Abstract
Introduction
The objective was to review the effectiveness of iodoform-based compared to noniodoform-based filling materials in the root canal treatment of deciduous teeth.
Methods
This systematic review and meta-analysis used randomized clinical trials with six months or more follow-up. The risk of bias of individual studies and the certainty of the evidence were evaluated (Cochrane risk of bias tool and GRADE, respectively).
Results
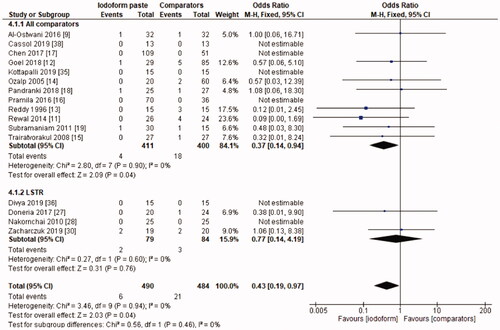
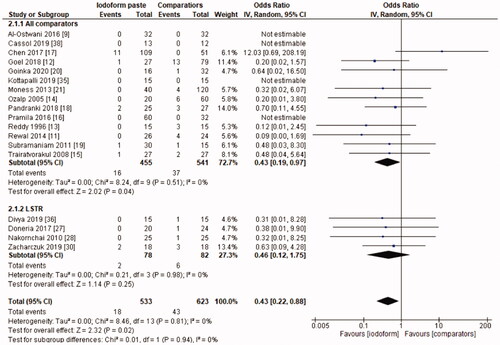
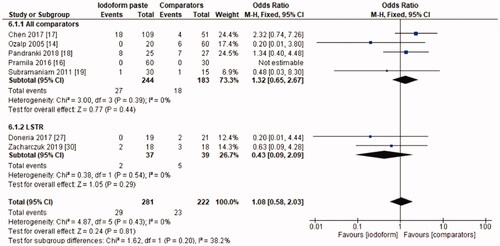
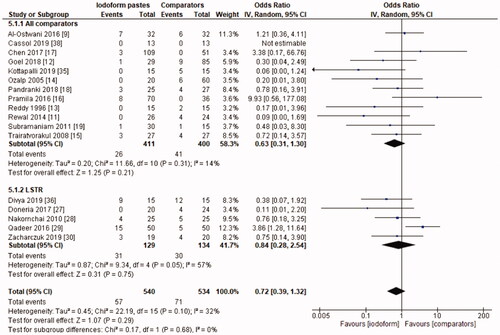
The initial search resulted in 5,127 studies after removal of duplicates. After screening by title and abstract, 34 full-text studies were eligible and 21 remained in the qualitative synthesis and 19 in the meta-analysis. Iodoform-based filling materials resulted in fewer clinical failures when compared to noniodoform-based filling materials at the 6 months (OR = 0.43, 95%CI: 0.19–0.97, p = .04) and 9–12 months (OR = 0.46, 95%CI: 0.23–0.93, p = .03), but not at the 18–30 months follow-up (OR = 1.08, 95%CI: 0.58–2.03, p = .81). When considering radiographic failures, there was no statistical difference between iodoform-based and noniodoform-based filling materials at the 6 months (OR = 0.72, 95%CI: 0.39–1.32, p = .29) and 18–30 months follow-ups (OR = 1.06, 95%CI: 0.51–2.21, p = .87), but fewer radiographic failures were detected at the 9–12 months follow-up (OR = 0.49, 95%CI: 0.29–0.80, p = .005).
Conclusion
Iodoform-based filling materials showed better clinical and radiographic performance when compared to non-iodoform-based filling materials in the short term, and similar performance in the long term. However, most of the studies exhibited unclear or high risk of bias and the overall certainty of the evidence ranged from low to very low. Therefore, new randomized clinical trials must be accomplished to corroborate this conclusion.
Keywords:
Introduction
Endodontic treatment with complete pulp removal—pulpectomy—is commonly used in deciduous teeth with irreversible pulpitis or necrotic pulp [Citation1,Citation2]. Usually, after the chemomechanical preparation of the root canals, an absorbable and biocompatible material must be used to favor repair and allow the permanence of the tooth in the mouth till its physiological exfoliation [Citation1]. In an attempt to simplify the steps, Lesion Sterilization and Tissue Repair (LSTR) therapy has been investigated for the treatment of primary tooth canals. This technique implies nonmechanical preparation of the root canals and placement of a paste made of a mixture of antibiotics at the entrance of the root canals [Citation3].
The complex morphology of root canal systems in primary teeth can hamper mechanical instrumentation, irrigation and/or disinfection [Citation4]. For this reason, the use of substances with antimicrobial properties is generally used to increase the chances of a successful endodontic treatment [Citation1]. Even in LSTR therapy, the use of a mixture of antibiotics is considered sufficient to respond to the periapical lesions [Citation3].
A good filling material must be absorbable and able to accompany the deciduous tooth root resorption and not interfere with the germ of the successive permanent tooth [Citation1,Citation2]. For a long time, zinc oxide eugenol (ZOE) was the material most used [Citation4–21] but its use was reduced [Citation22] due to its limited antimicrobial action [Citation22–24], slower resorption compared to that of the deciduous teeth [Citation8,Citation24], and its ability to generate a foreign body-type reaction if the material overflows the root apex [Citation25]. The use of other filling materials has grown, mainly calcium hydroxide with iodoform [Citation4–7,Citation9,Citation10,Citation14–17,Citation19,Citation20,Citation22,Citation23,Citation26–30], since they exhibit pronounced antimicrobial action, are easily absorbed when overflowing the tooth apex [Citation31–33], are radiopaque, and can be purchased as a pre-mixed paste for easy application [Citation4].
