Abstract
Ceramic foams present special features that do not have the corresponding dense materials and hence they are widely used in a broad variety of applications. Titania has exceptional photoinduced properties with applications in membranes, bioceramics and photocatalysis and photovoltaics. However, in the photoinduced applications, the anatase phase is more active, but the sintering at high temperature leads to the transformation to rutile. The maintenance of anatase would require low sintering temperatures leading to a lack of consistency and very weak struts. To avoid this problem a possible alternative is to produce sintered rutile foams and to coat the strut walls with nanoparticles of anatase. This is a very simple and inexpensive method. In this work rutile foams are obtained by the replica method after a full optimization of the rheological behaviour of titania suspensions that are subsequently infiltrated with a colloidal titania suspension either in vacuum or using microwaves. The infiltrated foam can be sintered at 650 °C while maintaining the anatase phase.
1. Introduction
Ceramics with tailored porosity are increasingly demanded because they can provide special properties and features that could not be obtained with dense materials (Liu & Chen, Citation2014; Rice, Citation1998; Scheffler & Colombo, Citation2005). Porous ceramics are used in applications requiring a combination of properties related to the structure of the pores such as pore size distribution and shape, connectivity, particle size, etc., and the properties of the material in service such as chemical stability, thermal and electrical conductivity, mechanical resistance, etc. The broad variety of materials, designs, structures, and available manufacturing processes make it possible the application in very different areas, such as filters and membranes, catalytic supports, electrodes in cells and batteries, structural light materials for heating elements and burners, piezoelectrics, and biomaterials, among others.
The properties of the porous material strongly depend on the processing route used for their manufacture (Colombo, Citation2006; Montanaro, Jorand, Fantozzi, & Negro, Citation1998; Studart, Gonzenbach, Tervoort, & Gauckler, Citation2006). The most direct method to obtain porous parts is through the incomplete sintering using heating temperatures below that needed to fully densify the compact (Jean, Sciamanna, Demuynck, Cambier, & Gonon, Citation2014). However, this can lead to defects and low uniformity if the process is not well controlled. In addition to this, there are several methods for producing porous ceramics, which can be grouped into the following four different families (Studart et al., Citation2006):
the replica method, based in the impregnation of a cellular structure into a suspension that fills the open pores and reproduces the shape of the template once it is burned out by a thermal treatment (Saggio-Woyansky, Scott, & Minnear, Citation1992; Sousa et al., Citation2005). Natural sponges, coral or wood (Sieber, Hoffmann, Kaindl, & Greil, Citation2000), or synthetic carbon and polymer foams such as polyurethane are frequently used (Luyten et al., Citation2005).
the use of sacrificial templates, using a dispersed sacrificial phase in a matrix of the ceramic or precursor phase that is removed during a chemical or a thermal treatment generating the pores (Alves, Tari, Fonseca, & Ferreira, Citation1998; Wang, Sung, Li, & Kim, Citation2004). Again, the sacrificial templates can be biomolecules (for example, starches, dextrins, alginates, cellulose, etc.) or synthetic (such as beads of different polymers, e.g., poly(styrene), poly(ethylene oxide), poly(methylmetacrylate), etc (Abe, Seki, Fukunaga, & Egashira, Citation1994; Thijs, Luyten, & Mullens, Citation2004). In opposition to organic templates, the water contained in a suspension can be also used as a template when it is frozen and subsequently sublimated. This is the well-known freeze casting or ice-templating method (Araki & Halloran, Citation2005; Deville, Citation2008; Tallón, Moreno, & Nieto, Citation2009).
direct foaming of ceramic suspensions or precursors, based in the introduction of air into a suspension or liquid precursor solution, for example adding a surfactant with energetic mechanical mixing to create bubbles (Dhara & Bhargava, Citation2003; Ortega, Sepulveda, Innocentini, & Pandolfelli, Citation2001; Santacruz, Moreno, & Rodrigues-Neto, Citation2008) and some organic additive capable to retain the bubbles in the bulk during the consolidation by sol-gel setting, gel-casting, in situ coagulation or flocculation of suspensions, etc. Some inorganic materials display also sol-gel transitions, such as silica (Rincón, Giacomello, Pasetto, & Bernardo, Citation2017).
additive manufacturing by direct and indirect methods, depending on whether the material is directly deposited to obtain the final piece or not (Chen et al., Citation2019; Deckers, Vleugels, & Kruth, Citation2014; Zocca, Colombo, Gomes, & Günster, Citation2015). Although some of the techniques can produce dense parts, they are better suited for the production of cellular materials and foams with open cell structures.
