (Zeiss, Oberkochen, Germany) and calibrated software (Deltapix InSight, Smorum, Denmark) (). Subsequently, 10 restorations were made in each group using the same restorative materials and procedures described earlier in detail and according to . Grinding with silicon carbide paper #1000 was performed to remove excess material above cavity margins. Thereafter, the specimens were polished wet on felt using aluminium oxide particles and finally were rinsed with pressurized water to remove possible residues from the surface.
Figure 1. Schematics of the cavity preparations for marginal assessments with margins in enamel (left) or dentine (right).
The specimens were stored in phosphate-buffered saline solution at 37 °C for 1–2 h and initial marginal assessment was performed. The specimens were then thermocycled for 6000 cycles between 5 °C and 55 °C water baths and a second marginal assessment was carried out. Subsequently, the specimens were stored in phosphate-buffered saline solution at 37 °C for 28 days, when the final marginal assessment was made.
The marginal adaptation was assessed with a light microscope at ×64 magnification. When a gap occurs in a cavity with a circular opening, the gap has a crescent shape and it is obvious to identify the position of the largest gap by pre-inspecting the margins. After pre-inspecting the marginal perimeter, the widest gap of each specimen was identified and measured. Thereafter, the directly opposite margin was also assessed and any gap present there was measured. Considering the total gap measured linearly from both opposing margins, wall-to-wall contraction of the restoration was calculated as a percentage of the cavity diameter [Citation14].
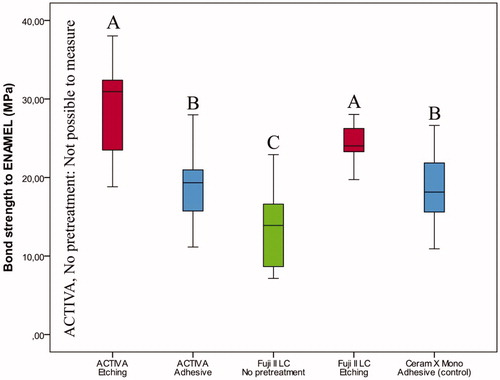
Figure 2. Box-plot for shear bond strength (MPa) of the restorative materials according to pretreatment of enamel after thermocycling and storage for 28 days. The boxes include median and 25–75% quartiles, the whiskers represent the interquartile range. Different letters represent statistical significance.
Volumetric contraction
The volumetric contraction of the materials was measured using the bonded-disk method [Citation15], as described in details elsewhere [Citation7]. In short, standard amounts of the restorative materials (0.22 ± 0.02 g) were placed on a glass plate attached to a metallic ring. On top of the ring, a thin glass lamina was positioned on the surface of which a linear variable differential transformer (LVDT; 7DCDT-100, Hewlett-Packard, Waltham, MA) rested, connected to a power output of 5 V. When the bottom of the restorative material was light-cured for 40 s using the light-curing LED lamp, the glass lamina deflected. Values of vertical linear displacement of the LVDT after 60 min were converted to polymerization contraction. Triplicates of each restorative material were measured.
Statistical methods
Shapiro–Wilk’s tests showed non-normal distribution in shear bond strength, marginal adaptation and volumetric contraction for some groups; therefore, non-parametric tests were used to analyse the data at the level of significance α = 0.05. Median and interquartile range were calculated for each group. A Kruskal–Wallis test was applied to identify possible differences between groups, followed by post hoc multiple pairwise comparisons using Mann–Whitney’s U tests. Possible differences between repeated wall-to-wall contraction data obtained before thermocycling, immediately after thermocycling and after storage for 28 days within groups were investigated using Friedman’s tests and Wilcoxon’s signed ranks tests.
Results
The results are summarized in . Additional data are presented in Tables S1 and S2 in the supplementary information.
Figure 3. Box-plot for shear bond strength (MPa) of the restorative materials according to pretreatment of dentine after thermocycling and storage for 28 days. The boxes include median and 25–75% quartiles, the whiskers represent the interquartile range. Different letters represent statistical significance.