An ideal filling material for deciduous teeth has not been found yet [Citation4–7]. However, the use of iodoform in different filling materials stands out due to the resorption capability and good antimicrobial properties [Citation8,Citation10,Citation34]. In the market, there are different formulations with iodoform, including ZOE [Citation9,Citation11–13,Citation16–19,Citation21,Citation35,Citation36], calcium hydroxide [Citation9–12,Citation14–20,Citation22,Citation23,Citation27–29,Citation35], antibiotic agents [Citation37,Citation38], or other substances [Citation39,Citation40].
Previous systematic reviews have focused on different filling materials for deciduous teeth after endodontic treatment [Citation4–7], on the chemomechanical technique or LSTR therapy [Citation3]. However, there is no review investigating possible advantages of iodoform addition to filling materials, independent of the endodontic technique used. The objective this study was to review the effectiveness of iodoform-based compared to non-iodoform-based filling materials in the root canal treatment of deciduous teeth.
Materials and methods
Protocol and registration
The study protocol was registered in the PROSPERO database (CRD42019123937), and the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement were followed [Citation41]. The study was accomplished from December 2018 to June 2019 and updated in March 2021.
Information sources and search strategy
P (Population) = children with deciduous teeth that received root canal treatment;
I (Intervention) = root canal treatment using iodoform-based filling materials;
C (Comparison) = root canal treatment using noniodoform-based filling materials;
O (Outcomes) = clinical and/or radiographic success/failure;
S (Type of studies) = randomized clinical trials.
The search used the following electronic data bases: MEDLINE via PubMeb, Scopus, Web of Science, Latin American and Caribbean Literature on Health Sciences (LILACS), Biblioteca Brasileira em Odontologia (BBO) (Brazilian Dentistry Library) and Cochrane Library.
The search strategy (Supplement 1) was based on controlled vocabulary (MeSH terms) of the PubMed database along with free keywords retrieved from titles and abstracts. MeSH terms and free keywords were initially combined in each item using the Boolean operator ‘OR’. The Population and Intervention were combined to build the search strategy by the Boolean operator ‘AND’. The search strategy developed for PubMed was adapted to other electronic databases. We also hand-searched the reference lists of all primary studies for additional relevant publications.
The grey literature was searched using the databases System for Information on Grey Literature in Europe (SIGLE) and Scholar Google. Dissertations and theses were searched using the ProQuest Dissertations and Theses Full‐Text databases and the Periódicos Capes Thesis database.
Eligibility criteria
Randomized Clinical Trials (RCT) were included. The studies excluded comprised noncontrolled clinical studies, editorial letters, literature reviews, in vitro or animal studies, observational studies, case reports and case series. Studies written in Chinese and Japanese were also excluded. No restrictions on publication dates were applied.
Study selection and data collection process
The retrieved studies were imported into a reference management software (EndNote X9 - Clarivate Analytics, Philadelphia, USA). After removal of duplicates, studies were excluded after title and abstract reading according to the exclusion criteria previously described. This process was performed independently by two reviewers (M.F.S.J. and A.C.R.C.); in case of disagreement, a third reviewer was consulted (L.M.W.).
The studies were selected by title and abstracts accordingly to the described eligibility criteria. Full-text studies were obtained when there was insufficient information in the title and abstract to make a clear decision.
Eligible studies received an identification that combined the first author’s name and the year of publication. The data from the studies were extracted to customized extraction forms that comprised the study design, participants, interventions and outcomes. Studies reporting different follow-ups of the same research were only considered once to prevent data overlapping. This process was performed independently by two reviewers (M.F.S.J. and A.C.R.C.); in case of disagreement, a third reviewer was consulted (L.M.W.).
Risk of bias in individual studies
The evaluation of the risk of bias of the selected studies was carried out by two independent reviewers (M.F.S.J. and A.C.R.C.), using the Cochrane Collaboration tool to evaluate the risk of bias in RCT [Citation42]. The evaluation criteria comprised five items: (1) sequence generation, (2) allocation concealment, (3) blinding of result evaluators, (4) incomplete result data, and (5) selective result reports. In case of discrepancies between the evaluators, a third reviewer was consulted (L.M.W.).
The risk of bias was rated following the recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. Each criterion was graded as having ‘low’, ‘unclear’ or ‘high’ risk of bias, accordingly to the information retrieved in the text regarding potential bias.
The study was judged as ‘high’ risk if at least one key domain was not achieved adequately. At the study level, a study was judged as having a ‘low’ risk of bias if all domains were considered at ‘low’ risk. If the information could not be retrieved or was incomplete on one of these domains, without presenting a ‘high’ risk of bias in any domain, the study was considered as having an ‘unclear’ risk of bias.
Meta-analysis
The outcomes assessed were clinical or radiographic failure (yes or no) for the different follow-ups (6, 9–12 and 18–30 months) and techniques for tooth preparation (chemomechanical and LSTR). The results were summarized using the random-effects model to estimate the Odds Ratio (OR) using a 95% confidence interval (95%CI). The heterogeneity was assessed using the Cochran Q test and the I2 statistics. All analyses were performed using the software Revman 5 (Review Manager ver. 5, The Cochrane Collaboration, Copenhagen, Denmark).