As in any other slurry-based processing, the optimization of the suspensions is a critical step to achieve the desired microstructural uniformity and the ability to reproduce the pore structure (Moreno, Citation2020). Of course, the distribution, size and concentration will depend on the shaping method and the characteristics of the pore formers. In the case of the replica method, there is a variety of sponges with different pore diameters and the rheological behaviour has to be studied to have the proper solids loading and viscosity and a good adhesion to the sponge during the impregnation process.
TiO2-based materials are widely studied owing to their broad range of applications in many different areas of technology and therefore, the literature on this material is extremely abundant. Some of the most important applications are those involving purification filters and membranes and those based in their excellent photoactivity, such as photocatalytic and photovoltaic devices (Fujishima, Zhang, & Tryk, Citation2008; Kang et al., Citation2019). The main problem of bulk titania is the high temperature required for sintering (normally above 1400 °C), at which the anatase phase transforms into rutile (Gouma & Mills, Citation2001). The manufacture of anatase scaffolds is complex because it requires the preparation of concentrated suspensions to obtain the foam, which is a hard limitation when using nanoparticles.
To solve these problems some authors have proposed several approaches to enhance the photocatalytic activity. One is to enhance the activity in the visible range, normally doping with some non-metal or metal atoms, mainly rare-earth transition metals and to produce the foam with the doped material. In this case all the material contains the dopant atoms thus difficulting synthesis and processing and increasing the cost. Another approach is to produce a backbone of a macroporous reticulated material and to coat it with a few micrometres film of TiO2 anatase with a high surface area. For example, Zr-doped titania films have been deposited on ceramic foams prepared with alumina and alumina-mullite (Plesch, Vargová, Vogt, Gorbár, & Jesenák, Citation2012) serving as excellent carriers for titania films. Nickel foams have been also used as carrier support for the dip-coating of anatase TiO2 (Hu et al., Citation2007). In this case, the heat treatment results in the formation of a NiO layer and if properly controlled, the Ni2+ ions can increase the absorption edge of TiO2 to the visible light region. However, the thickness of the NiO layer and the concentration of Ni2+ ions acting as a dopant in the titania film are difficult to control. In addition, for specific shapes in which no commercial wafers can be used, the manufacture of the reticulated nickel foams is more difficult. Another approach is the use of a reticular material of carbon foam as a support (Wang et al., Citation2017), which is furtherly impregnated with nanosized titania. However, some of these support materials can lead to incompatibilities derived from the different thermal expansion coefficient of the materials, or the different densification kinetics. A simple approach to avoid this phase incompatibility is the preparation of a reticulated foam of the same material, in this case TiO2, which is then sintered and transforms to the rutile phase. This rutile foam is used as the backbone to be impregnated with an anatase sol, which is cured at a temperature low enough to avoid transformation to rutile. This procedure is simple and versatile due to the availability of titania powders, the simplicity of the foam shaping and the efficiency of the impregnation by conventional dip-coating.
Accordingly, the aim of this work was to optimize the rheological behavior of concentrated titania suspensions for the manufacture of cellular structures by the replica method using polyurethane foam. After sintering the titania skeleton consists of rutile phase. The objective is to coat this rutile foam with anatase nanoparticles. For so doing, a nanoparticulate suspension of titania-anatase was infiltrated by a conventional impregnation route with a nanoparticulate sol by dip-coating using a vacuum pump. In addition, an innovative approach for the impregnation of anatase is proposed, consisting in the use of a microwave oven at 180 °C to promote the immobilization of anatase on the rutile supporting foam and a subsequent curing step at 650 °C, in order to avoid the phase transformation of anatase to rutile. This allows the formation of an anatase layer on the rutile backbone, with good mechanical resistance and thermal compatibility.
2. Materials and methods
As starting material, a commercially available submicron-sized titania powder (Merck, 808, Germany) was used. This powder has a specific surface area of 9 m2/g and an average particle size of 0.35 µm, and crystallographically, it consists of anatase phase. Surface area was measured by N2-adsorption using the one-point method (Monosorb Surface Area Analyser MS-13, Quantachrome Co., Boynton Beach, USA), and particle size distributions were measured by laser diffraction (Mastersizer S, Malvern, UK).
