Abstract
Objectives
To compare infiltration, sealing and microleakage in root dentin with a self-conditioning adhesive system combined with dual curing resin (resin-based cement) to a conventional epoxy-resin-based sealer using confocal microscopy imaging.
Methods
26 roots were enlarged and disinfected. Dentin tubules of 24 teeth were labelled with a red fluorophore (Rhodamine B) (two samples served as controls). Root canal samples were sealed in group AH (n = 11) with a conventional sealer (AH Plus Root Canal Sealer, Dentsply DeTrey) and in group RC (n = 11) with a resin-based cement (Parabond combined with Paracore, Coltène). Roots were then sectioned horizontally and immersed in H2O2 to remove the Rhodamine B not fixed by the sealers. The empty dentin spaces were labeled with a green fluorophore (Fluorescein) enabling the evaluation of infiltration as well as microleakage by confocal microscopy. Two additional samples were fractured in vertical direction for observation under SEM.
Results
Group RC presented significantly more infiltration in the middle third than in the middle and apical thirds of group AH. Microleakage was significantly higher in group AH than in group RC. SEM images revealed more dentin plugs and a homogenous resin layer in group RC in contrast to group AH.
Conclusion
The resin-based cement revealed promising outcomes compared to a traditional epoxy resin based sealer.
Key messages
Infiltration and microleakage in infiltrated and sealed root dentin samples are higher in middle than apical root thirds.
Root dentin infiltration and sealing with a self-conditioning adhesive system and a dual-curing resin cement revealed less microleakage than with an epoxy-resin-based sealer.
1. Introduction
Despite huge progress in research and development on clinical protocols for endodontic treatments, long-term success rates of endodontically treated teeth are still quite variable, sensitive to the diagnostic methods applied, and depending on the definition of success. [Citation1, Citation2]
The main goal of endodontic treatments is to clean the root system and to create a structure free from micro-organisms and toxins. Until now, there is no method to fully eradicate the bacteria that have colonized the whole root system including dentin tubules. Most attempts are more or less successful to clean the internal surface of the root canal wall, accessory canals and the entrances of dentinal tubules. However, attempts to clean all dentinal tubules in their entire length by penetrating into deep dentin layers, to create a real sterile environment, have not been success-full. [Citation3] As a consequence, current treatment protocols aim to control endodontic infections with an additional seal of the root dentin walls and filling of the main root canals by accepting remaining bacteria, or remnants of them, in deep dentin layers. The root canal seal and filling aim to stop bacterial colonization by preventing microleakage that potentially allows an undetectable passage of bacteria, fluids, molecules, or ions as nutrition supply between the tooth and restorative material and thus a re-infection of the root canal system. Literature has shown, that the quality of the root canal obturation in terms of the tightness of the seal and the working length directly influences the long-term success rates of the endodontic treatment. [Citation4]
Presently, hydrophobic epoxy resin sealer like AH Plus (Dentsply DeTrey, Konstanz, Germany), are considered the gold standard and are often applied as a control in research. [Citation5] AH Plus provides characteristics such as relatively short setting time (16-17 hours), minimal polymerization shrinkage, low dimensional changes and low dissolution < 3%. [Citation6] Biocompatibility is controversially discussed as it was shown, that freshly mixed AH-Plus sealer released small amounts of formaldehyde and bisphenol. [Citation5]
Literature investigating the marginal seal of AH Plus to dentin walls compared to other endodontic sealers found low values of leakage over a period of several weeks up to 3 months. [Citation7] Due to its weak acidity, the AH Plus sealer has the ability to etch the dentin surface and collagen fibrils, leading to a covalent binding of the epoxy ring of the resin to the amine groups of the collagen and forming a micro-mechanical lock with the dentin tissue. [Citation8]
However, especially in moist conditions of complex root canal anatomies, literature reported voids and gaps between the AH Plus sealer and the root canal or tubule wall, allowing leakage and bacteria growth that can eventually lead to endodontic treatment failure and tooth extraction. [Citation9, Citation10]
Dual-curing resin-based cements have shown promising results of adhesion on root dentin tissues. Depending on the system applied, dentin is therefore first etched and then infiltrated with an adhesive system, that aims to penetrate the exposed collagen fibrils as well as the dentin tubules and to enable a flowable composite to bond. [Citation11]
Until now, the indication of these materials is limited to the cementation of posts on dentin in the coronal and middle root third. However, to our knowledge no study has investigated whether the entire root canal length could be sealed and infiltrated with such materials.
This study aimed to compare infiltration and sealing of root dentin in the middle and apical root third with a traditional epoxy-resin based sealer (AH Plus) and a chemical, self-conditioning adhesive system (Parabond) in combination with a dual curing glass reinforced post cement (Paracore).
2. Materials and methods
2.1 Shaping and Cleaning
For this study 26 intact and caries-free upper third molars with a straight palatal root of 11-12mm length from the apex to the CEJ with completed apexification were included. Sample size calculation was based on unpublished preliminary tests, that detected an absolute difference of 12% of infiltration between the materials (mean infiltration conventional sealer apical 48%). With a power of 90% and a two-sided alpha error of 5% we calculated a required sample size of n = 10 roots per group. In order to prevent unintentional drop-outs, we included one additional tooth sample for each group resulting in n = 11 teeth per group. Additional samples served as negative controls and for SEM investigations as described in details in the following sections.
