Abstract
Background: Worldwide an increasing number of transgender and gender diverse individuals are requesting genital Gender Affirming Surgery (gGAS). For both masculinizing and feminizing gGAS various procedures and techniques are employed. Current literature on gGAS reports heterogeneous, non-standardized and often ill-defined outcomes. Presently, no consensus exists on what outcomes should be evaluated in order to assess the clinical results and the effectiveness of these procedures, which precludes development of evidence-based treatment guidelines.
Aims: This international consensus study aims to develop Core Outcome Sets (COS) for both masculinizing and feminizing gGAS. These represent the minimum sets of outcomes recommended to be measured and reported in all clinical trials pertaining to gGAS.
Methods: Two Core Outcome Sets for masculinizing and feminizing gGAS will be developed in parallel by following the Core Outcome Measures in Effectiveness Trials (COMET) guidelines. The stages of development for each set are: i) Identify outcomes measured and reported in previous research through a systematic review of the literature; ii) Identify outcomes suggested by transgender and gender diverse individuals during focus groups and interviews; iii) Combine and structure the outcomes into a preliminary outcome list; iv) Conduct e-Delphi surveys among stakeholders (i.e. professionals in transgender healthcare and transgender individuals) in which all potential outcomes will be rated on level of importance; and v) Decide on the final COS during an online consensus meeting.
Discussion: This study will produce minimum, core sets of relevant outcomes for gGAS, through reaching international consensus with key stakeholders, including transgender individuals. Development of these COS will enable the measurement and reporting of relevant and standardized outcomes, facilitating continued scientific advancement of this field.
Introduction
The number of transgender people seeking gender-affirming medical care continues to increase annually (Wiepjes et al., Citation2018). Gender affirming care, including psychological, hormonal and surgical interventions, has been shown to improve the wellbeing and quality of life of transgender individuals (Javier et al., Citation2022; van de Grift et al., Citation2018).
As techniques in genital gender affirming surgery (gGAS) continue to improve, more transgender individuals are pursuing surgery (Chaya et al., Citation2022). However, while there are some recognized gold standard gGAS operations, the field is fraught with variability in techniques and surgical training (Frey et al., Citation2017). As more surgeons perform these procedures, it is paramount that we use evidence-based recommendations to optimize patient care (Coleman et al., Citation2022). gGAS lacks high quality evidence to drive these recommendations and many leading publications in this field are non-generalizable as they are single center and single technique-based, and most commonly retrospective. Even when higher quality evidence publications are available, they suffer from minimal follow-up and ad hoc approaches to assessment of satisfaction or outcomes (Chen et al., Citation2019; Horbach et al., Citation2015; Li et al., Citation2022). Due to these limitations, surgeons and patients are often left without proper guidance on the optimal techniques for a given scenario (Selvaggi & Bellringer, Citation2011; Wroblewski et al., Citation2013). Moreover, there is a lack of evidence on how various techniques compare with regard to safety and effectiveness (Chaya et al., Citation2022; Frey et al., Citation2017). In order to drive evidence-based recommendations and standardization in the field of gGAS, well documented comparative studies are urgently needed. Furthermore, there should be a consensus on which outcomes are necessary and required to capture the essential clinical and patient reported outcomes (Barone et al., Citation2018; Oles et al., Citation2022). A Core Outcome Set (COS) can be instrumental in achieving this.
Feminizing gGAS
Transgender women and gender diverse individuals may opt for feminizing gGAS (Hadj-Moussa et al., Citation2018). Creation of the female external genitalia (vulva) includes labiaplasty, urethral and meatal reconstruction, clitoroplasty and perineal flap augmentation. Technical variants exist for each of these procedures. Vaginoplasty is the collective name for surgical techniques in which a vaginal cavity is created. The main difference between vaginoplasty techniques is the choice of tissue used to line the neo-vaginal cavity; i.e. (scrotal) skin, penile skin, pedicled small or large intestinal segments, pedicled peritoneal tissue or a combination (Horbach et al., Citation2015; Li et al., Citation2021; Pariser & Kim, Citation2019). If a vaginal canal is not desired, vulvoplasty (also known as minimal-depth vaginoplasty) is an option. In this case, only the vulva is created (Jiang et al., Citation2018). Penile inversion vaginoplasty has been described as the gold standard, however, this is primarily based on expert opinion. Currently, due to the lack of evidence, there is no consensus on indications for specific feminizing gGAS procedures nor on the optimal techniques (Castanon et al., Citation2022; Horbach et al. Citation2015). Standardizing outcomes and conducting high-quality comparative studies is necessary to enable evidence-based decision making with regard to feminizing gGAS (Horbach et al., Citation2015; van der Sluis et al., Citation2022).