For infiltration a commercial TiO2-anatase particulate sol (Aerodisp® W740X, Degussa-Evonik, Germany) was used. This suspension, based in the well-known titania P25 powder, has a solids content of 40 wt% and pH 5–7. The dry powder has a ratio of anatase-rutile phases of about 75/25 and an average particle size of 20 nm and a surface area of 100 m2/g. Foams of polyurethane with 30 ppi (pores per inch) were used for this study.
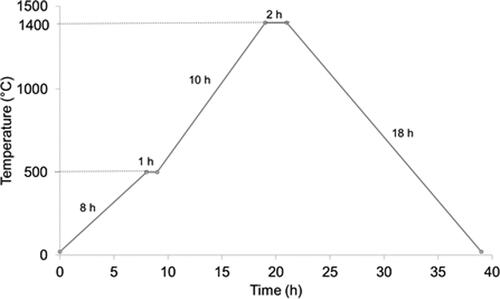
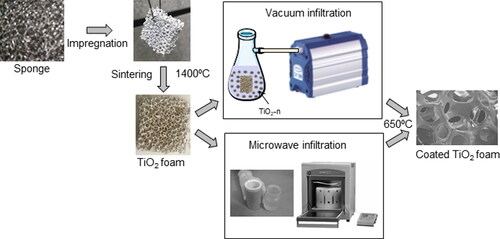
The experimental procedure is schematically shown in . The polyurethane foams were immersed into the slurry of submicron-sized titania and subjected to manual compression. This operation was repeated three times to fill the pores and the excess of slurry deposited in the cavities was removed with jet air. After impregnation, the samples were left in air to dry for 24 h and subsequently fired using the thermal cycle shown in , with a step at 500 °C/1h to promote burning out of the organic foam and a final sintering temperature of 1400 °C/2h to obtain high density in the skeleton of the foam. The sintered ceramic foam was immersed in a colloidal suspension of nanosized titania in order to promote the coating of the nanometric titania onto the sintered foam. The infiltration was performed by either application of vacuum using a pump or by introducing the foam and the colloidal suspension in a microwave oven. A commercial microwave digestion system (Milestone ETHOS One, Sorisole, Italy) operating at microwave frequency of 2.45 GHz and pressure of 1 Pa was used, with a holding time of 20 min and a maximum temperature of 180 °C. Finally, the infiltrated samples were treated at 650 °C, in order to densify the nanosized fraction avoiding a massive transformation of anatase to rutile.
Figure 1. Schematic flow chart of the replica process to obtain titania foams and their subsequent infiltration with colloidal titania by using either vacuum infiltration or microwave drying at 180 °C.
To produce the foams titania suspensions were prepared in water at different solids loadings ranging from 20 to 45 vol.% using a concentration of 0.2 wt% (referred to dry solids) of a polyacrylic-based polyelectrolyte (Duramax D3005, Dow Chemicals, USA). This polyelectrolyte is supplied as an aqueous solution with 35 wt% active matter and has an average molecular weight of 3500 Dalton. Fresh suspensions were prepared by adding the powder slowly in deionized water containing the required amount of deflocculant under mechanical agitation, which was maintained for 30 min. Then, consecutive cycles of ultrasonication of 1 min were applied using a sonicator (Dr. Helscher UP400S, Germany) with a power of 400 W. To evaluate the effect of mixing and sonication, the rheological behaviour was measured before and after each minute of sonication.
To achieve the desired adhesion after impregnation a poly(vinyl alcohol) solution (PAF Optapix 35, Zschimmer-Schwarz, Germany) with an active matter content of 35%, a density of 1.08 m2/g and a viscosity of 1300 mPa.s, was added in concentrations of 1 and 2 wt%.
Rheological measurements were performed with a rotational rheometer (Haake Mars II, Thermo, Germany) under controlled rate conditions at 25 °C using a double-cone and plate measuring system with a cone angle of 2° and a diameter of 60 mm. The measuring cycle consisted on a first step with an increase of shear rate from 0 to 1000 s−1 in 5 min, 1 min at maximum shear rate and a down curve from 1000 to 0 s−1 in 5 min.