Teeth were cleaned with an ultrasonic scaler (EMS Piezo Master 400, EMS SA Nyon, Switzerland) prior the preparation of access to the pulpal chamber with a diamond bur (40 µm, Intensiv, Montagnola, Switzerland) in a highspeed (red) contra-angle handpiece. Palatal canal orifices were localized, and size of the apical constriction was controlled with a size 10 and 15 taper C-pilot file (K Files, MICRO-MEGA, Besançon Cedex, France), diameters of larger than 15 were excluded from the study and replaced, and working length was determined visually with a K file 10 (apex-1mm). Root canals were mechanically enlarged with a crown down technique in a micromotor (CanalProTM Jeni, Coltène/Whaledent GmbH, Langenau, Germany) to a size of 40/.04 (Hy FlexTM EDM, Coltène/Whaledent GmbH, Langenau, Germany) under constant rinsing with 2ml NaOCl (3%, Hänseler Swiss Pharma, Herisau, Switzerland) after each filing step. Final irrigation was performed with 3ml EDTA (17%, Pharma24 SA, Geneva, Switzerland), 2ml distilled water, 3ml NaOCl (3%, Hänseler Swiss Pharma, Herisau, Switzerland) and again 2ml distilled water. Roots were dried subsequently with paper points (Hy FlexTM EDM Paper Points, Coltène/Whaledent GmbH, Langenau, Germany).
To visualize the infiltration and seal of the later-on applied materials into dentin tubules, we did not add a dye to the sealers but intended moreover to detect the material indirectly. We therefore adapted a protocol published in work on infiltration of natural caries cavities [Citation12] and labeled all dentin porosities including dentinal tubules with a red fluorophore, an ethanolic solution of 0.1% RITC (Rhodamine B isothiocyanate, 283924-100MG, SIGMA-ALDRICH Chemistry, Steinheim, Germany), for 24h. The sealer “fixed” the Rhodamine B in the dentin and hindered hydrogen peroxide -as described in detail below- to bleach the tissue. In contrast, parts of the dentin that were not or incompletely infiltrated were however bleached and we could thus visualize the infiltration of the sealers.
2.2 Root canal obturation and preparation for confocal microscopy analysis
After the root dentin was labeled with Rhodamine B, root canals were subsequently dried with paper points and divided in two groups with 11 teeth each.
In group AH a conventional sealer (AH Plus Root Canal Sealer, Dentsply DeTrey, Konstanz, Germany) was applied in the labeled root canal with a paper point up to the working length. Then, a calibrated gutta-percha point (Hy FlexTM EDM Guttapercha Points, Coltène/Whaledent GmbH, Langenau, Germany) was introduced, cut at the canal entrance with a heated instrument and plugged.
In group RC, Parabond Non-Rinse Conditioner (Coltène/Whaledent GmbH, Altstätten, Switzerland) was applied for 30 seconds with a paper point on the labeled canal walls up to the working length, slightly airdried for 2 seconds, then Parabond was mixed in a relation of 1:1 bottle A (chemical cured adhesive A and B Coltène/Whaledent GmbH, Altstätten, Switzerland) and applied actively for 30 seconds in the root canal. The excess bond was removed with paper points and slightly airdried for 2 seconds. Paracore DENTIN SLOW (dual curing core & resin cement (Coltène/Whaledent GmbH, Altstätten, Switzerland)) was applied onto the canal walls in a small quantity and spread out with a Gutta-percha point (Hy FlexTM EDM Guttapercha Points, Coltène/Whaledent GmbH, Langenau, Germany) that had been impregnated with Vaseline (Favodent Karl Huber, Karlsruhe, Germany), so that it could be easily removed after setting of the resin and before cutting the samples in horizontal sections.
Two additional teeth served as negative controls. Their roots were mechanically enlarged, rinsed and labeled with Rhodamine B. They were then cut and treated as described in the following paragraphs without any root canal sealing and obturation.
All obturated teeth were stored at 370C under 100% humidity for at least 7 days until further use.
Horizontal sections (300µm) of 24 teeth were cut at 2 and 4mm distance from the apex representing a sample from the apical and middle root canal third (Diamond Cut-off Wheel M1D13, 127mm dia. X 0.4mm, Struers, Ballerup, Denmark) under constant water cooling.
To bleach the red fluorophore, that had not been enclosed by the root canal filling, air-dried samples were immersed for 24h in 35% hydrogen peroxide solution (35%, Drogerie du Jura, Nyon, Switzerland), and abundantly rinsed with water for 60 seconds.
To visualize microleakage (not or partly infiltrated porosities) in the root dentin, we then immersed the bleached root sections in a 50% ethanolic solution with 100 μM sodium fluorescein (Fluorescein sodium salt, 46970-100G-F, SIGMA-ALDRICH Chemistry, Steinheim, Germany) for 3 minutes to label all porosities and imperfections of the root dentin seal. Labelled specimens were then washed in deionized water for 10 seconds.
All sections were fixed and mounted between slides and coverslips and stored in the dark until observation.
2.3 Confocal microscopy analysis
Images were recorded under a confocal microscope (Zeiss Confocal Line scan LSM 800 Airyscan, Karl Zeiss Microscopy, Cambridge, UK) at a 10-fold magnification and at a wavelength of λemission=580 nm for Rhodamine B and λemission= 516 nm for Fluorescein. Each section was analyzed entirely (i.e. the entire canal/lumen scope) and an average of four captures digitally assembled was used to obtain the entire canal at x10 magnification.