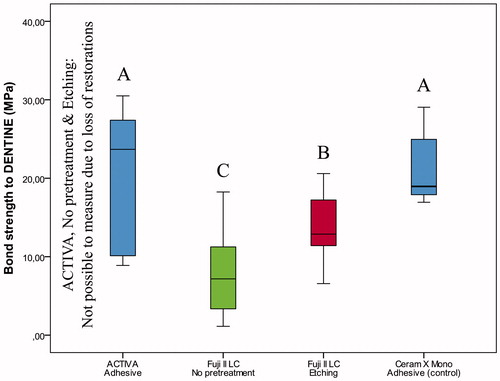
Figure 4. Box-plot for wall-to-wall contraction (%) of the restorative materials according to pretreatment of cavities with margins on enamel after thermocycling and storage for 28 days. The boxes include median and 25–75% quartiles, the whiskers represent the interquartile range. Different letters represent statistical significance.
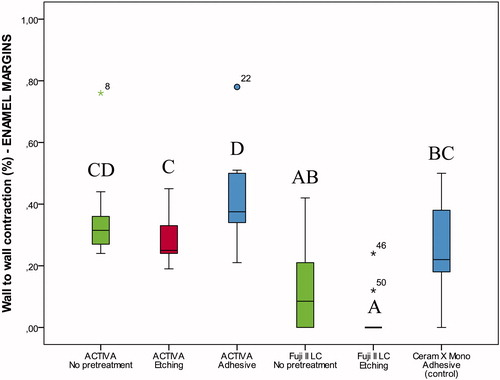
Figure 5. Box-plot for wall-to-wall contraction (%) of the restorative materials according to pretreatment of cavities with margins on dentine after thermocycling and storage for 28 days. The boxes include median and 25–75% quartiles, the whiskers represent the interquartile range. Different letters represent statistical significance.
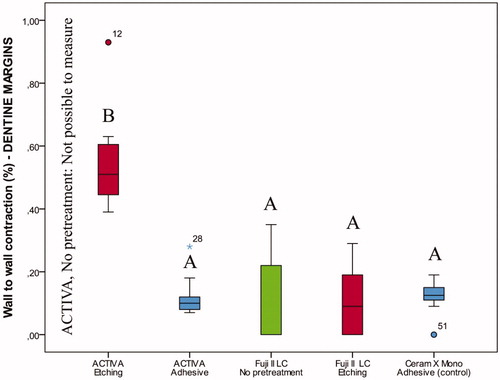
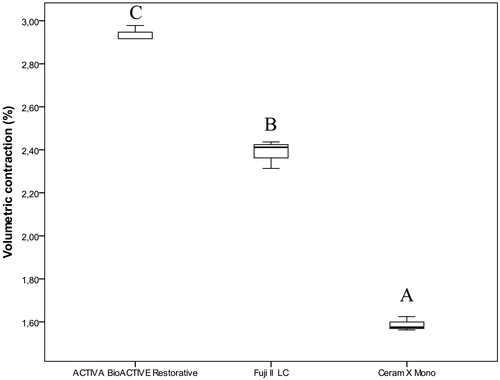
Figure 6. Box-plot for volumetric contraction (%) of the investigated restorative materials. The boxes include median and 25–75% quartiles, the whiskers represent the interquartile range. Different letters represent statistical significance.
Shear bond strength
If no previous etching was performed or self-etch adhesive applied prior to restoration with ACTIVA BioACTIVE Restorative, all restorations were lost and measurements were not possible. Previous etching with phosphoric acid enabled adhesion of ACTIVA BioACTIVE Restorative to enamel and resulted in higher bond strength than the control; however, previous etching was not sufficient to secure restorations to dentine ( and , Table S1). Similar bond strengths were observed for ACTIVA BioACTIVE Restorative placed with the self-etch adhesive and the control resin composite (enamel: p = 1.000, dentine: p = .657) (Table S1, and ).
For Fuji II LC, etching with polyacrylic acid significantly improved bond strength of restorations on both enamel and dentine ( and , Table S1).