Microstructural observations were performed with a field emission gun scanning electron microscope (FE-SEM) with energy dispersive X-ray (FE-SEM-EDX, Hitachi S-4700 type I, Tokyo, Japan) microanalysis. X-ray diffraction experiments were performed using a Brooker Advance DM8 using Cu Kα radiation at a working power of 30 kV and 40 mA, a range of 2θ between 20° and 80°, a step size of 0.02° and a scanning speed of 0.5 s/step. Due to the little fraction of coating phase and its very small size the anatase phase cannot be detected in the diffractograms. Therefore, in order to determine whether the phase is retained at this temperature or transforms to rutile the colloidal anatase slurry was dried and calcined at 700 °C for the XRD analysis.
3. Results and discussion
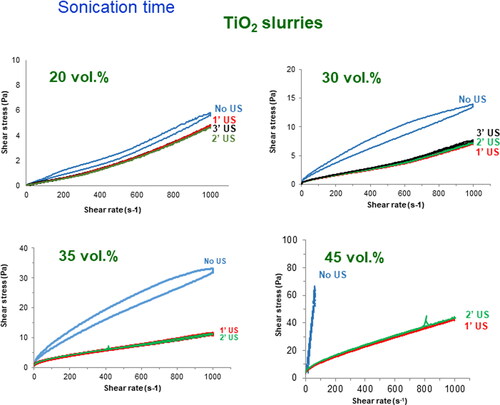
In order to optimize the suspension characteristics for a good impregnation in the polyethylene foam, the rheological behaviour of submicron sized titania suspensions was first studied. As reported in a previous work (López-López, Baudín, & Moreno, Citation2008), the isoelectric point of this titania powder occurs at pH 4.2. Homogeneous concentrated suspensions were obtained using a polyacylic based deflocculant at a concentration of active matter of 0.2 wt% on a dry solid basis. These suspensions were prepared by mechanical agitation and further ultrasonication for successive cycles of 1 min, up to a total of 3 min. Suspensions were prepared at similar conditions using solids loadings of 20, 30, 35 and 45 vol.%. shows the flow curves of the suspensions with those solids loadings just after mechanical mixing and after the application of 1 to 3 min of sonication. As it can be seen the suspensions with low solids contents (20 vol.%) exhibit an apparent shear thickening behaviour and, prior to the application of ultrasounds, they have some hystheresis cycle, demonstrating its lack of homogeneity. After application of ultrasounds the slurries become stable without any time dependency but they still show and apparent shear thickening and have viscosity values much lower than desired for the impregnation. This apparent shear thickening has been reported previously (Carnicer, Alcázar, Orts, Sánchez, & Moreno, Citation2021) and is due to the error in the measurement and is related with the wall slippage occurring in very low viscosity suspensions. In the 30 vol.% suspensions the slurry prepared without sonication show a shear thinning behaviour with a clear thixotropic cycle, which is removed after sonication, confirming that stability is only achieved in sonicated suspensions. However, no apparent shear thickening is observed here because the viscosity values are larger and fall within the measuring ranges of rotational rheometers. These effects are more evident in the suspensions with higher solids loadings, and for concentrations as high as 45 vol.% solids the sonicated suspensions exhibit a low viscosity of about 44 mPa.s at a shear rate of 1000 s−1 and no thixotropic hystheresis in spite of the high viscosity of the non-sonicated suspensions, in which the torque of the rheometer is exceeded at very low shear rate.
Figure 3. Flow curves of titania suspensions prepared to solids loadings of 20 to 45 vol.% prior and after successive sonication cycles of 1 min.
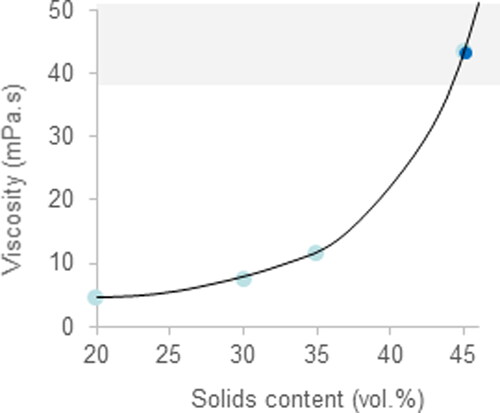
The variation of viscosity at the maximum shear rate with the solids content is plotted in . It can be seen that the viscosity increases exponentially, as expected for particulate dispersions. From 45 vol.% solids, the curve becomes practically asymptotic so that greater solids contents would give excessively high viscosities. This indicates that suspensions with 45 vol.% solids are the most appropriate for impregnation.
Figure 4. Variation of viscosity at a shear rate of 1000 s−1 as a function of the solids content of the slurries.
