Abstract
Aim
To summarize and report laboratory studies of adhesion in eroded substrates, which used bond strength as an outcome measure. To determine the strategies available to overcome bonding difficulties, the quality and consistency of the methodology and to find evidence gaps.
Materials and Methods
The present review followed PRISMA-ScR guidelines. A search was conducted on PubMed/Medline, Scopus and EMBASE (Ovid) databases to identify published peer-reviewed papers (2010–2020). For final qualitative synthesis, 29 articles were selected which respected the inclusion criteria. Data charting was carried out, independently, by two reviewers and quality assessment of the articles was performed.
Results
The primary studies included fall into four major categories: comparison of restorative materials and application modes, enzymatic inhibitors, surface pretreatments or remineralization strategies. Most studies found evaluated dentin (76%), while 17% evaluated enamel, and 7% evaluated both substrates. The majority of the studies reported an effective intervention (83%). Bond strength to eroded dentin is significantly reduced, while in enamel erosion is beneficial. The bond strength to eroded dentin is material-dependent and favored in systems containing 10-MDP. Great disparities among the erosion models used were found, with citric acid in different concentrations being the preferred method, although standardization is lacking.
Conclusions
Adhesives containing 10-MDP show beneficial results in eroded dentin, and surface preparation methods should be considered. Studies which evaluated adhesion to eroded enamel/dentin show high heterogeneity in what concerns aims and methodology. Strategies that focus on remineralizing dentin and strategies to protect bond longevity in this substrate require further research.
1. Introduction
Erosion in enamel and dentin is considered an increasingly complex challenge in dentistry [Citation1]. According to Bartlett, Okunseri and Lussi, the prevalence of dental erosion is high and is present in approximately 30% of the world population. Dental erosion is also more common among men [Citation2–5].
The success of operative dentistry is largely determined by the correct understanding of the chemical and biological processes that govern the tooth structure. Only this way is possible to understand pathological changes in the oral cavity and, consequently, adapt clinical procedures to the case [Citation6].
Enamel and dentin are highly mineralized tissues, made up of an organized inorganic matrix of hydroxyapatite crystals [Citation7]. Enamel comprises 96 wt% of hydroxyapatite crystals, while the remaining 4% are water and residual organic content [Citation7–9]. Alike enamel, there is also an inorganic matrix in dentin, although in lesser quantity, surrounding and protecting the organic content. This is mostly type-I collagen, responsible for making dentin a challenging substrate to bond to [Citation10]. Despite their apparent similarities, they each have different coping mechanisms and regeneration potentials, in response to the various aggressions they may be subjected to in the oral environment [Citation8]. These include trauma, caries, abrasion, attrition and erosion [Citation11].
Erosion is described in the literature as a noncarious progressive lesion linked to the dissolution of hard tissues by acids which are not bacterial by-products [Citation12]. This gradual dissolution leads to the weakening of the enamel and increased susceptibility to abrasion or attrition, yet it remains remineralizable [Citation13]. However, a prolonged exposure to acids may render the enamel unable to regenerate, leaving it permanently affected, ultimately impacting on the underlying dentin [Citation14]. Thus, and taking into account the prevalence of this phenomenon, it is important to adopt rehabilitation strategies in order to guarantee the protection of the dental hard tissues [Citation15]. Erosive defects may even elicit pain, in certain clinical scenarios, where dentin is severely affected, requiring immediate intervention [Citation1].
In light of the current available evidence, the existing strategies rely on adhesive protocols and novel biomimetic approaches which benefit from advances in nanotechnology. These include new bioactive polymers, fillers or toothpastes which aid calcium-phosphate remineralization [Citation6,Citation16–20].
Bonding to an eroded substrate and its predictability will vary depending on whether it is enamel or dentin. Due to aforementioned factors, they behave differently during the adhesive process [Citation21,Citation22]. In enamel, the erosive process seems to be beneficial for adhesion since it promotes the creation of micro and macroporosities that facilitate resin penetration and retention, in a high surface energy substrate [Citation13,Citation17,Citation21]. In contrast, there is increased difficulty in bonding to eroded dentin [Citation21,Citation23,Citation24]. Occurrences such as a hypermineralization layer and tubular occlusion, lead to a weak reactionary ability of this substrate. Due to this, resin impregnation is impaired, ultimately leading to a compromise in the bonding procedure [Citation11,Citation25–27]. Consensus is yet to be reached regarding the best materials or strategies available to improve bond strength to eroded substrates. Furthermore, optimization of such bonding strategies contribute to durable restorations, as questions may arise regarding longevity of bonded eroded substrates [Citation23,Citation26]. Such interventions are pivotal in severe erosion cases, which involve deep dentin, as this disfavors the long-term prognosis of the restoration.
Therefore, taking into account that dental erosion reflects one of the greatest challenges today in oral rehabilitation [Citation1,Citation28], the objective of this review is to sum and report laboratory studies of adhesion in eroded substrates, in order to understand what has been done, to summarize the knowledge in the field, assess the quality of the studies performed and to identify gaps in the evidence. This will inform and direct future research.