Assessment of the certainty of the evidence
The certainty of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [Citation43]. This stage was accomplished to determine the overall certainty of the evidence for each meta-analysis. The evidence can be graded in 4 levels (very low, low, moderate, high). When a meta-analysis is graded as ‘high quality’, it means that the authors are very confident that the true effect lies close to the estimate of the effect.
Results
Selection of studies
The initial search in the databases resulted in 6,049 registers (Supplement 1). The removal of duplicates resulted in 5,127 registers. After the selection based on title, the number of registers was reduced to 96. A total of 62 registers was excluded after the abstract reading, resulting in 34 full text for the eligibility assessment. Thirteen registers were excluded due to: (1) nonrandomized clinical trial [Citation22] (2) absence of a group using iodoform-based filling material [Citation44,Citation45], (3) all groups used iodoform-based filling materials [Citation34,Citation46–49], (4) original dissertation of an included study [Citation50], (5) publication of preliminary results of an included study [Citation51,Citation52], and (6) text in Chinese [Citation53,Citation54] (Supplement 2).
Characteristics of the included studies
The characteristics of the 21 selected studies are listed in . The randomization unit of the clinical studies was either the patient [Citation10–12,Citation20,Citation27,Citation29], or the tooth [Citation9,Citation13–18,Citation21,Citation28,Citation30,Citation36–38]. In some studies, the randomization unit was not identified [Citation19,Citation35].
Table 1. Summary of some methodologic characteristics of the included studies.
The number of patients included in the studies ranged from 27 to 120 children, and only three studies presented a sample calculation [Citation12,Citation17,Citation29]. The age of the patients ranged between 3 and 13 years old. Thirteen studies reported exclusively pulpectomy resulting from complications of carious lesions [Citation9,Citation12,Citation13,Citation15–19,Citation21,Citation27,Citation29,Citation35,Citation36], whereas other studies included deciduous teeth with pulpectomy associated with pulp lesions caused by carious lesions or trauma [Citation10,Citation38], chronic infection [Citation20] or necrosis [Citation30]. Finally, other studies did not clearly explain the clinical reason for root canal treatment [Citation11,Citation14,Citation28,Citation37].
Most studies included upper and lower molars [Citation9,Citation11,Citation12,Citation14,Citation17,Citation19,Citation27–30,Citation35,Citation36], others only second molars [Citation20], lower molars [Citation15], anterior and posterior teeth [Citation10,Citation13,Citation37,Citation48], or only upper incisors [Citation21]. Two studies did not report on the type of teeth included [Citation16,Citation18].
The root canal treatments were carried out by one operator [Citation11,Citation12,Citation15,Citation16,Citation18,Citation21,Citation27,Citation28,Citation35,Citation36,Citation38], two [Citation14] or four [Citation30] operators or by an unreported number of operators [Citation9,Citation10,Citation13,Citation17,Citation19,Citation20,Citation29,Citation37]. The procedure was carried out in a single session [Citation9,Citation11,Citation15,Citation16,Citation19,Citation21,Citation35] or in two sessions [Citation10,Citation13,Citation17]. A further two studies used individualized protocols for deciding the number of root canal treatment sessions [Citation14,Citation38], while in the remaining nine studies the number of sessions was not reported [Citation12,Citation18,Citation20,Citation27–30,Citation36,Citation37].
In most studies, the chemomechanical technique was employed for the root canal treatment [Citation9–12,Citation14–21,Citation27–30,Citation35–38], while the LSTR therapy without instrumentation was employed in fewer studies [Citation28–30,Citation36,Citation37]. Finally, one study did not clearly report on the technique employed [Citation13].
Several iodoform-based filling materials had been used in the studies. Iodoform with calcium hydroxide was present in the commercial brands: Metapex [Citation9,Citation10,Citation19,Citation20] and Vitapex [Citation14–17,Citation27–29] and Maisto-Capurro paste [Citation30]. Iodoform associated with zinc oxide, eugenol and calcium hydroxide was synthesized using all these components [Citation17], without addition of chlorophenol [Citation9], with addition of propolis [Citation36], or as the commercial brand (Endoflas) [Citation11,Citation12,Citation18,Citation19,Citation35]. One study used iodoform, zinc oxide and eugenol (RCFill) [Citation16] and another study used iodoform, zinc oxide eugenol (ZOE), bismuth subcarbonate, resins, barium sulphate, eugenol and excipients (Zical) [Citation21]. Two studies used different versions of the modified Guedes-Pinto paste [Citation37,Citation38], and finally one study used Maisto paste [Citation13].
Several positive control groups (noniodoform-based filling materials) were found in the studies. ZOE was standard [Citation9–21]; alone or in addition to other components such as chloramphenicol and tetracycline (CTZ) [Citation37]. Other versions of zinc oxide (ZO) without eugenol were found, but with the addition of other active components such as calcium hydroxide [Citation38], propolis [Citation9], ozonized oil [Citation27], aloe vera [Citation12,Citation20], sodium fluoride 10% [Citation12] and nanohydroxyapatite [Citation35]. Calcium hydroxide–based pastes were also used: Apexcal (calcium hydroxide, bismuth carbonate, polyethylene glycol, glycerine and water) [Citation21], Sealapex [Citation14] and Calcitur [Citation14]. The material used in the LSTR technique was 3Mix paste (ciprofloxacin, metronidazole and minocycline) [Citation28–30,Citation36,Citation37].