First, the stack of the assembled images were corrected with Imaris 9.7.2 (Andor Technology Limited, Belfast, UK) a microscopy image analysis software, that enabled adjusting the yaw, pitch and roll angles to have the tooth section parallel to the focal plane (xy-plane) ().
In a second step, a custom-made program, based on the software Matlab R2021a (MathWorks, Natick, MA, US), served for the selection of the most appropriate frames with maximum red and green fluorescence inside of the dentin tubuli. We hereby considered the stack with maximum red fluorescence inside the dentin tubules as reference – also for the green fluorescence- and selected with the help of the program additional 4 images around the red fluorescence peak (+/-2 frames), resulting in five slices with a z-resolution of 7 µm for each sample in each red and green fluorescent channels.
Further, the program projected all five images on one plane (see ), that was then analyzed.
Figure 1. Confocal images loaded into image analysis software (Imaris). Sections of the 3D stack are visualized within the xy, xz and yz planes before (a) and after (b) the correction of parallelism via the oblique slicer tool of Imaris.
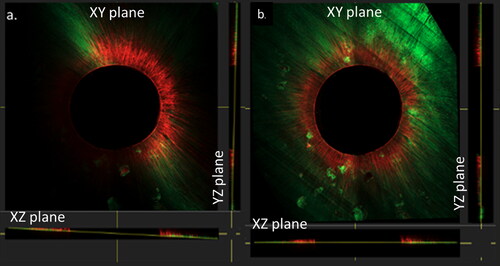
Figure 2. Confocal images with an overview (a) of the root section, (b) magnification of the white box in (a) with 1: working area of 20μm and 2: exclusion of 5 μm; (c) working area with both fluorophores, fluorescein sodium salt (representing microleakage) and Rhodamine B isothiocyanate (representing infiltration) (from the left to the right)
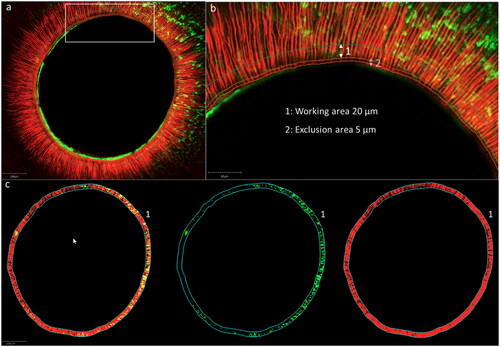
For the quantification of red and green fluorescence, a working area around the lumen () was manually delimited within an image analysis software QuPath (Bankhead, P. et al. QuPath: Open-source software for digital pathology image analysis. Scientific Reports (2017). To avoid any overestimation of red or green fluorescence within the dentin tissue (due to staining of the sealer layer on the root canal walls) we excluded the inner 5 µm (exclusion area) and quantified the dentin infiltration in an area at a depth of the following 20 µm around the lumen (working area) ().
For the quantification of red and green fluorescence in the working area, images, representing both fluorophores were first spit in an image with the green and another with the red fluorescence (). The percentage of fluorescence was then automatically calculated by the program based on the ratio of the number of red or green pixels and the number of pixels without fluorescence in the defined working area.
To avoid a maximum of bias due to the color of the images’ background, the quantification for each sample was performed by two operators independently. Results were then discussed until finding an agreement.
2.4 SEM Images
One sample of each group served for electron microscopy examination. The apical 4 mm of the palatal root was removed with a diamond disk (Diamond Cut-off Wheel M1D13, 127mm dia. X 0.4mm, Struers, Ballerup, Denmark) under constant water cooling. A groove was then prepared with the same device at one external surface of the root fragment in order to split it in longitudinal direction along the root canal with a hammer and chisel into two halves, that were fixed in a solution of 0.1M cacocylate (Sodium cacodylate trihydrate ≥ 98%, Sigma-Aldrich Chemie, Steinheim, Germany) and 2.5% glutaraldehyde (glutaraldehyde solution 50%, Sigma-Aldrich Chemie, Steinheim, Germany) and dried in increasing concentrations of ethanolic solutions (Ethanol, EMSURE, Merck KGaA, Darmstadt, Germany). The split root halves were then glued on custom made specimen holders with double-sided tape and then further dried in a vacuum desiccator (Kartell S.p.A., Noviglio, Italy) for 3 d. Specimens were then gold sputtered (layer of 50 nm thickness with 24 karat gold, HHV Ltd, Crawley, UK) and subjected to SEM (Zeiss Gemini – Sigma 300 VP, Karl Zeiss Microscopy, Cambridge, UK). Photos @ x1.5K were taken along the fracture line of both samples.