2. Materials and methods
2.1. Search strategy
This scoping review was conducted according to the PRISMA-Scr Statement criteria (Preferred Reporting Items for Scoping Reviews) [Citation29]. To identify the primary review question, the PCC framework of the Joanna Briggs Institute was adopted, where P (Population) was defined as restorations in enamel/dentin, C (Concept) was defined as bonding to eroded enamel/dentin and C (Context) were laboratory studies [Citation30]. A search strategy was developed for OVID (EMBASE), Medline/PubMed and Scopus databases, with keywords obtained from Medical Subject Headings (MeSH) and additional free keywords. These were combined with Boolean operators as follows: ((Dental erosion) OR (Tooth erosion)) AND (Adhes* OR Bond* OR Materials testing OR Tensile strength OR Dental bonding* OR Dentin-Bonding Agents* OR resin-dentin). The electronic search covered peer-reviewed papers that were published in the last 10 years (2010–2020), as ideas in adhesive dentistry are rapidly abandoned, the most relevant research will be the latest. There was no language restriction. The last search was conducted on 16 September 2020. Records were retrieved and potentially relevant titles and abstracts were selected, followed by full-text reading and inclusion.
2.2. Eligibility criteria
Only laboratory studies that tested bonding of a dental material to eroded enamel or dentin were considered for this review. To respect the inclusion criteria, the bonding procedure had to be carried out in an already eroded substrate. Substrates that suffered erosion protocols after bonding were excluded, as this is not the aim. Studies were only included if their outcome measured any setup of bond strength test (tensile, shear or push-out), which is a gold-standard measure of dental adhesive longevity. The focus were studies of eroded substrates for restorative purposes, using resin composite. Studies which focused on bond strength of orthodontic brackets were excluded. Only studies that used human permanent teeth or bovine teeth were considered eligible. Clinical studies or other animal studies were excluded.
2.3. Study selection and data processing
The data were retrieved from the databases and organized using Mendeley Desktop software (v.1.19.4), where duplicates were removed. Screening was done by three reviewers, in triplicate, and disagreements were resolved by consensus. Data charting was developed by two reviewers (M.B.C. and A.D), which was then independently charted. All relevant information was extrapolated to a Microsoft Excel spread sheet (v. 16.37, Microsoft, USA) which included: author and date of study, country, substrate used, sample size, materials, intervention, test and conclusion. These are summarized in . Reasons for exclusion of studies following full-text reading were recorded.
Table 1. Laboratory adhesion studies in eroded substrates included in the scoping review.
2.4. Quality assessment – risk of bias
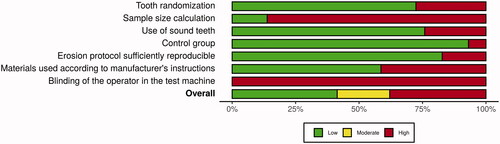
Although risk of bias assessment is optional, according to the PRISMA extension for Scoping Reviews, it can be performed depending on the nature of the review question [Citation29]. In this case, a descriptive analysis of methodological quality in the laboratory research conducted in the primary studies is relevant. Thus, to assess this, in order to guide future research, seven parameters were chosen to be analysed in a Yes/No scale, similarly to Montagner et al. [Citation31]. When insufficient information was provided, the item was classified with a ‘No’. These were: sample randomization, sample size calculation, use of sound teeth, the presence of a control group, reproducibility of the erosion protocol, use of materials according to the instructions and blinding of the operator on the test machine. Studies that scored at least three parameters with ‘No’ were classified as moderate risk, while more than three parameters were classified as having high risk of bias due to methodological flaws or uncertainty. Plots were built using the RoBvis 2.0 visualization tool (https://mcguinlu.shinyapps.io/robvis/).
3. Results
3.1. Study inclusion
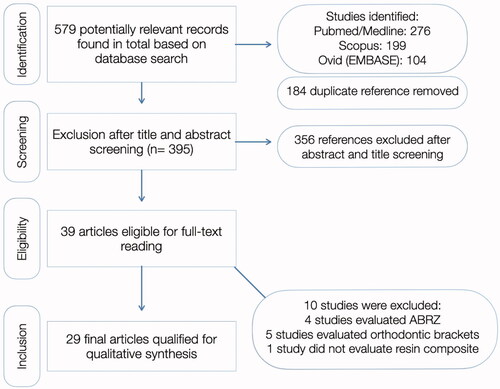
In total, 579 articles were identified in all databases. Out of these, 184 were duplicates and were subsequently removed. A total of 39 records remained after title and abstract screening, out of which 10 were excluded for reasons mentioned in , which illustrates the flow diagram of study selection. All primary studies included and data recorded for each are shown in . One study was also excluded from this analysis because the author could not be contacted and the full-text could not be retrieved.
Figure 1. PRISMA-ScR flowchart used for the scoping review. Reasons for study exclusion included: studies which looked at acid-base resistance zones (ABRZ), evaluating erosion after the restorative procedure, studies which evaluated adhesion to orthodontic brackets, or studies which did not use resin composite as a restorative material.
3.2. Study characteristics
Most studies that investigated adhesion in eroded substrates focused on dentin (22/29 − 76%). Only five studies used enamel as a substrate (17%), and only two evaluated both (7%). As a bond strength testing setup, microtensile bond strength was the preferred choice (18/29 − 62%), followed by microshear (6/29 − 21%), shear (4/29 − 14%), and one study used a macrotensile setup (3%). For studies in which long-term bond strength was studied in addition to immediate bond strength (24 h), storage in water was the preferred method (8/12 − 66%). This was followed by artificial saliva (3/12 − 25%), and one study used 5000 cycles of thermocycling.