The number of follow-up sessions included a minimum of two [Citation9,Citation10,Citation15,Citation28,Citation29] and a maximum of seven [Citation14] clinical and/or radiographic appointments. The interval of between follow-ups sessions varied greatly from 15 days [Citation37] to 30 months [Citation16].
Clinical and/or radiographic evaluations were carried out by a single evaluator [Citation15,Citation19] or by two [Citation16,Citation38] or three evaluators [Citation9,Citation20]. Some studies reported that the evaluators were blinded or double-blinded for both the clinical and radiographic evaluations [Citation9,Citation14,Citation16,Citation20,Citation38] others only for the clinical [Citation28] or the radiographic evaluations [Citation11,Citation27]. Evaluators were previously calibrated to identify clinical and radiographic success in three studies [Citation9,Citation16,Citation38] or only for radiographic success [Citation11,Citation27,Citation28]. However, most of the studies did not report on the calibration process [Citation10,Citation12,Citation13,Citation15,Citation17–19,Citation21,Citation29,Citation30,Citation35–37].
The clinical failure was assessed by the presence of: (1) pain [Citation9–15,Citation17–21,Citation27,Citation28,Citation30,Citation35–38], (2) mobility [Citation9,Citation10,Citation12–15,Citation17–20,Citation27,Citation28,Citation30,Citation35–37]; (3) tenderness on percussion [Citation9,Citation11,Citation12,Citation14,Citation19–21,Citation30,Citation35,Citation38]; (4) tenderness [Citation13,Citation18]; (5) fistula [Citation10–12,Citation14,Citation17,Citation18,Citation21,Citation28,Citation30,Citation37,Citation38]; (6) swelling [Citation10–12,Citation14,Citation15,Citation19,Citation21,Citation30,Citation35,Citation36,Citation38]; (7) sinus tract [Citation11,Citation12,Citation15,Citation19,Citation21,Citation36]; (8) redness [Citation11,Citation12,Citation15,Citation19]; (9) purulent exudate expressed from the gingival margin [Citation19]; (10) premature exfoliation [Citation36]; (11) loss of clinical crown/coronal restoration or recurrent caries [Citation21]. Some researchers also noted the absence of normal mucosa [Citation9,Citation20,Citation27,Citation37] or the health of tissues surrounding the teeth [Citation13,Citation21]. In the present review, it was not possible to access the modified American Association of Endodontists criteria applied by Pramila et al. [Citation16].
The radiographical failure was assessed by: (1) the presence of radiographic lesions [Citation10,Citation17]; (2) absence of reduction in the size of radiolucent area in the intra-radicular [Citation27,Citation36] or inter-radicular region [Citation9,Citation11–13,Citation19,Citation29,Citation30,Citation36–38], including the furcation [Citation14,Citation28,Citation36,Citation37] or periapical region [Citation12,Citation14,Citation15,Citation19,Citation21,Citation28–30]; (3) the absence of continuity of lamina dura [Citation12,Citation14,Citation15,Citation27]; (4) the absence of normal periodontal ligament space [Citation21,Citation37]; (5) the presence of external [Citation12,Citation17,Citation21,Citation27,Citation28,Citation30,Citation38] or internal [Citation12,Citation21,Citation27,Citation28,Citation30,Citation38] root resorption [Citation14,Citation19,Citation37]; (6) the presence of new radiolucency formed after of treatment [Citation10,Citation28]; (7) the presence of radiolucency involving the successor tooth germ [Citation19]; (8) the presence of change in the direction of the successor tooth [Citation17,Citation35,Citation36]; (9) the absence of bone regeneration [Citation9,Citation12,Citation13,Citation15,Citation20,Citation27,Citation30]; (10) the absence of filling material in the root canal [Citation17]; (11) the absence of extruded material extraradicularly [Citation17]; (12) the absence of resorption of extravasated material [Citation13,Citation35,Citation36] with physiologic root resorption [Citation35,Citation36].
Risk of bias evaluation
Out of the 21 eligible studies, two [Citation17,Citation38] were evaluated as having a ‘low’ risk of bias, 13 as having an ‘unclear’ risk [Citation9,Citation11–15,Citation18,Citation19,Citation27–29,Citation35,Citation36], and six studies as having a ‘high’ risk of bias [Citation10,Citation16,Citation20,Citation21,Citation30,Citation37] (Supplement 3).
Meta-analysis
Two studies were excluded from the quantitative synthesis because it was not possible to extract the primary data needed to compare the outcomes studied [Citation10,Citation37]. Therefore, 19 studies remained for the meta-analysis of clinical or radiographic failures [Citation9,Citation11–21,Citation27–30,Citation35,Citation36,Citation38]. In the meta-analysis, LSRT was compared to iodoformed filling material. Thus, the result of LSRT group was not compared to conventional treatment subgroup without iodoform (0 failures in 20 cases in 6 and 12 months in clinical and radiographic evaluation).