2.5 Statistics
All statistical analyses were run with Minitab 19.2020.1 (Minitab LLC, State College, PA, USA). The penetration and microleakage were evaluated using a two ways ANOVA test followed by Fisher’s LSD post-hoc test. Significance levels was set to p-values < 0.001
3. Results
3.1 Sealer infiltration
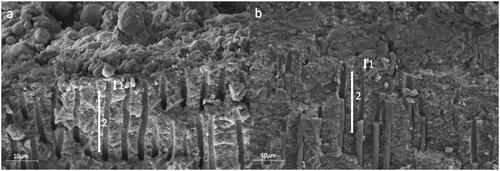
Neither of the two materials resulted in complete dentin infiltration along the entire root wall thickness (). Both materials sealed the root dentin wall and infiltrated the proximal root dentin tubules (marked with a red fluorophore) homogeneously along the root canal lumen in middle and apical root regions in confocal microscopy scans (, 3). However, SEM images revealed areas of not penetrated dentin tubules and gaps between the sealer and the dentin walls in group AH in contrast to group RC. () The resin-based cement created a complete layer in intimate contact with the root canal walls with resin plugs in the dentin tubules. ()
Figure 3. Confocal images with homogeneous sealer infiltration of dentin tubules for both groups in the middle root sections, group AH (photos a, b and c) conventional sealer (AH Plus Root Canal Sealer) and group RC (photos d, e and f) resin-based cement (ParaBond and ParaCore DENTIN SLOW). Left images: overview scans, middle images: fluorescence for quantification in working area and right images: magnifications of white rectangles in left images.
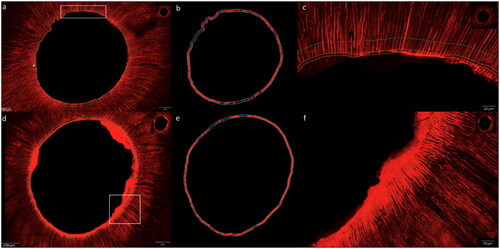
Figure 4. Representative SEM images with a vertical fracture of the roots along the root filling showing the sealer-dentin interface in the apical root third. group AH (a) conventional sealer (AH Plus Root Canal Sealer) and group RC (b) resin-based cement (ParaBond and ParaCore DENTIN SLOW); exclusion zone of 5 μm (1) and working area of 20μm (2).
Percentages of sealer infiltration in the predetermined area (“working area”) for both sealers in the apical and middle root thirds are represented in and .
Figure 5. Box-Whisker Plot with percentages of infiltration by both sealers (group AH conventional sealer AH Plus Root Canal Sealer) and group RC resin-based cement (ParaBond and ParaCore DENTIN SLOW)) at the middle and apical root sections, respectively. Asterisk indicates statistically significant differences between group RC Middle and the other experimental groups.
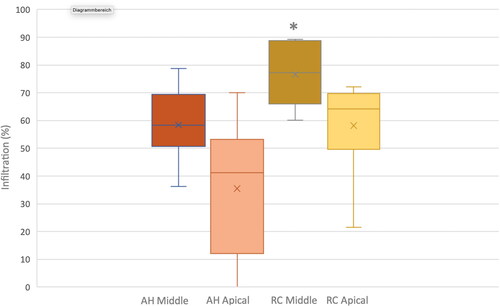
Figure 6. Confocal images representing the microleakage along the dentin tubules for both experimental groups in the middle root sections, group AH (photo a, b, and c) conventional sealer AH Plus Root Canal Sealer and group RC (photo d, e, and f) resin-based cement (ParaBond and ParaCore DENTIN SLOW). Left images: overview scans, middle images: fluorescence for quantification in working area and right images: magnifications of white rectangles in left images.
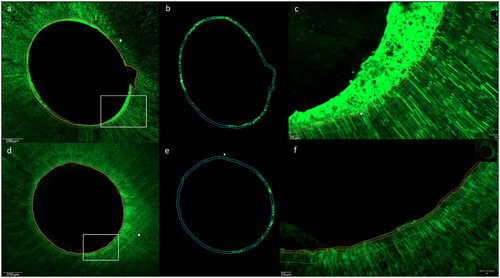
Figure 7. Box-Whisker Plot with percentages of microleakage in both experimental groups (group AH conventional sealer (AH Plus Root Canal Sealer) and group RC resin-based cement (ParaBond and ParaCore DENTIN SLOW)) analyzed in middle and apical root sections. Asterisks indicate statistically significant differences between group RC Middle and group RC Apical as well as between those two groups and the other experimental groups.
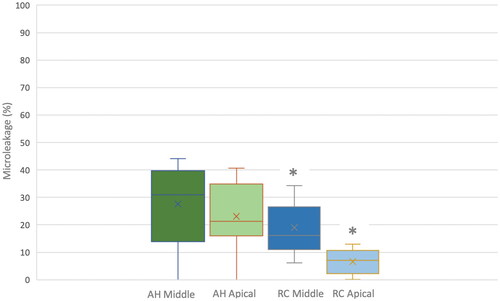
Table 1. Material composition of conventional sealer (AH Plus Root Canal Sealer) and adhesive sealers (ParaBond and ParaCore DENTIN SLOW)
Table 2. Results of sealer infiltration (mean values and standard deviations). Different uppercase letters indicate statistically significant differences based on Fisher’s LSD Test (p<0,001). Group AH conventional sealer (AH Plus Root Canal Sealer) and group RC resin-based cement (ParaBond and ParaCore DENTIN SLOW).
Table 3. Results of microleakage (mean values and standard deviations). Different uppercase letters indicate statistically significant differences based on Fisher’s LSD Test (comparison between groups p<0,0001, comparison between middle and apical root third p<0,006)). Group AH conventional sealer (AH Plus Root Canal Sealer) and Group RC resin-based cement (ParaBond and ParaCore DENTIN SLOW).