3.3. Materials and interventions
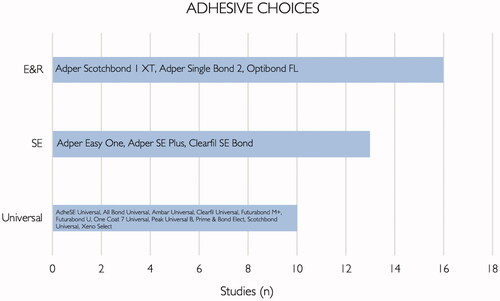
Regarding the choice of adhesives in the primary studies that were identified, most authors used etch-and-rinse adhesives (16/29 − 55%), followed by self-etch adhesives (13/29 − 45%) and finally universal adhesives (10/29 − 34%) – . Two studies evaluated glass ionomer cements (7%), and one study evaluated a luting cement (3%). The interventions investigated in primary studies included: testing of matrix metalloproteinases (MMP) inhibitor strategies, comparison of restorative materials and application modes, remineralization strategies and surface pretreatments.
3.4. Erosion protocols
The different erosion protocols used in the primary studies and respective frequencies can be seen in . The citric acid used in the 16 studies that were included varied between pH 2.1-3.75.
Table 2. Erosion protocols discriminated by study and frequency of appearance (n).
3.5. Quality assessment – risk of bias
The output of the quality assessment showing the results for each parameter that was evaluated is shown in . Twelve studies were classified as having low risk of bias (12/29 − 41%), and six studies were classified as moderate risk (6/29 − 21%). The remaining 11 studies were classified as having high risk of bias (11/29 − 38%). Only Yabuki et al. [Citation37], Augusto et al. [Citation41], Siqueira et al. [Citation42] and Flury et al. [Citation55] reported sample size calculation. The last parameter evaluated, blinding of the operator in the test machine, was not performed on any of the studies evaluated in this review. The weighted summary plot of each parameter can be seen in .
Table 3. Quality assessment of the primary studies included in this review, using a Yes/No scale for seven different domains.
4. Discussion
The number of patients that show signs of erosion has been increasing and therefore, it is very common to find eroded substrates clinically [Citation36,Citation54,Citation59]. The importance of finding the best strategy and clinical plan to rehabilitate these cases has gained a significant weight. Gathering all the current evidence is key to clarify which is the best adhesive strategy when dealing with a clinical scenario of erosion.
Even though enamel is the first anatomic barrier exposed to acid challenge, and therefore subject to an erosion phenomenon, the majority of primary studies focused on dentin. In fact, the latter is commonly affected by erosion and evidence tells us that adhesion remains a challenge when held in dentin [Citation21,Citation32,Citation44,Citation56]. Moreover, most cavity preparations involve dentin, hence the need for evidence that can guide clinical in this substrate. When bond strength is the outcome, eroded enamel seemed not to be a challenge [Citation37,Citation50]. Eroded dentin, however, showed significant differences when compared to sound dentin [Citation44,Citation46]. Furthermore, authors that compared the performance of a self-etch and an etch-and-rinse adhesive [Citation34], an etch-and-rinse with a universal adhesive [Citation38] or a self-etch with an universal adhesive [Citation39] all advocate that in the presence of erosion, the bonding performance may be compromised in dentin, even if some adhesives showed better results. Other studies also highlighted the fact that bond strength might be higher when enamel is eroded [Citation46]. This is expected as the rougher surface that forms plays a favorable role in securing interlocking of the resin in enamel [Citation13,Citation37,Citation54,Citation56]. Giacomini et al. [Citation50] even states that no additional treatment is required in eroded enamel, and pre-conditioning with 37% phosphoric acid may be enough to guarantee a successful and conservative bond. Despite this, Wang et al. [Citation13] and Casas-Apayco et al. [Citation54] argue that the changes in substrate such as loss of structure as well as disorganization present in eroded enamel should not be ignored. These alterations could lead to wear, mineral and consequently hardness deficiency, all factors that contribute to weakening of the substrate [Citation13,Citation37,Citation54].
As for reestablishing mineral loss in dentin and trying to revert alterations caused by acid erosion, different pretreatments were tested. Some remineralizing agents like stannous-chloride and amine fluoride (SnCl2/AmF) were not persistent and did not increase the bond strength to eroded dentin nor did arginine-containing toothpastes [Citation38,Citation48]. In spite of these results, other types of remineralizing agents may lead to precipitation of calcium-fluoride-like deposits on the tooth surface, thus reducing the erosive mineral loss in dentin, as shown by Flury while experimenting with NaF and Sn/F solutions [Citation55]. Krithi [Citation34], on one hand, also demonstrated that sodium fluoride (NaF) showed improvement in bond strength results. On the other hand, the authors stressed the need for more studies regarding NovaMin [Citation34], a type of bioglass composed of calcium sodium phosphor-silicate and usually indicated for dentin hypersensitivity [Citation60]. Since remineralizing agents have a vast intervention field in operative dentistry and very few studies investigated these agents, further studies should be considered.