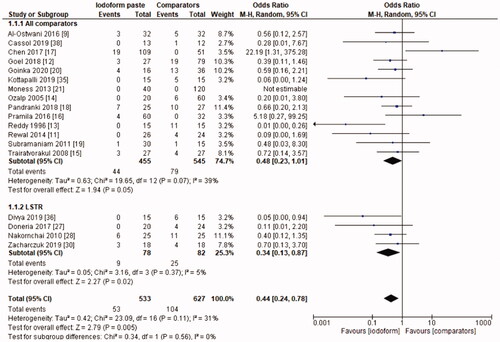
In the clinical evaluation, iodoform-based filling materials showed fewer failures at the 6 months (OR = 0.43, 95%CI: 0.19–0.97, p =.04) and 9–12 months follow-ups (OR = 0.46, 95%CI: 0.23–0.93, p = .03), but not at the 18–30 months follow-up (OR = 1.08, 95%CI: 0.58–2.03, p = .81). In the subgroup using chemomechanical preparation, the iodoform-based filling materials performed better than noniodoform-based materials both at the 6 months (OR = 0.37, 95%CI: 0.14–0.94, p = .04) and 9–12 months follow-ups (OR = 0.43, 95%CI: 0.19–0.97, p = .04), but not at the 18–30 months follow-up (OR = 1.32, 95%CI: 0.65–2.67, p =.44). In the subgroup using the LSTR technique, there was no difference between iodoform-based and noniodoform-based materials at any of the follow-ups (6 months [OR = 0.77, 95%CI: 0.14–4.19, p = 0.76], 9–12 months [OR = 0.57, 95%CI: 0.15–2.12, p = .2]), 18–30 months [OR = 0.43, 95%CI: 0.09–2.09, p = .44]). The results did not present heterogeneity for clinical failures (test χ2, p > .05, I2=0%) ().
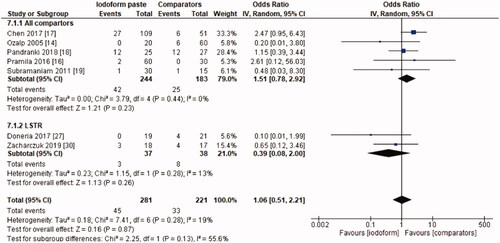
In the radiographic evaluation, there was no statistical difference between iodoform-based and non-iodoform-based materials at the 6 months (OR = 0.72, 95%CI: 0.39–1.32, p=.29) and 18–30 months follow-ups (OR = 1.06, 95%CI: 0.51–2.21, p = .87), but fewer radiographic failures were detected for iodoform-based materials at the 9–12 months follow-up (OR = 0.49, 95%CI: 0.29–0.80, p = .005). In the chemomechanical preparation subgroup, there was no difference between iodoform-based and noniodoform-based materials at any of the follow-ups (6 months [OR = 0.63, 95%CI: 0.31–1.30, p = .21], 9–12 months [OR = 0.55, 95%CI: 0.30–1.02, p = .06], 18–30 months [OR = 1.51, 95%CI: 0.78–2.92, p = .23]). In the subgroup using the LSTR technique, there was no difference between iodoform-based and noniodoform-based materials at the 6 months (OR = 0.84;95%CI: 0.28–2.54, p = .75) and 18–30 months follow-ups (OR = 0.39; 95%CI: 0.08–2.00, p = .26), but iodoform-based materials showed lower failures rates at the 9–12 months follow-up (OR = 0.34; 95%CI: 0.13–0.87, p = .02). The results did not present heterogeneity (test χ2; p >.05; I2<32%), with the exception of the analysis of the subgroup using the LSTR technique at 6 months (test χ2; p = .05; I2 = 57%) ().
Assessment of the certainty of the evidence
The certainty of the evidence was assessed according to the evaluated outcomes: clinical and radiographic failure in the different follow-up periods (6, 9–12 and 18–30 months) and for the techniques for tooth preparation (chemomechanical and/or LSTR).
For the outcome ‘clinical failure’, the certainty of the evidence was graded as ‘very low’ for all follow-up periods, with the exception of the total (chemical-mechanical technique and LSTR) and LSTR subgroup analysis at the 6 and 9–12 months, which was rated ‘low’ ().
Table 2. Summary of findings in Grading of Recommendations, Assessment, Development and Evaluation (GRADE) for clinical or radiographic evaluation at 6 months (A), 9-12 months (B) or 18-30 months (C) in deciduous teeth.
For the outcome ‘radiographic failure’, the certainty of the evidence was graded as ‘very low’ for all follow-up periods, with the exception of the LSTR subgroup at 9–12 months, which was rated ‘low’ ().
Discussion
This systematic review and meta-analysis compared iodoform-based and noniodoform-based filling materials for root canal treatment of deciduous teeth. Iodoform-based filling materials showed fewer clinical failures when compared to noniodoform-based materials after 6 and 9–12 months, and similar performance after 18–30 months. There were fewer radiographic failures of iodoform-based filling materials at the 9–12 months follow-up, but similar performance of the two groups of materials at the 6 and the 18–30 months follow-ups.
The similarity between the filling materials with and without iodoform at the 6 months radiographic evaluation may derive from the insufficient time to detect significant changes in bone neo-formation and lesion healing, particularly when the evaluation criteria were based on visual examination of the radiographs. More sensitive methods, like radiographic subtraction is known to better detect subtle changes in radiopacity that supersede human eye examination capacity [Citation55]. Although they exhibit low heterogeneity, the loss of patients during follow-up was not explained by the authors, resulting in incomplete data outcome and this probably have influence in the results.