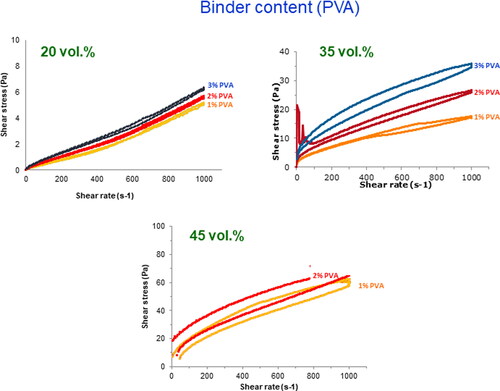
However, in order to retain the particles adhered to the walls of the foam it is necessary to add also a binder, which also contributes to the viscosity. In this case an aqueous solution of poly(vinyl alcohol) (PVA) was used to concentrations of 1 and 2 wt% (referred to dry solids). In order to check the influence of the binder addition on the slurries viscosity, the rheological behaviour was also measured. shows the flow curves of suspensions with 1, 2, and 3 wt% PVA and different solids loadings. Once again the viscosity of 20 vol.% suspensions is too low for the impregnation process, and the most concentrated suspensions still presents a relatively low viscosity and some thixotropy, which in this case may help to maintain the adherence of the particles to the foam walls. It is also seen that viscosity is slightly higher for the suspension with 2 wt% PVA.
Figure 5. Flow curves of suspensions with different solids contents and binder concentrations prepared with 1 min sonication.
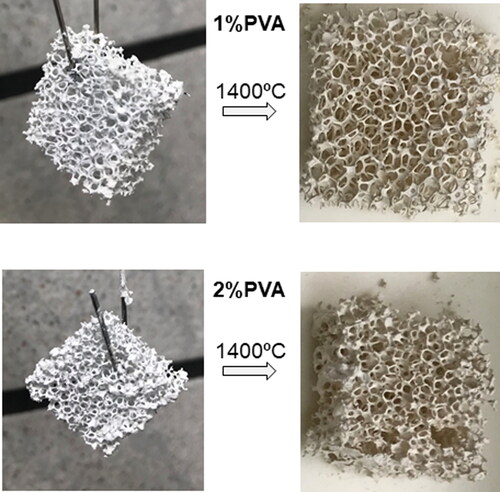
In order to evaluate which content of binder must be used, both suspensions were used for the impregnation of titania slurries. The aspect of the foams impregnated with 1 and 2 wt% PVA containing slurries can be seen in just after impregnation and after debinding and sintering. Although both conditions could be used for the process it seems that 1 wt% is better, as the higher viscosity obtained with more binder amount leads to some zones of poor homogeneity with viscous slurry adhered in the zones between the struts that tend to close the pores. Accordingly, 1 wt% PVA was selected for further studies.
Figure 6. Titania foams obtained with 1 and 2 wt% of binder (PVA) prior and after sintering at 1400 °C.
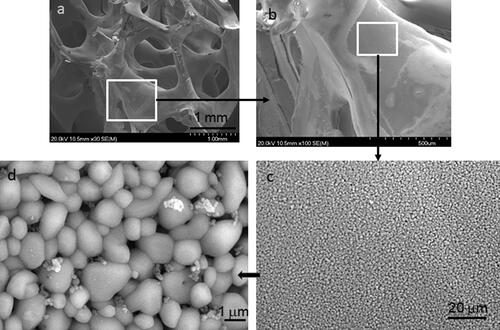
The microstructure of the sintered foam obtained with the vacuum pump is shown in at different magnifications. shows the general morphology of the foam, with the open cells and dense struts, one of them is presented at higher magnification in . The microstructure of the dense struts can be seen in the detailed microphotograph of , where it can be stated that the sample has a great uniformity with small grain size and no defects like pores, agglomerates, grain growth, etc. This large homogeneity demonstrates the great stability of the optimized suspensions. In a detailed view at higher magnification is shown, and it can be appreciated that the surface contains some small particles as a consequence of the infiltration under vacuum.
Figure 7. Scanning electron microscopy images showing the macro and the microstructure of the sintered titania foams coated with the anatase colloidal suspension by vacuum infiltration and heated at 650 °C. The excellent homogeneity of the struts microstructure can be observed as well as the formation of anatase nanoparticles on the titania surfaces.