While remineralizing agents have not been consistent, deproteinizing agents demonstrate some significant results. Use of NaOCl previous to the application of the adhesive minimized the degradation of the latter, in long-term studies [Citation39]. In fact, NaOCl pretreatment is capable of partially removing the organic superficial layer in eroded dentin and thinning the smear layer. This could be a solution to promote resin infiltration and thus bond strength to eroded dentin [Citation41,Citation43]. It is wise to underline that two of these three studies used NaOCl at 10% and the other one chose a concentration of only 5.2%, and therefore, a strict protocol is needed for more consistent results [Citation39,Citation41,Citation43]. Another valid pretreatment is laser irradiation to reduce the superficial layer, affected by erosion, and modify the substrate. This prepares the surface for bonding without negatively affecting the substrate. Er,Cr:YSGG laser associated with a self-etch adhesive has shown higher bond strength results when compared to other surface pretreatments including diamond bur [Citation57]. Maeda et al. [Citation49] also demonstrated that Nd:YAG laser seems to have benefits in bond strength results, under erosive challenges. Finally, although conservative preparations and noninvasive treatments need to be respected, some treatments advocating the use of a fine-grift diamond bur led to better long-term results when dealing with eroded dentin. The rationale for this is the removal of the disorganized superficial layer, facilitating adhesion [Citation43,Citation58].
At baseline, bonding to sound dentin is already considered difficult and short-lasting. When dentin suffers erosion this difficulty increases even more, as explained above. As the literature shows, the establishment of a hybrid layer, composed of collagen, monomers and eventually debris (smear layer), is key to establish an appropriate bond, being directly related to the chemical stability and longevity of the restoration [Citation61,Citation62]. Regarding eroded dentin, there is a dissolution of peri and intertubular minerals, resulting in the exposure of a thick superficial organic layer and, after restoration, tag formation of under 3 μm whereas for sound dentin, these values tend to be between 9 and 15 μm [Citation25]. Collapsed demineralized fibrils and excess water content are also observable, leading to a deficient hybrid layer and impairing the penetration and in situ polymerization of the adhesive, ultimately affecting bond strength [Citation17,Citation47]. Since eroded dentin leads to an increase of water present in the matrix, nanoleakage becomes a bigger threat to hybrid layers [Citation42].Consequently, this water is partially responsible for accelerated activation of endogenous proteases, the so-called matrix metallo-proteinases (MMPs), capable of hydrolyzing the organic matrix [Citation63,Citation64]. In fact, this lengthened contact between water and monomers found in the adhesive can ultimately lead to an accelerated hybrid layer degradation and failure of the restoration [Citation58]. Events such as incomplete polymerization, resin plasticization or creation of water-rich channels seen under erosive conditions, are able to degrade chemical bonds within the polymer matrix and thus contribute to enzymatic degradation of the denuded collagen [Citation65,Citation66]. Accordingly, these studies highlighted the need to overcome the difficulties in infiltration of the adhesive to a thickened eroded organic layer as well as protecting a more vulnerable and challenged hybrid layer.
In order to answer and establish protocols that could protect the hybrid layer from early degradation, some authors led experiments and pretreatments with enzymatic inhibitors. As eroded dentin is more vulnerable to hybrid layer breakdown, it is important to stabilize the interface. In fact, MMPs can be activated by exposure to low pH and are able to progress dental erosion [Citation63,Citation67]. The successive pH demineralization-remineralization cycles are not sufficient to inhibit MMP activity, although their optimum pH to function is around neutral conditions [Citation67,Citation68]. Therefore, when dealing with erosive challenges, there is an accelerated activation of the proteases [Citation43]. Chlorhexidine (CHX) has been often tested, since it was proven that it can inhibit proteolytic enzymes and reduce chances of collagen fiber degradation. Since eroded dentin produces a sensitive hybrid layer, testing inhibitors could be the subject of promising investigations. Even though it has been proven that it is only able to retard degradation and not fully inhibit it, 2% CHX was tested in a few studies included [Citation36,Citation40,Citation43,Citation47,Citation51,Citation53]. The results, however, were conflicting and do not support any recommendation [Citation36,Citation47,Citation51]. Evidence has shown that even though CHX does not seem to have significant results on immediate bond strength, the substance might have beneficial effects over time in sound dentin and is considered a promising enzymatic inhibitor. However, CHX only remains effective when it is trapped in the dentin matrix, as the chemical bond is electrostatic and reversible [Citation51]. The results also seem to be dependent on the adhesive used. In fact, CHX and 10-MDP may compete over the calcium present in apatite. That being said, CHX may reduce the immediate bond strength when used simultaneously with a universal or self-etch adhesive [Citation36,Citation47]. The type of adhesive and whether it is susceptible to inhibitors must be taken into account when using this type of pre-treatment. Moreover, some studies show that CHX causes a reaction when in presence of dentin, forming a precipitate that ultimately reduces the depth of dentin etched [Citation36]. In the presence of eroded dentin, these results are even less predictable.
While CHX or benzalkonium chloride (BAC) showed that proteolytic inhibitors may not improve the durability of bond strength, other agents showed encouraging immediate bond strength results. In fact, cross-linking agents like proanthrocyanidins (PAA) and riboflavin (RFV) contributed to stabilize the collagen mesh, resulting in promotion of monomer infiltration, ultimately altering the bonding and nanomechanical properties of eroded dentin [Citation32].