All systematic reviews published so far were inconclusive in identifying the best choice of filling material for primary tooth pulpectomy [Citation4–7]. This systematic review was the first to detect significant differences between different filling materials. This result may indicate a positive effect on the clinical and radiographic outcomes of filling materials containing iodoform, an effect which can be explained by the high antimicrobial property of iodoform [Citation8,Citation10,Citation34]. In general, the odds ratio for the treatment effect was obtained from an adequate sample size and a good number of studies were included. Even so, the certainty of the evidence was classified as ‘low’ or ‘very low’ for the evaluated outcomes. This limits our confidence, that is, the true effect may be substantially different from the estimate of the effect. The ideal scenario for analyzing the effect of iodoform-based filling material would be a study comparing the same filling material with and without iodoform.
Most studies used commercially available iodoform-based filling materials, such as Vitapex [Citation14–17,Citation27–29], Metapex [Citation9,Citation10,Citation19,Citation20], Endoflas [Citation11,Citation12,Citation18,Citation19,Citation35], RC Fill [Citation16], Zical [Citation21], Maisto paste [Citation13] or Maisto-Capurro paste [Citation30]. Although many professionals choose premixed pastes due to ease of use, the professional must be aware of the material’s composition and follow manufacturer recommendations for a greater success rate. Only two studies [Citation37,Citation38] used an unmarketed, iodoform-based filling material in the form of a modified Guedes-Pinto paste. In this situation, the proportions of the components must be respected during handling to obtain the maximum benefit and the lowest risk of adverse effects. The resorption ability is an advantageous property of iodoform [Citation8,Citation10,Citation34]. However, when iodoform-based filling materials are not used correctly, they have the disadvantage of compromising aesthetics through a brown-yellowish pigmentation of the dental crown [Citation34]. To reduce pigmentation, during the performance of the clinical procedure, the operator must be careful cleaning of the pulp chamber after root canal obturation and before restoration.
The application of different eligibility criteria and different clinical protocols during endodontic treatment among the studies may have introduced some degree of variability and have influenced the rates of radiographic and clinical failures. Potentially influencing factors include: selection of the patient and tooth and the operator’s ability and preferred technique (manual, motorized files or LSTR). Also, the instrument size and the taper influence the flow of the root canal filling material and the removal of infectious radicular dentin [Citation56]. Incomplete information about such bias factors as observed in the included studies make comparison between studies difficult. To facilitate the comparison between the techniques, subgroup analysis considering the different root canal techniques (chemomechanical root canal preparation and LSTR therapy) was performed.
The LSTR involves the use of a triple antibiotic mixture, i.e. a paste based on ciprofloxacin, metronidazole and minocycline (3Mix-MP) [Citation3,Citation28–30,Citation36,Citation37], aiming to disinfect the root canal system without any mechanical preparation. The performance of iodoform-based filling materials was similar in the chemomechanical technique and the LSTR therapy at the 6 and the 9–12 months clinical evaluations, and in the radiographic analysis at 6 months. The iodoform-based filling material was superior only in the radiographic comparison at the 9–12 months of follow-up. Another systematic review has previously demonstrated that the two treatments showed comparable outcomes, regardless of the follow-up period (6, 12 or 18 months) and type of evaluation (clinical or radiographical) with certainty of evidence ranging from very low to moderate [Citation3].
It must be pointed out that the absence of difference in our metanalysis may be due to the limited number of studies and a small sample size that probably failed to identify differences between groups; also the certainty of evidence was graded as low and very low. Even so, it is worth noting that a simplified technique such LSTR can benefit professionals, as it will reduce clinical time and facilitate the treatment of non-collaborative children [Citation3]. For this reason, future studies with the use of LSTR are encouraged and should compare not only the techniques used, but include test groups with and without iodoform even with the chemomechanical technique.
As recommended in systematic reviews about filling materials in deciduous teeth [Citation3–7], there has been an increase in the number of randomized clinical trials in the last few years [Citation9,Citation11,Citation12,Citation17,Citation20,Citation21,Citation27,Citation29,Citation30,Citation35–38]. But it is relevant to point out that the quality of the trials was not improved sufficiently to strengthen the scientific evidence. This highlights the importance of best design and methodological accuracy of the primary studies, particularly regarding the randomization process and blinding of evaluators. Thus, further studies are still needed to increase the certainty of the evidence.
Conclusion
Iodoform-based filling materials showed better clinical and radiographic performance when compared to noniodoform-based filling materials in the short term and similar performance in the long term. However, most of the studies showed unclear or high risk of bias and the overall certainty of the evidence ranged from low to very low. Therefore, new randomized clinical trials must be accomplished to corroborate this conclusion.
Supplemental Material
Download MS Word (260.4 KB)Supplemental Material
Download MS Word (136.9 KB)Supplemental Material
Download MS Word (102.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- American Academy of Pediatric Dentistry. Guideline on pulp therapy for primary and immature permanent teeth. Pediatr Dent. 2016;38:52–288.
- American Academy of Pediatric Dentistry. Pulp therapy for primary and immature permanent teeth. Pediatr Dent. 2017;39:325–333.
- Duarte ML, Pires PM, Ferreira DM, et al. Is there evidence for the use of lesion sterilization and tissue repair therapy in the endodontic treatment of primary teeth? A systematic review and meta-analyses. Clin Oral Investig. 2020;24(9):2959–2972.
- Barcelos R, Santos MP, Primo LG, et al. ZOE paste pulpectomies outcome in primary teeth: a systematic review. J Clin Pediatr Dent. 2011;35(3):241–248.