Marginal assessment
Friedman tests identified significant differences within groups for wall-to-wall contraction assessments made before thermocycling, immediately after thermocycling and after storage for 28 days, both for restorations with enamel (p<.01) and dentine margins (p<.01). According to Wilcoxon’s signed ranks test, wall-to-wall contraction after storage for 28 days was significantly different than that obtained before and after thermocycling (p=.001). After storage for 28 days, the trend was that wall-to-wall contraction became smaller for Fuji II LC and Ceram X Mono. ACTIVA BioACTIVE Restorative, on the contrary, behaved more broadly and showed a tendency to increased wall-to-wall contraction after thermocycling and storage for 28 days.
Differences in wall-to-wall contraction were observed between groups (p<.05). For restorations with enamel margins stored for 28 days, ACTIVA BioACTIVE Restorative showed significantly higher wall-to-wall contraction than Fuji II LC and Ceram X Mono (, Table S2). If no previous etching was performed or adhesive applied in dentine prior to restoration with ACTIVA BioACTIVE Restorative, restorations were lost after thermocycling; thus, wall-to-wall contraction could not be measured (, Table S2). In restorations with dentine margins, the highest wall-to-wall contraction was seen for ACTIVA BioACTIVE Restorative after phosphoric acid etching; however, this improved significantly with the use of the self-etch adhesive, with results similar to the control (resin composite restorations).
The best marginal adaptation after 28 days was seen for Fuji II LC, both in restorations with enamel and dentin margins ( and , Table S2).
Volumetric contraction
Significant differences were observed between all three investigated restorative materials (p=.027). ACTIVA BioACTIVE Restorative showed the highest contraction followed by Fuji II LC. The lowest contraction was measured for Ceram X Mono ().
Discussion
This laboratory study addressed properties relevant to the clinical use and performance of ACTIVA BioACTIVE Restorative, a restorative material with claimed bioactivity. In this study, when ACTIVA BioACTIVE Restorative was placed directly without pretreatment of the hard dental tissues, most restorations were lost. Due to the flat surfaces used for shear bond strength testing, the loss of restorations was already noted immediately after fabrication of specimens or during thermocycling. These findings contradict the self-adhesion capability claimed by the manufacturer, who promises ‘a strong resin-hydroxyapatite complex and a positive seal against microleakage’ [Citation16]. When the material was applied in hemi-spherical cavities, restorations were retained to cavities with margins in enamel despite the lack of surface pretreatment. On the contrary, restorations placed in non-pretreated cavities with margins in dentine were lost due to poor adhesion combined with large volumetric contraction of ACTIVA BioACTIVE Restorative.
In this laboratory study, it was possible to assess the results on enamel and dentine separately. In clinical practice, most cavities show a combination of enamel and dentine margins. This fact in combination with the self-retentive configuration of most cavities after caries excavation and preparation can explain why the loss of restorations is not more frequently encountered. However, in a recent randomized clinical trial of Class I and II restorations using ACTIVA BioACTIVE Restorative, one of the main reasons for failure was loss of the restoration, followed by post-operative sensitivity and secondary caries, all indicating insufficient adhesion of the material [Citation6]. The clinical behavior of ACTIVA BioACTIVE Restorative using only phosphoric acid as pretreatment was shown to be catastrophic with a very high annual failure rate of 24%. The excessive number of failures occurred in spite of the long familiarization process of the operator with the material before the start of the study, the fact that retentive cavities were prepared, and that the material was used following the manufacturer’s recommendations [Citation17] at the time [Citation6].
In the instructions for use of ACTIVA BioACTIVE Restorative as per September 2017, the tooth surface should be etched for 10 s with phosphoric acid prior to insertion of the restorative material [Citation17]. According to our results, however, the use of phosphoric acid alone is not sufficient to ensure adhesion to dentine; this became evident by the loss of restorations. Only by applying the self-etch adhesive was it possible to retain ACTIVA BioACTIVE Restorative – and result in shear bond strengths similar to those of the control (resin composite restorations) in both enamel and dentine. In November 2017, the manufacturer recommends to ‘use a bonding agent in non-retentive cavity preparations’ [Citation18]. After discussions with the manufacturer regarding the poor adhesive ability of the material from our clinical and laboratory findings, the authors were pleased to see that the use of an adhesive (type not specified) was included in the manufacturer’s instructions for use as per March 2019 [Citation19].