A variety of adhesive systems were taken into account in the studies included. The conclusions regarding this question were not consistent, since some studies did not compare different types of adhesives, and many contemporary adhesives were not featured. As mentioned before, bonding to enamel does not pose a problem and does not seem to be adhesive-dependent. Conversely, in dentin, most studies demonstrate that for several types of adhesives tested, bond strength in eroded dentin was compromised [Citation34,Citation42]. Nevertheless, the majority of authors suggest that the type of adhesive influences bond strength to eroded dentin, although the best adhesive strategy is still unknown with this amount of evidence [Citation33,Citation42,Citation57]. As previously seen, one of the main obstacles is the lack of proper infiltration by the monomers in eroded dentin. Functional monomers such as 10-MDP have the ability to chemically interact with calcium present in hydroxyapatite. An intermediate layer where MDP molecules are bonded to the calcium in the surrounding solution and to another MDP molecule may be formed. This promotes long-term stability of the adhesive interface and higher short-term bond strength results. This so-called ‘nanolayering’ phenomenon is an advantage towards adhesives that only benefit from mechanical adhesion [Citation69,Citation70]. In eroded dentin, there is only a partial demineralization of the inorganic matrix and therefore, calcium is left to interact with 10-MDP. Thus, MDP-containing adhesives may be useful in this condition [Citation42]. Furthermore, other materials such as self-adhesive flowable composites were tested. These presented very similar bond strength when compared to traditional composite bonded via an adhesive [Citation35] and may be a promising strategy. Cruz et al. [Citation59] and Lenzi et al. [Citation56] compared adhesives to glass ionomer cements, although the aim is redundant as longevity associated to glass ionomers is limited. Despite this, a trend in certain types of adhesive choices was noted, especially in what concerns 3 M adhesives such as Adper Single Bond 2 or Scotchbond Universal. Further research should be conducted with other adhesives such as Clearfil Protect, Clearfil S3 and more studies featuring Optibond FL should be led, regarded as the gold standard of the etch-and-rinse category, and other formulations also used clinically.
Some questions still remain unanswered due to the lack of standardized protocols. All studies followed a specific erosive protocol but all showed differences. Most authors preferred citric acid [Citation33,Citation34,Citation36–39,Citation41,Citation43,Citation45,Citation46,Citation48,Citation55,Citation57,Citation58], in most cases at a 1% concentration. Yet, not only the concentrations differed from one another but every study had a distinct management of the erosive cycling applied. While some applied citric acid to the samples four times a day for 5 min [Citation46], others did the same but six times a day [Citation58]. For instance, Cruz [Citation59] concluded that bond strength was not affected when dealing with eroded dentin although when in 2015 [Citation52] the same author changed erosive protocols, the results led to the belief that erosion had indeed compromised the quality of the bond. This reinforces the idea that the disparity of experimental conditions leads to conflicting results. Also, other authors operated with soft drinks such as Coca-Cola [Citation13,Citation32,Citation35,Citation42,Citation44,Citation51,Citation53,Citation54,Citation56,Citation59] or Sprite [Citation49,Citation52]. Some also used orange juice to simulate the effects of citric acid [Citation47,Citation50], while others chose hydrochloric acid. Differences in concentration of the acid, pH of the solutions used, demineralization times, total amount of days and type of sample all contributed to great disparities among the studies. A consensus for models of erosion in dental research, published in 2011 sets out recommendations [Citation71]. The authors consider that citric acid is to be used as a model solution, preferred over commercial beverages due to its reproducibility and pH control. The duration of the challenge should be concordant with the aim of the study but should not last more than minutes, when it is a model for extrinsic erosion, and pH should be within an acceptable range found in real-life acidic challenges. The details of these protocols should be published in sufficient extent in these studies [Citation71].
Eleven studies which used bovine teeth as substrate in alternative to human teeth were identified [Citation13,Citation36,Citation40,Citation43,Citation45,Citation48,Citation49,Citation51,Citation53,Citation55,Citation58]. Past studies have documented that bonding to bovine teeth is comparably different than to human teeth, and results have to be cautiously interpreted [Citation72,Citation73]. Accordingly, it is always preferable to research with the most realistic conditions possible and in this case, some studies may lack that nature, eventually leading to suboptimal results. The duration of the acid challenge has to be adapted if a bovine substrate is used, as acid suscpetibility is different to that of human mineralized tissues [Citation71].
Regarding bond strength tests, the preferred setup was the microtensile bond strength test [Citation13,Citation32,Citation36,Citation38–43,Citation46–48,Citation50,Citation51,Citation54,Citation55,Citation57,Citation58]. Most authors chose this type of test mainly because it is highly reproducible, useful for material screening and has good clinical translation. Even though it lacks the ability to simulate intraoral conditions, since it does not take into account the C-factor, it is considered the gold standard in bond strength testing [Citation74,Citation75]. Additionally, Murase et al. [Citation35] tested a new bond strength setup based on a new scratch and tensile test and future studies should take the opportunity to validate this method.
Gaps in the evidence surrounding adhesion studies in eroded substrates were identified. Novel remineralising composites, such as commercialized Activa Bioactive (Pulpdent) or experimental composites [Citation76] are yet to be researched in the context of erosion. Sensitivity analysis of disparities in erosion protocols should be carried out, along with formulation of a standardized erosion protocol as guidance. A definitive need for in-depth research remains regarding adhesive options and clarifying the need for pretreatments is essential. Research in this topic should firstly focus on identifying methods to secure initial bonding to eroded dentin, rather than evaluating degradation. Mapping the preclinical evidence is important to guide future clinical studies by identifying which strategies are viable and which should not be further tested. Clear guidelines should be established in order to give clinicians better treatment options when dealing with erosive conditions. This can ultimately lead to better bond strength results which can translate to durable, successful restorations.