The ANOVA test detected statistically significant differences in penetration between root regions and between the two sealers (p-values = 0.001; ). The post-hoc analyses showed that only group RC (resin-based cement) in the middle root section, with a mean infiltration percentage of 76.5, was significantly different from the other three groups. No significant differences were found between those three groups, displaying mean infiltration percentages ranging from 49.93 to 58.44%.
3.2 Sealing quality (microleakage)
Microleakage along the sealer tags in the root dentin, which was measured with the help of a green fluorophore, was also distributed homogeneously along the root canal lumen in both experimental groups. The two root samples, which served as negative controls without any sealing or obturation of the enlarged and cleaned root dentin tissues proved the effectiveness of the green fluorophore that marked the microleakage in the experimental groups as it penetrated the entire root dentin.
The ANOVA test detected statistically significant difference in microleakage between root regions (p-values = 0.006) and between the two sealers (p-values = 0.0001; ). The post-hoc analyses showed that the microleakage percentage was higher for group AH than for group RC. While there was no statistically significant difference between the two root sections for group AH, microleakage was significantly higher in the middle root section than in the apical root section for group RC.
4. Discussion
This study aimed to compare a traditional epoxy-resin based sealer (AH Plus) and a resin-based cement (Parabond + Paracore) with regard to infiltration and sealing of root dentin in middle and apical root thirds.
Infiltration in dentin has been evaluated previously by adding a dye into the material -investigated and applying the modified materials on root dentin; however, this approach may alter the material’s properties. [Citation12] In this study we used a novel methodology that intended to analyze the infiltration of different sealers into root canal dentin without changing the materials’ properties. We indirectly visualized the materials by labeling the dentin with a dye prior to sealer application and then bleached the samples after setting to remove the dye, that was not fixed with the sealer on the dentin. In contrast to previous studies that evaluated the penetration in dentin tubules and the microleakage in separate root samples, the methodology proposed in this study combined the analysis of both aspects of interest in the same samples. This might be seen as an advantage as we could directly compare both values. However, there are also some drawbacks due to the applied protocol - especially with regard to the microleakage values, as described below.
The process for visualization of infiltration and microleakage includes several steps of intensive drying and application of ethanolic solutions that might have an impact on the properties of the root dentin and in consequence on the seal.
AH Plus is a hydrophobic material. However, literature described low bond strength values after the application of high concentrations of ethanolic solutions. In contrast, 70% isopropyl alcohol application revealed promising results compared to ethanol or conventional drying of the root dentin with paper points. It has been speculated that ethanol promotes better removal of the water from the dentin tissue, but also to impair sealer infiltration into the exposed collagen and consequently impacting the hybrid layer formation and sealer adhesion. [Citation13] Thus, it seemed, that either too moist and too dry conditions can impact the adhesion of AH Plus to dentin.
From that point of view, it is possible that the infiltration values tended to be lower and the microleakage values along the sealer dentin interface tended to be higher for AH plus than that what would be achieved in a clinical setting.
In contrast, the resin-based cement Parabond, which contains ethanol as a solvent might have been positively influenced by the application of ethanol. Literature reports on a technique called ethanol-wet-bonding that intends to replace rinse-water from the etched tissues with ethanol. Ethanol based adhesives can thus easily and effectively infiltrate the collagen and create a tight hybrid layer. [Citation14]
Moreover, it is interesting to note, that ethanol application can lead to widening of the interfibrillar spaces through dehydration and shrinkage of proteoglycans filling the interfibrillar space and allow therefore a higher infiltration rate of resins. [Citation15]
However, it is possible that the sealer adhesion is in addition influenced by the application of sodium hypochlorite within the preparation of the main root canals. Sodium hypochlorite has two effects that can impact on adhesion and increase leakage: to remove organic material (collagen) from the exposed dentine surface [Citation16] and to break down to sodium chloride and oxygen. Consequently, the collagen might be removed from the dentin and ideal interaction of the sealers with the organic components of the tissues might be negatively impacted.
Infiltration of the sealer into dentin tubules intends to encapsulate remaining micro-organisms as well as to cut them off from the nutrition supply in order to maintain a sterile environment in the main root canal and finally to improve long-term infection control of the root space.
However, we found that neither of the tested sealers penetrated the full length of the dentinal tubules in the root surface, and that the degree of infiltration depended on the location of the root section (apical or middle third).
Sealing and infiltration of root dentin with adhesives and resins is challenging due to issues related to the anatomy of the root canals- particularly with respect to the configuration factor (C-factor), a tool to quantitatively measure the geometry of cavities. The C-factor represents the ratio of bonded to unbonded surfaces in a cavity [Citation17]; values higher than 5 are considered to have an increased risk to resin detaching during the polymerization process. [Citation17, Citation18] Literature reports on values of >200 for root canal configurations, which can explain the difficulties reported in studies on application of adhesives and composites[Citation18–21]
In order to overcome these issues when applying the resin-based cement on the root dentin walls we first intended to reduce the thickness of the resin layer and to decrease the resin volume and consequently its volumetric contraction while polymerization with the help of a gutta-percha master point.[Citation22] The volumetric contraction is reported to enhance the formation of cracks and spaces between the resin and the dental tissue- and finally to promote the failure of the adhesive interface. [Citation23]
In contrast, AH Plus is reported to show higher infiltration rates and bond strength values when applied in thicker layers. [Citation24] The SEM observations in the present study showed large areas of non-infiltrated root dentin when AH Plus was used, whereas the adhesive sealing revealed a good infiltration in dentin tubules and a tight seal of the main canal. A possible explanation for this finding might be an obstruction of the dentin tubules entrances by a remaining smear layer that was not entirely removed with the irrigation protocol and could thus hinder sealer infiltration into the tubules. In contrast to AH Plus, the protocol for the resin-based cement included an initial application of a non-rinse conditioner which may have contributed to a better smear layer removal and in consequence to a higher infiltration rate. An additional explanation for an impaired sealer infiltration might be the obturation technique applied. Lateral compaction technique is reported to apply higher forces on the sealer, promoting better infiltration in the dentin tubules. [Citation25] In the present study we introduced a single master gutta-percha point in both experimental groups. However, as we observed the proximal root dentin of only one sample per group under the SEM, these findings do not allow a final conclusion.