- Barja-Fidalgo F, Moutinho-Ribeiro M, Oliveira MA, et al. A systematic review of root canal filling materials for deciduous teeth: is there an alternative for zinc oxide-eugenol? ISRN Dent. 2011;2011:367318.
- Smaïl-Faugeron V, Glenny AM, Courson F, et al. Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst Rev. 2018; 5:CD003220.
- Najjar RS, Alamoudi NM, El‐Housseiny AA, et al. A comparison of calcium hydroxide/iodoform paste and zinc oxide eugenol as root filling materials for pulpectomy in primary teeth: a systematic review and meta-analysis. Clin Exp Dent Res. 2019;5(3):294–310.
- Primosch RE, Glomb TA, Jerrell RG. Primary toothpulp therapy as taught in predoctoral pediatric dentalprograms in the United States. Pediatr Dent. 1997;19:118–122.
- Al-Ostwani AO, Al-Monaqel BM, Al-Tinawi MK. A clinical and radiographic study of four different root canal fillings in primary molars. J Indian Soc Pedod Prev Dent. 2016;34(1):55–59.
- Mortazavi M, Mesbahi M. Comparison of zinc oxide and eugenol, and vitapex for root canal treatment of necrotic primary teeth. Int J Paediatr Dent. 2004;14(6):417–424.
- Rewal N, Thakur AS, Sachdev V, et al. Comparison of endoflas and zinc oxide eugenol as root canal filling materials in primary dentition. J Indian Soc Pedod Prev Dent. 2014;32(4):317–321.
- Goel H, Mathur S, Sachdev V. Clinical and radiographic evaluation of four different zinc-oxide integrated root canal obturating materials used in primary teeth. Pediatric Dent J. 2018;28(2):73–86.
- Reddy VV. Fernandes . Clinical and radiological evaluation of zinc oxide-eugenol and maisto's paste as obturating materials in infected primary teeth–nine months study. J Indian Soc Pedod Prev Dent. 1996; 14:39–44.
- Ozalp N, Saroğlu I, Sönmez H. Evaluation of various root canal filling materials in primary molar pulpectomies: an in vivo study. Am J Dent. 2005; 18:347–350.
- Trairatvorakul C, Chunlasikaiwan S. Success of pulpectomy with zinc oxide-eugenol vs calcium hydroxide/iodoform paste in primary molars: a clinical study. Pediatr Dent. 2008;30(4):303–308.
- Pramila R, Muthu MS, Deepa G, et al. Pulpectomies in primary mandibular molars: a comparison of outcomes using three root filling materials. Int Endod J. 2016;49(5):413–421.
- Chen X, Liu X, Zhong J. Clinical and radiographic evaluation of pulpectomy in primary teeth: a 18-months clinical randomized controlled trial. Head Face Med. 2017;13(1):12.
- Pandranki J, V Vanga NR, Chandrabhatla SK. Zinc oxide eugenol and endoflas pulpectomy in primary molars: 24-month clinical and radiographic evaluation. J Indian Soc Pedod Prev Dent. 2018;36(2):173–180.
- Subramaniam P, Gilhotra K. Endoflas, zinc oxide eugenol and metapex as root canal filling materials in primary molars-a comparative clinical study. J Clin Pediatr Dent. 2011;35(4):365–369.
- Goinka C, Reddy KS, Ganapathi A, et al. Comparative evaluation of three different an obturating materials in pulpectomy; an in vivo study. Indian J Dent Sci. 2020;12:68–72.
- Moness AM, Khattab NN, Waly NG. Evaluation of three root canal filling materials for primary teeth (in vivo and in vitro study). Egypt Dent J. 2012; 59:1–13.
- Gupta S, Das G. Clinical and radiographic evaluation of zinc oxide eugenol and metapex in root canal treatment of primary teeth. J Indian Soc Pedod Prev Dent. 2011;29(3):222–228.
- Dunston B, Coll JA. A survey of primary tooth pulptherapy as taught in US dental schools and practiced bydiplomates of the american board of pediatric dentistry. Pediatr Dent. 2008;30:43–48.
- Tchaou WS, Turng BF, Minah GE, et al. Inhibition of pure cultures of oral bacteria by root canal filling materials. Pediat Dent. 1996;18:444–449.
- Reddy S, Ramakrishna Y. Evaluation of antimicrobial efficacy of various root canal filling materials used in primary teeth: a microbiological study. J Clin Pediatr Dent. 2007;31(3):193–198.
- Sadrian R, Coll JA. A long-term follow-up on the retention rate of zinc oxide–eugenol filler after primary tooth pulpectomy. Pediatr Dent. 1993;15(4):249–253.
- Doneria D, Thakur S, Singhal P, et al. In search of a novel substitute: clinical and radiological success of lesion sterilization and tissue repair with modified 3mix-mp antibiotic paste and conventional pulpectomy for primary molars with pulp involvement with 18 months follow-up. Contemp Clin Dent. 2017;8(4):514–521.
- Nakornchai S, Banditsing P, Visetratana N. Clinical evaluation of 3Mix and vitapex as treatment options for pulpally involved primary molars. Int J Paediatr Dent. 2010;20(3):214–221.
- Qadeer I, Munir B, Dar SY. Comparison of effectiveness of triple antibiotic paste (3mix) and vitapex for root canal treatment of pulpally involved primary molars. Pak Oral Dent J. 2016; 36:654–657.