As a limitation, this scoping review deliberately focused in laboratory studies and did not include any type of clinical study, which could be interesting to compare with our present results in the future, in another scoping review or systematic review with a convergent research question. However, clinical studies evaluating adhesion in eroded susbtrates are scarce, and a scoping review with an aim of pre-clinical studies should precede clinical reviews. As one paper could not be retrieved, it also adds to the limitation of the present review, which would have contributed to the findings and qualitative synthesis.
5. Conclusion
A considerable amount of evidence was found regarding adhesion studies which measured bond strength in eroded substrates. Based on the evidence mapping performed in this study, the following conclusions can be drawn:
Bond strength to eroded dentin is substantially reduced, compared to sound dentin, and this was a finding transversal to all studies. Enamel on the other hand, benefits from an erosion challenge during the adhesive procedure.
Bond strength to eroded dentin is material-dependent, with best results seen in adhesive systems containing functional monomer 10-MDP.
Surface pre-treatments such as bur preparation, laser irradiation (Er,Cr:YSGG; Nd:YAG) and NaOCl were able to improve bond strength to eroded dentin. Remineralization strategies and novel self-adhesive composites also showed promising results and warrant further research.
MMP inhibitors in eroded dentin show conflicting results, with some authors supporting their use, and others reporting no effect at short and long-term or adverse effect in bond strength, specifically concerning 2% CHX.
Standardization of laboratory studies is recommended. Studies should confirm the use of sound teeth prior to erosion protocols and properly randomize samples and allocate them to experimental groups. A non-eroded control group is highly recommended to serve as a baseline for data analysis. The erosion study model should specify whether it is extrinsic or intrinsic erosive simulation, and for extrinsic, citric acid is encouraged over soft drinks.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Disclosure statement
No potential competing interest was reported by the author(s).
References
- Carvalho TS, Colon P, Ganss C, et al. Consensus report of the European Federation of Conservative Dentistry: erosive tooth wear-diagnosis and management. Clin Oral Investig. 2015;19(7):1557–1561.
- Bartlett D, O'Toole S. Tooth wear and aging. Aust Dent J. 2019;64(S1):S59–S62.
- Bartlett DW, Lussi A, West NX, et al. Prevalence of tooth wear on buccal and lingual surfaces and possible risk factors in young European adults. J Dent. 2013;41(11):1007–1013.
- Lussi A, Schaffner M, Hotz P, et al. Dental erosion in a population of Swiss adults. Community Dent Oral Epidemiol. 1991;19(5):286–290.
- Okunseri C, Wong MCM, Yau DTW, et al. The relationship between consumption of beverages and tooth wear among adults in the United States. J Public Health Dent. 2015;75(4):274–281.
- Neel EAA, Aljabo A, Strange A, et al. Demineralization-remineralization dynamics in teeth and bone. Int J Nanomedicine. 2016;11:4743–4763.
- Ding C, Chen Z, Li J. From molecules to macrostructures: recent development of bioinspired hard tissue repair. Biomater Sci. 2017;5(8):1435–1449.
- De Dios Teruel J, Alcolea A, Hernández A, et al. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch Oral Biol. 2015;60(5):768–775.
- Veis A, Dorvee JR. Biomineralization mechanisms: a new paradigm for crystal nucleation in organic matrices. Calcif Tissue Int. 2013;93(4):307–315.
- Goldberg M. Dentin structure composition and mineralization. Front Biosci. 2011;E3(2):711–735.
- Warreth A, Abuhijleh E, Almaghribi MA, et al. Tooth surface loss: a review of literature. Saudi Dent J. 2020;32(2):53–60.
- Marqués ML, Leyda MAM, Ribelles LM, et al. Dental erosion. Etiologic factors in a sample of Valencian children and adolescents. Cross-sectional study. Eur J Paediatr Dent. 2019;20:189–193.
- Wang L, Casas-Apayco LC, Hipólito AC, et al. Effect of simulated intraoral erosion and/or abrasion effects on etch-and-rinse bonding to enamel. Am J Dent. 2014;27:29–34.
- Viana Í, Alania Y, Feitosa S, et al. Bioactive materials subjected to erosion/abrasion and their influence on dental tissues. Oper Dent. 2020;45(3):E114-E123
- Wilder-Smith CH, Materna A, Martig L, et al. Longitudinal study of gastroesophageal reflux and erosive tooth wear. BMC Gastroenterol. 2017;17(1):113.
- Braga RR. Calcium phosphates as ion-releasing fillers in restorative resin-based materials. Dent Mater. 2019;35(1):3–14.
- Moda MD, Briso ALF, Oliveira R. d, et al. Effects of different toothpastes on the prevention of erosion in composite resin and glass ionomer cement enamel and dentin restorations. J Appl Oral Sci. 2020;28:e20200493.
- Neel EAA, Bozec L, Perez RA, et al. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int J Nanomedicine. 2015;10:6371–6394.
- Skrtic D, Antonucci JM, Eanes ED, et al. Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53(4):381–391.
- Skrtic D, Antonucci JM. Effect of chemical structure and composition of the resin phase on vinyl conversion of amorphous calcium phosphate-filled composites. Polym Int. 2007;56(4):497–505.