Our findings corroborate those of Omidi et al. [Citation11] and Kaushik and Yadav [Citation12], who employed microleakage as a means of assessing marginal integrity and cavity seal. In both these previous studies, significantly higher dye penetration was observed when ACTIVA BioACTIVE Restorative was used without previous phosphoric acid etching and use of adhesive. In the latter as well as in the present study, only when adhesive was applied was microleakage similar to that of restorations made with a resin composite – though these results depended on the employed adhesive [Citation12]. Their results seem to be contradicted by the >99% intact margins reported by Hughes et al. [Citation8], measured approximately 1 h after placement of restorations ACTIVA BioACTIVE Restorative. Whereas the present study also found a more positive picture for ACTIVA BioACTIVE Restorative immediately after placement of the restorations, these results deteriorated after thermocycling and storage. This deterioration is likely due to the development of contraction stresses resulting from continued polymerization reaction in combination with thermal stresses induced by thermocycling, stresses which could not be compensated for. On the other hand, the reduction in wall-to-wall contraction for Fuji II LC and Ceram X Mono after storage for 28 days can be explained by the lower contraction stresses in both of these materials in combination with water sorption, which partly compensated for the size of the gap. Since the exact composition of ACTIVA BioACTIVE Restorative is not disclosed, it is difficult to discuss why this material does not perform as the manufacturer claims or why it differs from the other investigated RMGIC (Fuji II LC).
In the present study, the use of the self-etch adhesive prior to restoration with ACTIVA BioACTIVE Restorative resulted in comparable marginal adaptation with that of control composite restorations, as observed by Kaushik and Yadav [Citation12]. However, ACTIVA BioACTIVE Restorative still showed high wall-to-wall contraction in restorations with enamel margins. Additionally, the contraction measured for ACTIVA BioACTIVE Restorative in our study was significantly higher (median 2.9%) than that reported by the manufacturer (i.e. 1.7%) [Citation19]. In fact, a concave surface visible to the naked eye could be noted on restorations made with ACTIVA BioACTIVE Restorative immediately after light-curing, resulting in polymerization stresses that can in part explain debonding. The unsatisfactory performance of the material observed in vitro and in vivo, in spite of following the manufacturer’s recommendations at the time of initiating the study, is alarming. This is aggravated when considering the climbing sales worldwide and consecutive product awards that ACTIVA BioACTIVE Restorative has received in 2016, 2017 and 2019 [Citation20]. The present results should be used constructively to enforce a more thorough testing of dental materials prior to launching onto the market and to regulate marketing claims that lack supporting evidence [Citation6,Citation21,Citation22]. Additionally, our findings recommend a more critical assessment from dental professionals when presented with newer, ‘marvellous’ solutions. The claimed bioactive potential of depositing hydroxyapatite in the occurrence of gaps at the tooth-restoration interface is not yet made available and the confirmation of the latter claim is hereby challenged by the findings of this study. Moreover, any adhesive applied on the cavity walls may be a barrier for the suggested bioactivity of the material.
Supplemental Material
Download MS Word (17.6 KB)Acknowledgments
This work was part of the research traineeship of Stavroula Michou, funded by the Erasmus+ EU Programme, European Commission. Additional expenses were covered by the Faculty of Health and Medical Sciences, Department of Odontology, University of Copenhagen. The authors acknowledge Lifco Dental AB, Sweden, for donating the investigated materials and some consumables used in this project. The results of this study were shared at the Academy of Dental Materials meeting in Porto de Galinhas, Brazil held on October 2018 [Citation23].
Disclosure statement
The authors report no conflict of interest.
References
- McCabe JF, Yan Z, Al Naimi OT, et al. Smart materials in dentistry. Aust Dent J. 2011;56(Suppl. 1):3–10.AQ3
- Pameijer CH, Garcia-Godoy F, Morrow BR, et al. Flexural strength and flexural fatigue properties of resin-modified glass ionomers. J Clin Dent. 2015;26(1):23–27.