Furthermore, the filler particle size of AH Plus has been reported to be with up to 8 μm too large to enter dentin tubules which have an average diameter of 1.7μm. [Citation26] It was therefore assumed, that only the unfilled resin components were able to penetrate the dentin tubules and covered the dentin with a very thin layer. [Citation26]
Besides spreading out the sealer, we further intended to apply the gutta-percha point in group RC (resin-based cement) as a “stress breaker” within the polymerization process. To avoid any adhesion between the master point and the resin, the master point was impregnated with a thin layer of vaseline. This prevented the formation of a mono-block of the sealer and core material, which was intended to contribute to overcoming issues related to the unfavorably high configuration factors of the root canals [Citation20, Citation21]. Adhesive mono-block root canal fillings are reported to be prone to crack formation along the entire length of the adhesive interface without any preventive measures [Citation27].
Literature has reported of improved bond strength for posts with an indirect bonding procedure comprising a first step of coating the canal walls with hybridized resins, and then in a second step bond to the core material to the cured root canal wall coating. The separation of two steps was reported to compensate for polymerization stresses in root canals as well as in unfavorable configurations of inlay restorations in tooth crowns. [Citation28] In line with this approach, we intended to avoid any adhesion of the core material (guttapercha) in the main root canal to the sealer coating the root walls and infiltrating into dentin tubules. SEM images showed clearly that these issues were overcome and that a tight seal with a resin-based cement is possible. Similar to conventional sealers, the infiltration of resins into the dentin tubules is also impacted by the pressure created by the core material, which is due to inhomogeneities of the root canal anatomy and difficult to control.
Compared to coronal dentin, radicular dentin shows morphological variations with irregularities, that can influence the adhesion and might explain observed differences between the root regions. Literature reports of areas of resorption, pulp stones, smaller proportion of tubular area available for tag formation [Citation29] as the numbers and size of dentinal tubules decrease from coronal to apical due to physiological sclerosis, and the oblique orientation of about 50% of dentinal tubules [Citation30] that might affect the sealing possibilities of obturation materials. [Citation31] Both experimental groups showed higher values of penetration in the middle region with a higher number of dentinal tubules which is in line with the literature. [Citation32]
However, literature reveals that sealer infiltration into dentinal tubules might not directly be correlated to sealing ability and microleakage. [Citation33] After disinfection of the root system with endodontic irrigants, remaining bacteria in deep dentin layers are supposed to be cut off from nutrition supply by a tight root dentin seal. In case this seal is leaking, bacteria and their by-products might pass and maintain an infection. Thus, it is necessary to not only study sealer infiltration, but also to determine microleakage along the resin dentin interface.
To visualize the contrast of infiltrated to not infiltrated root tissues it was required to cut the infiltrated roots in horizontal slices and to bleach the entire root slices. It was not possible to apply the bleach and consequently the dye marking the microleakage only in the root main canal walls. Moreover, it was required to color the already cut root slices in order to not distort the microleakage due to washing out or spreading out of the dye on the dentin tissue during the cutting process. This could be considered as a disadvantage of this study because it is not possible to determine the precise origine of the microleakage. As the dye was not only applied on the root canal walls but also on the cut surface of the dentin and penetrated all dentin porosities after sealer infiltration, it might be that the microleakage values were overestimated.
Both sealers showed microleakage in the middle and the apical root third. Higher values of microleakage were recorded in the middle region than apically which might be attributed to the specific root dentin micromorphology with decreasing number of dentin tubules from coronal to apical. [Citation30]
Group RC with a resin-based cement showed significantly less microleakage along the resin tags than the conventional sealer in both tested root regions. This can be explained by the higher infiltration rate and the observation of a homogenic sealer layer on the root walls.
Literature concentrates on the investigation of either apical or coronal leakage and found also important values for AH Plus. [Citation4] To our understanding, the eventual presence of coronal or apical leakage becomes less important with a tight dentin tissue seal that closes up remaining bacteria and makes them inaccessible for tissue fluids. Coronal leakage can be controlled with adhesive restorations, and apical leakage of tissue fluids can be supposedly maintain infections only if intraradicular bacteria are accessible.