- Van Meerbeek B, Yoshihara K, Van Landuyt K, et al. From Buonocore’s pioneering acid-etch technique to self-adhering restoratives. A status perspective of rapidly advancing dental adhesive technology. J Adhes Dent. 2020;22:7–34.
- Pashley DH, Tay FR, Breschi L, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27(1):1–16.
- Ururahy MS, Curylofo-Zotti FA, Galo R, et al. Wettability and surface morphology of eroded dentin treated with chitosan. Arch Oral Biol. 2017;75:68–73.
- Özcan M, Dündar M, Erhan Çömlekoğlu M. Adhesion concepts in dentistry: tooth and material aspects. J Adhes Sci Technol. 2012;26(24):2661–2681.
- Kinney JH, Balooch M, Haupt DL, et al. Mineral distribution and dimensional changes in human dentin during demineralization. J Dent Res. 1995;74(5):1179–1184.
- Tay FR, Pashley DH. Resin bonding to cervical sclerotic dentin: a review. J Dent. 2004;32(3):173–196.
- Attin T, Wegehaupt FJ. Impact of erosive conditions on tooth-colored restorative materials. Dent Mater. 2014;30(1):43–49.
- Milosevic A, O'Sullivan E, Royal College of Surgeons of England. Diagnosis, prevention and management of dental erosion: summary of an updated national guideline. Prim Dent Care. 2008;15(1):11–12.
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467–473.,
- Peters M, Godfrey CM, Mcinerney P, et al. 2017 Guidance for the conduct of JBI scoping reviews. Joana Briggs Inst Rev Man. 2017. https://www.researchgate.net/publication/319713049_2017_Guidance_for_the_Conduct_of_JBI_Scoping_Reviews
- Montagner AF, Sarkis-Onofre R, Pereira-Cenci T, et al. MMP inhibitors on dentin stability: A systematic review and meta-analysis. J Dent Res. 2014;93(8):733–743.
- de Siqueira FSF, Hilgemberg B, Araujo LCR, et al. Improving bonding to eroded dentin by using collagen cross-linking agents: 2 years of water storage. Clin Oral Invest. 2020;24(2):809–822.
- Ferretti MA, Theobaldo JD, Pereira R, et al. Effect of erosive challenge and cigarette smoke on dentin microhardness, surface morphology and bond strength. BDS. 2020;23(3):1–8.
- Krithi B, Vidhya S, Mahalaxmi S. Microshear bond strength of composite resin to demineralized dentin after remineralization with sodium fluoride, CPP-ACP and NovaMin containing dentifrices. J Oral Biol Craniofacial Res. 2020;10(2):122–127.
- Murase Y, Kotake H, Kusakabe S, et al. Use of new scratch test and tensile test for evaluation of bond strength of selfadhesive flowable resin composite for repair of artificial tooth erosion. Dent Mater J. 2020;39(3):435–443.
- Costa C. d, Passos VF, Neri JR, et al. Effect of metalloproteinase inhibitors on bond strength of a self-etching adhesive on erosively demineralized dentin. J Adhes Dent. 2019;21:337–344.
- Yabuki C, Rikuta A, Murayama R, et al. Effect of acid erosion on enamel bond strength of self-etch adhesives and sonic velocity measurement of enamel. Dent Mater J. 2018;37(4):542–548.
- Zumstein K, Peutzfeldt A, Lussi A, et al. The effect of SnCl2/AmF pretreatment on short- and long-term bond strength to eroded dentin. Biomed Res Int. 2018:3895356
- Siqueira FSF, Cardenas AFM, Gomes GM, et al. Three-year effects of deproteinization on the in vitro durability of resin/dentin-eroded interfaces. Oper Dent. 2018;43(1):60–70.
- Moda MD, Fagundes TC, Briso ALF, et al. Analysis of the bond interface between self-adhesive resin cement to eroded dentin in vitro. PLoS One. 2018;13(11):e0208024.
- Augusto MG, Torres CRG, Pucci CR, et al. Bond stability of a universal adhesive system to eroded/abraded dentin after deproteinization. Oper Dent. 2018;43(3):291–300.
- Siqueira FSF, Cardenas AM, Ocampo JB, et al. Bonding performance of universal adhesives to eroded dentin. J Adhes Dent. 2018;20:121–132.
- Deari S, Wegehaupt FJ, Tauböck TT, et al. Influence of different pretreatments on the microtensile bond strength to eroded dentin. J Adhes Dent. 2017;19:147–155.
- Forgerini TV, Ribeiro JF, Rocha RO, et al. Role of etching mode on bonding longevity of a universal adhesive to eroded dentin. J Adhes Dent. 2017;19:69–75.
- Flury S, Lussi A, Peutzfeldt A. Long-term bond strength of two benzalkonium chloride-modified adhesive systems to eroded dentin. Biomed Res Int. 2017;2017:1–8.
- Frattes FC, Augusto MG, Torres CRG, et al. Bond strength to eroded enamel and dentin using a universal adhesive system. J Adhes Dent. 2017;19:121–127.
- Giacomini MC, Scaffa PMC, Chaves LP, et al. Role of proteolytic enzyme inhibitors on carious and eroded dentin associated with a universal bonding system. Oper Dent. 2017;42(6):E188–E196.
- Bergamin ACP, Bridi EC, Amaral FLB, et al. Influence of an arginine-containing toothpaste on bond strength of different adhesive systems to eroded dentin. Gen Dent. 2016;64(1):67–73.