- Zacharczuk GA, Toscano MA, López GE, et al. Evaluation of 3Mix-MP and pulpectomies in non-vital primary molars. Acta Odontol Latinoam. 2019;32(1):22–28.
- Ranly DM. Pulpotomy therapy in primary teeth: new modalities for old rationales. Pediat Dent. 1994; 16:403–409.
- Mani SA, Chawla HS, Tewari A, et al. Evaluation of calcium hydroxide and zinc oxide eugenol as root canal filling materials in primary teeth. ASDC J Dent Child. 2000;67(2):142–147.
- Ba-Hattab R, Al-Jamie M, Aldreib H, et al. Calcium hydroxide in endodontics: an overview. OJST. 2016;06(12):274–289.
- Garcia-Godoy F. Evaluation of an iodoform paste in root canal therapy for infected primary teeth. ASDC J Dent Child. 1987;54(1):30–34.
- Kottapalli P, Madu GP, Ambati NR, et al. Clinical and radiographic evaluation of mixture of zinc oxide powder and nanohydroxyapatite as an obturating material in primary molars. BDS. 2019;22(1):63–69.
- Divya DV, Prasad MG, Radhakrishna AN, et al. Triple antibiotic paste versus propolis: a clinical quest for the reliable treatment of periapical lesions in primary molars. Saudi Endod J. 2019; 9:34–39.
- Calixto-Chanca KS, Correa-Olaya EI, Anchelia-Ramírez SH. Efectividad clínica y radiográfica de dos pastasantibióticas empleadas en necrosis pulpar en niños de un hospital nacional del perú. KIRU. 2014; 11:115–122.
- Cassol DV, Duarte ML, Pintor AVB, et al. Iodoform vs calcium hydroxide/zinc oxide based pastes: 12-month findings of a randomized controlled trial. Braz Oral Res. 2019; 33:e002.
- Cunha C, Barcelos R, Primo L. Soluções irrigadoras e materiais obturadores utilizados na terapia endodôntica de dentes decíduos. Pesqui Bras Odontopediatria Clín Integr. 2005;5:75–83.
- Chawla H, Setia S, Gupta N, et al. Evaluation of a mixture of zinc oxide, calcium hydroxide, and sodium fluoride as a new root canal filling material for primary teeth. J Indian Soc Pedod Prev Dent. 2008;26(2):53–58.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583.
- Higgins JP, Altman DG, Gøtzsche PC, Cochrane Bias Methods Group; Cochrane Statistical Methods Group, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394.
- Coser RM, Giro EMA. Tratamento endodôntico de molares decíduos humanos com necrose pulpar e lesão periapical. PGR- Pós-Grad Rev Fac Odontol. 2002; 5:84–92.
- Zhou W, Zheng Q, Tan X, et al. Comparison of mineral trioxide aggregate and iroot bp plus root repair material as root-end filling materials in endodontic microsurgery: a prospective randomized controlled study. J Endod. 2017;43(1):1–6.
- Rontani MP, Peters CF, Worliczeck AM. Tratamento endodôntico de dentes decíduos com necrose pulpar. Rev Assoc Paul Cir Dent. 1994; 48:1235–1238.
- Silveira EG, Silva RHH. Tratamento dos canais radiculares de molares decíduos com uma pasta de iodofórmio e glicerina. Rev Odontopediatr. 1994; 3:65–72.
- Thomas AM, Chandra S, Chandra S, et al. Elimination of infection in pulpectomized deciduous teeth: a short-term study using iodoform paste. J Endod. 1994;20(5):233–235.
- Ramar K, Mungara J. Clinical and radiographic evaluation of pulpectomies using three root canal filling materials: an in-vivo study. J Indian Soc Pedod Prev Dent. 2010;28(1):25–29.
- Cassol DV. Comparação clínica e radiográfica de materiais obturadores utilizados no tratamento de canais radiculares de dentes decíduos. Dissertação (mestrado). Programa de Pós-graduação em Odontologia (Odontopediatria) da Faculdade de Odontologia da Universidade Federal do Rio de Janeiro; 2017.
- Chen XX, Lin BC, Zhong J, et al. [Degradation evaluation and success of pulpectomy with a modified primary root canal filling in primary molars]. Beij Xue Xue Bao Yi Xue Ban. 2015; 47:529–535.
- Doneria D, Thakur S, Singhal P, et al. Comparative evaluation of clinical and radiological success of zinc oxide-ozonated oil, modified 3mix-mp antibiotic paste, and vitapex as treatment options in primary molars requiring pulpectomy: an in vivo study. J Indian Soc Pedod Prev Dent. 2017;35(4):346–352.
- Zhang XF, Xu XB. [Clinical evaluation of zinc oxide eugenol and vitapex as root canal filling materials in primary teeth]. Shang Kou Qiang Yi Xue. 2003;12:377–379.
- Lei Z, Zhang M, Ding J. [Efficacy evaluation of primary teeth canal filling with coral paste]. Med J Wuhan Univ. 2006;27:220–222.
- Chibinski ACR, Reis A, Kreich EM, et al. Evaluation of primary carious dentin after cavity sealing in deep lesions: a 10-to 13-month follow-up. Pediatr Dent. 2013; 35:e107-112.
- Carrotte P. Endodontic treatment for children. Br Dent J. 2005;198(1):9–15.