However, it would be relevant to assess long-term microleakage in further studies as the adhesion interface of resin cements is impacted by the mechanical stress of functional forces due to mastication and hydrolysis. [Citation19] AH Plus has shown very low values for dimensional changes. [Citation34] This was explained by water sorption and swelling of the epoxy resin component after polymerization. [Citation34]
This experiment should be perceived as a proof of principle for the application of a resin-based cement as a sealer in root canal configurations and might open a new and promising field of research and treatment in endodontics. The underlying protocol is not suitable for clinical application but serves as base for new preferably bioinert dental materials. This is due to the fact, that a reintervention in case of failure of an endodontic treatment with resin cement will be very challenging and dependent on the resin layer thickness. Moreover, resin cements are susceptible for foreign body reaction of over pressed root filling materials. [Citation35]
5. Conclusions
Within the limitations of the study, it can be concluded, that a resin-based cement can infiltrate and seal tightly the dentin in middle and apical root thirds. Both tested materials infiltrated the dentin tubules of root dentin but not to the full tubule depth. The resin-based cement infiltrated more dentin tubules and revealed less leakage along the dentin tubules than the conventional sealer.
Author contribution
Conception and design (CIA, IK, LM, NL); Analysis and interpretation of data (CIA, IK, LM, EDB); Drafting of the paper (CIA, LM, EDB); Revising paper critically for intellectual content (AF, MA); final approval of version to be published (all authors agree to be accountable for all aspects of the work)
Acknowledgements
Isaline Rossier, Luciana Caseiro, Bioimaging facility (UNIGE), Olivier Brun et François Prodon
Disclosure statement
The authors report no conflict of interest
Data availability
The data that support the findings of this study are available from the corresponding author, [CIA], upon request.
References
- Fernandez R, Cardona JA, Cadavid D, Alvarez LG, Restrepo FA. Survival of Endodontically Treated Roots/Teeth Based on Periapical Health and Retention: A 10-year Retrospective Cohort Study. J Endod. 2017;43(12):1–12. doi: 10.1016/j.joen.2017.08.003.
- Kruse C, Spin-Neto R, Evar Kraft DC, Vaeth M, Kirkevang LL. Diagnostic accuracy of cone beam computed tomography used for assessment of apical periodontitis: an ex vivo histopathological study on human cadavers. Int Endod J. 2019;52(4):439–50. doi: 10.1111/iej.13020.
- Tabassum S, Khan FR. Failure of endodontic treatment: The usual suspects. Eur J Dent. 2016;10(1):144–7. doi: 10.4103/1305-7456.175682.
- Komabayashi T, Colmenar D, Cvach N, Bhat A, Primus C, Imai Y. Comprehensive review of current endodontic sealers. Dental Materials Journal. 2020;39(5):703–20. doi: 10.4012/dmj.2019-288.
- Borges Á H, Orçati Dorileo MC, Dalla Villa R, Borba AM, Semenoff TA, Guedes OA, et al. Physicochemical properties and surfaces morphologies evaluation of MTA FillApex and AH plus. ScientificWorldJournal. 2014;2014:589732. doi: 10.1155/2014/589732.
- Lee JK, Kwak SW, Ha JH, Lee W, Kim HC. Physicochemical Properties of Epoxy Resin-Based and Bioceramic-Based Root Canal Sealers. Bioinorg Chem Appl. 2017;2017:2582849. doi: 10.1155/2017/2582849.
- Huang Y, Celikten B, de Faria Vasconcelos K, Ferreira Pinheiro Nicolielo L, Lippiatt N, Buyuksungur A, et al. Micro-CT and nano-CT analysis of filling quality of three different endodontic sealers. Dentomaxillofac Radiol. 2017;46(8):20170223. doi: 10.1259/dmfr.20170223.
- Camargo RV, Silva-Sousa YTC, Rosa R, Mazzi-Chaves JF, Lopes FC, Steier L, et al. Evaluation of the physicochemical properties of silicone- and epoxy resin-based root canal sealers. Braz Oral Res. 2017;31:e72. doi: 10.1590/1807-3107BOR-2017.vol31.0072.
- Roggendorf MJ, Ebert J, Petschelt A, Frankenberger R. Influence of moisture on the apical seal of root canal fillings with five different types of sealer. J Endod. 2007;33(1):31–3. doi: 10.1016/j.joen.2006.07.006.
- Donnelly A, Sword J, Nishitani Y, Yoshiyama M, Agee K, Tay FR, et al. Water sorption and solubility of methacrylate resin–based root canal sealers. Journal of Endodontics. 2007;33(8):990–4. doi: 10.1016/j.joen.2006.03.021.
- Rosa WL, Piva E, Silva AF. Bond strength of universal adhesives: A systematic review and meta-analysis. J Dent. 2015;43(7):765–76. doi: 10.1016/j.jdent.2015.04.003.
- Paris S, Bitter K, Renz H, Hopfenmuller W, Meyer-Lueckel H. Validation of two dual fluorescence techniques for confocal microscopic visualization of resin penetration into enamel caries lesions. Microsc Res Tech. 2009;72(7):489–94. doi: 10.1002/jemt.20701.
- Dias KC, Soares CJ, Steier L, Versiani MA, Abi Rached-Júnior FJ, Pécora JD, et al. Influence of Drying Protocol with Isopropyl Alcohol on the Bond Strength of Resin-based Sealers to the Root Dentin. Journal of Endodontics. 2014;40(9):1454–8. doi: 10.1016/j.joen.2014.02.021.
- Pashley D, Tay F, Carvalho R, Rueggeberg F, Agee K, Carrilho M, et al. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. American journal of dentistry. 2007;20:7–20.
- Ayar MK. A review of ethanol wet-bonding: Principles and techniques. Eur J Dent. 2016;10(1):155–9. doi: 10.4103/1305-7456.175687.