- Alrahlah A. Diametral tensile strength, flexural strength, and surface microhardness of bioactive bulk fill restorative. J Contemp Dent Pract. 2018;19(1):13–19.
- Garoushi S, Vallittu PK, Lassila L. Characterization of fluoride releasing restorative dental materials. Dent Mater J. 2018;37(2):293–300.
- Bansal R, Burgess J, Lawson NC. Wear of an enhanced resin-modified glass-ionomer restorative material. Am J Dent. 2016;29(3):171–174.
- van Dijken JWV, Pallesen U, Benetti A. A randomized controlled evaluation of posterior resin restorations of an altered resin modified glass-ionomer cement with claimed bioactivity. Dent Mater. 2019;35(2):335–343.
- Benetti AR, Havndrup-Pedersen C, Honoré D, et al. Bulk-fill resin composites: polymerization contraction, depth of cure, and gap formation. Oper Dent. 2015;40(2):190–200.
- Hughes KO, Powell KJ, Hill AE, et al. Delayed photoactivation of dual-cure composites: effect on cuspal flexure, depth-of-cure, and mechanical properties. Oper Dent. 2019;44(2):E97–E104.
- Cannavo M, Harsono M, Finkelman M, et al. Microleakage of dental bulk fill, conventional and self-adhesive composites. J Dent Res. 2014;93(Spec Iss A):847.
- Zmener O, Pameijer CH, Della Porta R, et al. Marginal bacterial leakage in class I cavities filled with a new resin-modified glass ionomer restorative material. Final Report. April; 2013.
- Omidi BR, Naeini FF, Dehghan H, et al. Microleakage of an enhanced resin-modified glass ionomer restorative material in primary molars. J Dent. 2018;15(4):205–213.
- Kaushik M, Yadav M. Marginal microleakage properties of activa bioactive restorative and nanohybrid composite resin using two different adhesives in non carious cervical lesions – an in vitro study. J West Afr Coll Surg. 2017;7(2):1–14.
- Benetti AR, Asmussen E, Peutzfeldt A. Influence of curing rate of resin composite on the bond strength to dentin. Oper Dent. 2007;32(2):144–148.
- Asmussen E, Jørgensen KD. A microscopic investigation of the adaptation of some plastic filling materials to dental cavity walls. Acta Odontol Scand. 1972;30(1):3–21.
- Watts DC, Cash AJ. Determination of polymerization shrinkage kinetics in visible-light-cured materials: methods development. Dent Mater. 1991;7(4):281–287.
- ACTIVA™ BioACTIVE Overview-Product Review [cited 2019 Feb 3]. Available from: https://www.pulpdent.com/shop/category/a/
- Pulpdent Corporation. ACTIVA BioACTIVE-Restorative, BioACTIVE-BASE/LINER. Instructions for use. XP-TV-IN-01 REV: 08/2016; 2016.
- Pulpdent Corporation. ACTIVA BioACTIVE-Restorative, BioACTIVE-BASE/LINER. Instructions for use. XP-V-IN-08w REV: 11/2017; 2017.
- Pulpdent Corporation. ACTIVA BioACTIVE-Restorative, BioACTIVE-BASE/LINER. Instructions for use. XP-V-IN-10w REV: 03/2019; 2019.
- Cinical evaluations of ACTIVA™ BioACTIVE Restorative [cited 2019 Apr 4]. Available from: https://www.dentaladvisor.com/evaluations/activa-bioactive-restorative-2-yr-2018-product-award/
- van Dijken J. Three-year performance of a calcium-, fluoride- and hydroxyl-ions-releasing resin composite. Acta Odontol Scand. 2002;60(3):155–159.
- van Dijken JWV, Sunnegårdh-Grönberg K. A two-year clinical evaluation of a new calcium aluminate cement in Class II cavities. Acta Odontol Scand. 2003;61(4):235–240.
- Michou S, Larsen L, Benetti AR, et al. Adhesion and marginal integrity of bioactive restorative materials. Abstracts of the Academy of Dental Materials Annual Meeting, 04–06 October 2018, Porto de Galinhas, Brazil. Dent Mater. 2018;34(S1):e11.