- Maeda FA, Fukushima KA, Tedesco TK, et al. Effect of erosive challenge and Nd:YAGlaser irradiation on bond strength of adhesive systems to dentin. Int J Adhes Adhes. 2016;64:60–64.
- Giacomini MC, Casas-Apayco LC, Machado CM, et al. Influence of erosive and abrasive cycling on bonding of different adhesive systems to enamel: an in situ study. Braz Dent J. 2016;27(5):548–555.
- Francisconi-dos-Rios LF, Casas-Apayco LC, Calabria MP, et al. Role of chlorhexidine in bond strength to artificially eroded dentin over time. J Adhes Dent. 2015;17(2):133–139.
- Cruz JB, Bonini G, Lenzi TL, et al. Bonding stability of adhesive systems to eroded dentin. Braz Oral Res. 2015;29(1):1–6.
- Machado CM, Zamuner AC, Modena K. d S, et al. How erosive drinks and enzyme inhibitors impact bond strength to dentin. Braz Oral Res. 2015;29(1):S1806–S300.
- Casas-Apayco LC, Dreibi VM, Hipólito AC, et al. Erosive cola-based drinks affect the bonding to enamel surface: an in vitro study. J Appl Oral Sci. 2014;22(5):434–441.
- Flury S, Koch T, Peutzfeldt A, et al. The effect of a tin-containing fluoride mouth rinse on the bond between resin composite and erosively demineralised dentin. Clin Oral Investig. 2013;17(1):217–225.
- Lenzi T, Hesse D, Guglielmi C, et al. Shear bond strength of two adhesive materials to eroded enamel. J Contemp Dent Pract. 2013;14(4):700–703.
- Ramos TM, Ramos-Oliveira TM, de Freitas PM, et al. Effects of Er:YAG and Er,Cr:YSGG laser irradiation on the adhesion to eroded dentin. Lasers Med Sci. 2015;30(1):17–26.
- Zimmerli B, De Munck J, Lussi A, et al. Long-term bonding to eroded dentin requires superficial bur preparation. Clin Oral Investig. 2012;16(5):1451–1461.
- Cruz JB, Lenzi TL, Tedesco TK, et al. Eroded dentin does not jeopardize the bond strength of adhesive restorative materials. Braz Oral Res. 2012;26(4):306–312.
- Khijmatgar S, Reddy U, John S, et al. Is there evidence for Novamin application in remineralization?: a systematic review. J Oral Biol Craniofac Res. 2020;10(2):87–92.
- Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265–273.
- Nakabayashi N, Nakamura M, Yasuda N. Hybrid layer as a dentin-bonding mechanism. J Esthet Dent. 1991;3(4):133–138.
- Betancourt DE, Baldion PA, Castellanos JE. Resin-dentin bonding interface: Mechanisms of degradation and strategies for stabilization of the hybrid layer. Int J Biomater. 2019;2019:5268342–5268311.
- Frassetto A, Breschi L, Turco G, et al. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability-a literature review. Dent Mater. 2016;32(2):e41–e53.
- Tezvergil-Mutluay A, Pashley D, Mutluay MM. Long-term durability of dental adhesives. Curr Oral Health Rep. 2015;2(4):174–181.
- Cadenaro M, Maravic T, Comba A, et al. The role of polymerization in adhesive dentistry. Dent Mater. 2019;35(1):e1–e22.
- Zarella BL, Cardoso CAB, Pelá VT, et al. The role of matrix metalloproteinases and cysteine-cathepsins on the progression of dentine erosion. Arch Oral Biol. 2015;60(9):1340–1345.
- Amaral S. d, Scaffa PMC, Rodrigues RDS, et al. Dynamic influence of pH on metalloproteinase activity in human coronal and radicular dentin. Caries Res. 2018;52(1-2):113–118.
- Tian F, Zhou L, Zhang Z, et al. Paucity of nanolayering in resin-dentin interfaces of MDP-based adhesives. J Dent Res. 2016;95(4):380–387.
- Carrilho E, Cardoso M, Ferreira MM, et al. 10-MDP based dental adhesives: Adhesive interface characterization and adhesive stability-a systematic review. Materials (Basel. 2019;12(5):790–718.
- Shellis RP, Ganss C, Ren Y, et al. Methodology and models in erosion research: discussion and conclusions. Caries Res. 2011;45(s1):69–77.
- Yassen GH, Platt JA, Hara AT. Bovine teeth as substitute for human teeth in dental research: a review of literature. J Oral Sci. 2011;53(3):273–282.
- de Carvalho MFF, Leijôto-Lannes ACN, Rodrigues Mcn de S, et al. Viability of bovine teeth as a substrate in bond strength tests: a systematic review and meta-analysis. J Adhes Dent. 2018;20(6):471–479.
- Sano H, Chowdhury AFMA, Saikaew P, et al. The microtensile bond strength test: Its historical background and application to bond testing. Jpn Dent Sci Rev. 2020;56(1):24–31.
- Armstrong S, Breschi L, Özcan M, et al. Academy of dental materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (μTBS) approach. Dent Mater. 2017;33(2):133–143.
- Delgado AHS, Almuusa A, Eshmawi Y, et al. Novel self-bonding composites: resin-dentin interfacial chemistry. Ann Med. 2019;51(sup1):97–97.