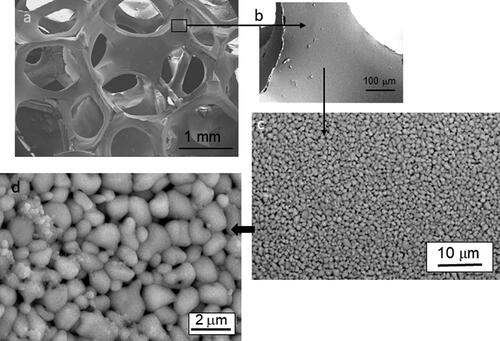
Similarly, shows the microstructure of the foam infiltrated using the microwave technique. In , a general view of the foam can be observed, with an open cell structure and dense struts (). As in the previous case, the microstructure of the struts is extremely homogeneous without pores, agglomerates and exaggerated grain growth. The higher magnification picture () shows the presence of the infiltrated nanoparticles, and the concentration is higher than in the case of vacuum infiltration. More concentrated suspensions would be desirable, however, to achieve a higher infiltration degree.
Figure 8. Scanning electron microscopy images showing the macro and the microstructure of the sintered titania foams coated with the anatase colloidal suspension by microwave heating and heated at 650 °C. The excellent homogeneity of the struts microstructure can be observed as well as the formation of anatase nanoparticles on the titania surfaces.
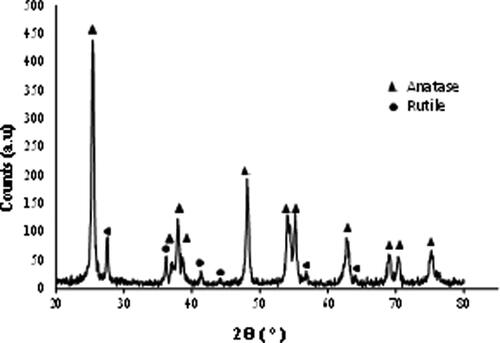
The samples were ground for the XRD analysis, but only rutile phase could be detected. This is expected because the foam is rutile and only the attached nanoparticles are anatase, but their concentration is low and the size is also very small. Since it is not possible to determine the presence of the anatase phase in this way, a sample of the colloidal suspension was dried and calcined at 700 °C. The corresponding spectrum for this treated powder is shown in , where it can be seen that the phases ratio is the same as in the colloidal particles, with a clear predominance of the anatase phase. So it can be expected that the nanoparticles coating the rutile foam are in the anatase phase, as desired.
Figure 9. XRD spectrum of the nanoparticles dried from the colloidal suspension after calcination at 700 °C.
Some studies suggest that non-sintered foams can have good mechanical properties and therefore there would be no needing for this long sintering step, or at least, the treatment corresponding to the curing of the anatase phase on the rutile skeleton. Hristov, Lesov, Mihaylov, Denkov, & Tcholakova (Citation2023) studied the effect of particle size on the compressive strength of green ceramic foams concluding that the materials prepared with smaller particles exhibited much higher compressive strength. However, they used spherical, smooth particles to produce the foam with sizes as fine as 4.5 nm. However, in most cases, the problem of ceramic materials is the bending strength rather than the compressive strength. Moreover, the foams in the green state have not the required consistency for handling and the desired stability in wet environments, which lead to the redispersion of the green body in the liquid. Also, the deposition of anatase requires a thermal treatment to allow the particles adhesion. Otherwise, they can peel out by mechanical effect or water/air erosion.