- Marending M, Luder HU, Brunner TJ, Knecht S, Stark WJ, Zehnder M. Effect of sodium hypochlorite on human root dentine–mechanical, chemical and structural evaluation. Int Endod J. 2007;40(10):786–93. doi: 10.1111/j.1365-2591.2007.01287.x.
- Feilzer AJ, De Gee AJ, Davidson C. Setting stress in composite resin in relation to configuration of the restoration. Journal of dental research. 1987;66(11):1636–9. doi: 10.1177/00220345870660110601.
- Özcan M, Volpato CAM. Current perspectives on dental adhesion: (3) Adhesion to intraradicular dentin: Concepts and applications. Japanese Dental Science Review. 2020;56(1):216–23. doi: 10.1016/j.jdsr.2020.08.002.
- Schwartz RS. Adhesive dentistry and endodontics. Part 2: bonding in the root canal system-the promise and the problems: a review. J Endod. 2006;32(12):1125–34. doi: 10.1016/j.joen.2006.08.003.
- Bouillaguet S, Troesch S, Wataha JC, Krejci I, Meyer J-M, Pashley DH. Microtensile bond strength between adhesive cements and root canal dentin. Dental Materials. 2003;19(3):199–205. doi: 10.1016/s0109-5641(02)00030-1.
- Breschi L, Mazzoni A, De Stefano Dorigo E, Ferrari M. Adhesion to intraradicular dentin: a review. Journal of Adhesion Science and Technology. 2009;23(7-8):1053–83. doi: 10.1163/156856109X440957.
- Tay FR, Loushine RJ, Lambrechts P, Weller RN, Pashley DH. Geometric Factors Affecting Dentin Bonding in Root Canals: A Theoretical Modeling Approach. Journal of Endodontics. 2005;31(8):584–9. doi: 10.1097/01.don.0000168891.23486.de.
- Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24(1):90–101. doi: 10.1016/j.dental.2007.02.009.
- Rahimi M, Jainaen A, Parashos P, Messer HH. Bonding of resin-based sealers to root dentin. J Endod. 2009;35(1):121–4. doi: 10.1016/j.joen.2008.10.009.
- Al-Hadlaq SM, Al-Jamhan A, Alsaeed T. Comparison of the single cone and cold lateral compaction techniques in sealing 0.04 taper root canal preparations. Gen Dent. 2010;58(5):e219-22.
- Asawaworarit W, Pinyosopon T, Kijsamanmith K. Comparison of apical sealing ability of bioceramic sealer and epoxy resin-based sealer using the fluid filtration technique and scanning electron microscopy. J Dent Sci. 2020;15(2):186–92. doi: 10.1016/j.jds.2019.09.010.
- Pirani C, Chersoni S, Foschi F, Piana G, Loushine RJ, Tay FR, et al. Does hybridization of intraradicular dentin really improve fiber post retention in endodontically treated teeth? Journal of Endodontics. 2005;31(12):891–4. doi: 10.1097/01.don.0000164853.92310.e7.
- Bouillaguet S, Bertossa B, Krejci I, Wataha JC, Tay FR, Pashley DH. Alternative Adhesive Strategies to Optimize Bonding to Radicular Dentin. Journal of Endodontics. 2007;33(10):1227–30. doi: 10.1016/j.joen.2007.05.016.
- Chu C-Y, Kuo T-C, Chang S-F, Shyu Y-C, Lin C-P. Comparison of the microstructure of crown and root dentin by a scanning electron microscopic study. Journal of Dental Sciences. 2010;5(1):14–20. doi: 10.1016/S1991-7902(10)60003-7.
- Mjör IA, Smith MR, Ferrari M, Mannocci F. The structure of dentine in the apical region of human teeth. Int Endod J. 2001;34(5):346–53. doi: 10.1046/j.1365-2591.2001.00393.x.
- Ekambaram M, Yiu CKY, Matinlinna JP. Bonding of adhesive resin to intraradicular dentine: A review of the literature. International Journal of Adhesion and Adhesives. 2015;60:92–103. doi: 10.1016/j.ijadhadh.2015.04.003.
- Boing TF, Gomes GM, Gomes JC, Reis A, Gomes OM. Is the bonding of self-adhesive cement sensitive to root region and curing mode? J Appl Oral Sci. 2017;25(1):2–9. doi: 10.1590/1678-77572015-0430.
- Bergmans L, Moisiadis P, De Munck J, Van Meerbeek B, Lambrechts P. Effect of polymerization shrinkage on the sealing capacity of resin fillers for endodontic use. Journal of Adhesive Dentistry. 2005;7(4).
- Versiani MA, Carvalho-Junior JR, Padilha MI, Lacey S, Pascon EA, Sousa-Neto MD. A comparative study of physicochemical properties of AH Plus and Epiphany root canal sealants. Int Endod J. 2006;39(6):464–71. doi: 10.1111/j.1365-2591.2006.01105.x.
- Wisniewska-Jarosinska M, Poplawski T, Chojnacki CJ, Pawlowska E, Krupa R, Szczepanska J, et al. Independent and combined cytotoxicity and genotoxicity of triethylene glycol dimethacrylate and urethane dimethacrylate. Mol Biol Rep. 2011;38(7):4603–11. doi: 10.1007/s11033-010-0593-1.