4. Conclusions
The rheological behaviour of submicron-sized titania aqueous suspensions was optimized, achieving solids loadings of 45 vol.% with 0.2 wt% of a polyacrylic polyelectrolyte while maintaining relatively low viscosities (by 45 mPa.s at 1000 s−1). The best suspensions for the impregnation of polyurethane foams contained 1 wt% of a poly(vynil alcohol). Homogeneous foams of TiO2-rutile without defects and clean open cells were obtained after sintering at 1400 °C/2h, with a step at 500 °C/1h for debinding. The sintered foams were introduced in a commercial nanoparticulate suspension of TiO2-anatase and infiltrated by application of vacuum or by using a microwave heating to drive the adhesion of the nanoparticles to the cell walls. The microstructural evaluation performed by scanning electron microscopy reveals a very uniform microstructure without macropores, exaggerated grain growth or other defects, demonstrating the high dispersion level of the suspensions. The nanoparticles of anatase are deposited onto the titania walls, although more concentrated suspensions would be required to achieve greater nanoparticles concentration. This opens a very simple and reliable process for the facile manufacture of dense walled foams of rutile with the presence of anatase at the surface.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abe, H., Seki, H., Fukunaga, A., & Egashira, M. (1994). Preparation of bimodal porous mullite ceramics. Journal of Materials Science, 29(5), 1–16. doi:10.1007/BF00975068
- Alves, H. M., Tari, G., Fonseca, A. T., & Ferreira, J. M. F. (1998). Processing of porous cordierite bodies by starch consolidation. Materials Research Bulletin, 33(10), 1439–1448. doi:10.1016/S0025-5408(98)00131-7
- Araki, K., & Halloran, J. W. (2005). Porous ceramic bodies with interconnected pore channels by a novel freeze casting technique. Journal of the American Ceramic Society, 88(5), 1108–1114. doi:10.1111/j.1551-2916.2005.00176.x
- Carnicer, V., Alcázar, C., Orts, M. J., Sánchez, E., & Moreno, R. (2021). Microfluidic rheology: A new approach to measure viscosity of ceramic suspensions at extremely high shear rates. Open Ceramics, 5, 100052. doi:10.1016/j.oceram.2020.100052
- Chen, Z., Li, Z., Li, J., Liu, C., Lao, C., Fu, Y. … He, Y. (2019). 3D printing of ceramics: a review. Journal of the European Ceramic Society, 39(4), 661–687. doi:10.1016/j.jeurceramsoc.2018.11.013
- Colombo, P. (2006). Conventional and novel processing methods for cellular ceramics. Philosophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences, 364(1838), 109–124. doi:10.1098/rsta.2005.1683
- Deckers, J., Vleugels, J., & Kruth, J.-P. (2014). Additive manufacturing of ceramics: a review. Journal of Ceramic Science and Technology, 5(4), 245–260.
- Deville, S. (2008). Freeze-casting of porous ceramics: A review of current achievements and issues. Advanced Engineering Materials, 10(3), 155–169. doi:10.1002/adem.200700270
- Dhara, S., & Bhargava, P. (2003). A simple direct casting route to ceramic foams. Journal of the American Ceramic Society, 86(10), 1645–1650. doi:10.1111/j.1151-2916.2003.tb03534.x
- Fujishima, A., Zhang, X., & Tryk, D. A. (2008). TiO2 photocatalysis and related surface phenomena. Surface Science Reports, 63(12), 515–582. doi:10.1016/j.surfrep.2008.10.001
- Gouma, P. I., & Mills, M. J. (2001). Anatase-to-rutile transformation in titania powders. Journal of the American Ceramic Society, 84(3), 619–622. doi:10.1111/j.1151-2916.2001.tb00709.x
- Hristov, M., Lesov, I., Mihaylov, L., Denkov, N., & Tcholakova, S. (2023). Role of particle size on the cohesive strength of non-sintered (green) ceramics. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 658(5), 130653. doi:10.1016/j.colsurfa.2022.130653
- Hu, H., Xiao, W. J., Yuan, J., Shi, J. W., Chen, M. X., & Shang Guan, W. F. (2007). Preparations of TiO2 film coated on foam nickel substrate by sol-gel processes and its photocatalytic activity for degradation of acetaldehyde. Journal of Environmental Sciences (China), 19(1), 80–85. doi:10.1016/s1001-0742(07)60013-8
- Jean, G., Sciamanna, V., Demuynck, M., Cambier, F., & Gonon, M. (2014). Macroporous ceramics: Novel route using partial sintering of alumina-powder agglomerates obtained by spray-drying. Ceramics International, 40(7), 10197–10203. doi:10.1016/j.ceramint.2014.02.089
- Kang, X., Liu, S., Dai, Z., He, Y., Song, X., & Tan, Z. (2019). Titanium dioxide: From engineering to applications. Catalysts, 9(2), 191. doi:10.3390/catal9020191
- Liu, P. S., & Chen, G. F. (2014). Porous materials processing and applications. Oxford: Elsevier.
- López-López, E., Baudín, C., & Moreno, R. (2008). Synthesis of zirconium titanate-based materials by colloidal filtration and reaction sintering. International Journal of Applied Ceramic Technology, 5(4), 394–400. doi:10.1111/j.1744-7402.2008.02232.x
- Luyten, J., Thijs, I., Vandermeulen, W., Mullens, S., Wallaeys, B., & Mortelmans, R. (2005). Strong ceramic foams from polyurethane templates. Advances in Applied Ceramics, 104(1), 4–8. doi:10.1179/174367605225010990
- Montanaro, L., Jorand, Y., Fantozzi, G., & Negro, A. (1998). Ceramic foams by powder processing. Journal of the European Ceramic Society, 18(9), 1339–1350. doi:10.1016/S0955-2219(98)00063-6
- Moreno, R. (2020). Better ceramics through colloid chemistry. Journal of the European Ceramic Society, 40(3), 559–587. doi:10.1016/j.jeurceramsoc.2019.10.014
- Ortega, F. S., Sepulveda, P., Innocentini, M. D. M., & Pandolfelli, V. C. (2001). Surfactants: A necessity for producing porous ceramics. American Ceramic Society Bulletin, 80(4), 37–42.
- Plesch, G., Vargová, M., Vogt, U. F., Gorbár, M., & Jesenák, K. (2012). Zr doped anatase supported reticulated ceramic foams for photocatalytic water purification. Materials Research Bulletin, 47(7), 1680–1686. doi:10.1016/j.materresbull.2012.03.057
- Rice, R. W. (1998). Porosity of ceramics. properties and applications. New York: CRC Press.
- Rincón, A., Giacomello, G., Pasetto, M., & Bernardo, E. (2017). Novel ‘inorganic gel casting’ process for the manufacturing of glass foams. Journal of the European Ceramic Society, 37(5), 2227–2234. doi:10.1016/j.jeurceramsoc.2017.01.012
- Saggio-Woyansky, J., Scott, C. E., & Minnear, W. P. (1992). Processing of porous ceramics. American Ceramic Society Bulletin, 71 (11), 1674–1682.
- Santacruz, I., Moreno, R., & Rodrigues-Neto, J. B. (2008). Preparation of cordierite materials with tailored porosity by gelcasting with polysaccharides. International Journal of Applied Ceramic Technology, 5(1), 74–83. doi:10.1111/j.1744-7402.2008.02189.x
- Scheffler, M., & Colombo, P. (2005). Cellular ceramics: Structure, manufacturing, properties and applications. Weinheim: Wiley-VCH
- Sieber, H., Hoffmann, C., Kaindl, A., & Greil, P. (2000). Biomorphic cellular ceramics. Advanced Engineering Materials, 2(3), 105–109. doi:10.1002/(SICI)1527-2648(200003)2:3<105::AID-ADEM105>3.0.CO;2-P
- Sousa, E., Silveira, C. B., Fey, T., Greil, P., Hotza, D., & de Oliveira, A. P. N. (2005). LZSA glass ceramic foams prepared by replication process. Advances in Applied Ceramics, 104(1), 22–29. doi:10.1179/174367605225011043
- Studart, A. R., Gonzenbach, U. T., Tervoort, E., & Gauckler, L. J. (2006). Processing routes to macroporous ceramics: A review. Journal of the American Ceramic Society, 89(6), 1771–1789. doi:10.1111/j.1551-2916.2006.01044.x
- Tallón, C., Moreno, R., & Nieto, M. I. (2009). Shaping of porous alumina bodies by freeze casting. Advances in Applied Ceramics, 108(5), 307–313. doi:10.1179/174367608X369280
- Thijs, I., Luyten, J., & Mullens, S. (2004). Producing ceramic foams with hollow spheres. Journal of the American Ceramic Society, 87(1), 170–172. doi:10.1111/j.1151-2916.2004.tb19965.x
- Wang, C., Shi, Z. H., Peng, L., He, W. M., Li, B. L., & Li, K. Z. (2017). Preparation of carbon foam-loaded nano-TiO2 photocatalyst and its degradation on methyl orange. Surfaces and Interfaces, 7, 116–124. doi:10.1016/j.surfin.2017.03.007
- Wang, H., Sung, I. Y., Li, X. D., & Kim, D. (2004). Fabrication of porous SiC ceramics with special morphologies by sacrificing template method. Journal of Porous Materials, 11(4), 265–271. doi:10.1023/B:JOPO.0000046353.24308.86
- Zocca, A., Colombo, P., Gomes, C. M., & Günster, J. (2015). Additive manufacturing of ceramics: Issues, potentialities, and opportunities. Journal of the American Ceramic Society, 98(7), 1983–2001.