Masculinizing gGAS
Procedures for masculinizing gGAS include procedures to create a neo-phallus (with or without urethral lengthening), a neo-scrotum and a neo-corona (Al-Tamimi et al., Citation2020; Selvaggi et al., Citation2009; Sommeling et al., Citation2018). Masculinizing gGAS is considered the most challenging of gender affirming surgical procedures (Frey et al., Citation2017). Phalloplasty is a surgical procedure in which an average to large sized neo-phallus is created using (multiple) large sized flaps obtained from different donor sites throughout the body. The most frequently used flaps are the radial forearm free flap, superficial circumflex iliac artery perforator flap, and anterolateral thigh pedicled flap. Other less commonly used flaps are the abdominal flap, the suprapubic pedicled flap, fibula free flap, the lateral upper arm flap, and the latissimus dorsi free flap (Al-Tamimi et al., Citation2020; Boczar et al., Citation2021; Wang et al., Citation2022). Metoidioplasty is a surgical procedure which creates a below-average-sized neo-phallus out of hormonally hypertrophied clitoral and labial tissue. Described techniques are the simple, the ring, the extended and the Belgrade metoidioplasty (Djordjevic et al., Citation2019; Morrison et al., Citation2022). A wide variety of techniques are used for masculinizing gGAS. Due to the lack of evidence on their value, safety and effectiveness, the choice for performing a specific procedure is currently mostly based on the surgeon’s preferences and skill. The need for standardized reporting of outcomes is paramount (Wang et al., Citation2022).
Core Outcome Set (COS)
A COS is defined as the minimum set of outcomes that should be measured in a standardized manner and reported consistently in all clinical trials for a specific health condition/health area (Williamson et al., Citation2017). Herein we describe the methodology we propose to use to define two COS: feminizing- and masculinizing gGAS.
Materials and methods
Registration and ethics
The project is prospectively registered with the Core Outcome Measures in Effectiveness Trials (COMET) database under study numbers 2064 and 2067 (Core Outcome Measures in Effectiveness Trials (COMET) database: registration study number 2064; Core Outcome Measures in Effectiveness Trials (COMET) database: registration study number 2067). It received ethical approval from the Amsterdam UMC, location VUmc Ethical board (Reference number: 2022.0102). The systematic reviews which are part of the project are registered in the International Prospective Register of Systematic Reviews (PROSPERO) under numbers CRD42020223430 and CRD42022347400.
Adhering to the standards for COS development
For the development of both COS within this project, the COMET guidelines will be followed (Williamson et al., Citation2017). According to the Core Outcome Set-STAndards for Development (COS-STAD), methodology relating to the scope, the stakeholders, and the consensus process should be explicitly described in the protocol for COS development. For this protocol we will adhere to the Core Outcome Set-STAndarised Protocol Items (COS-STAP) checklist, which can be found in Appendix 1 (Kirkham et al., Citation2019).
Study Steering Group
To guide the development of these COS (here on referred to as “The GenderCOS project”), an international Study Steering Group (SSG) was formed. The SSG consists of experts by lived experience (i.e. transgender individuals) (AB., BC.) and experts by profession (i.e. health care professionals and/or senior researchers) (TR., JBell., JB., MB., SM., WPB., M-BB. and MM.). The Study Management Group (SMG) consisting of physician-scientists (PR., MV., TP. and AC.) is responsible for the day-to-day management of the project. In the event that any protocol modifications need to be made to the protocol by the SSG and SMG during the course of the study, this will be clearly stated in the final publication of the COS. If any protocol modifications are made during phase 2 (see The GenderCOS project development process below) of The GenderCOS project all participants will be notified at the start of the next e-Delphi survey round.
Scope
The scope of The GenderCOS project is presented in .
Table 1. COS-STAD as applied to the domain scope specification.
Stakeholders and recruitment
Stakeholders who will be recruited to participate in the development of The GenderCOS project are described in . Details of the inclusion and exclusion criteria for each stakeholder group can also be found in .
Table 2. COS-STAD as applied to the domain, and inclusion- and exclusion criteria of, involved stakeholder groups.
Experts by profession will be recruited internationally. Promotion to participate in the project will be done via the website (www.gendercos.org), at international meetings (e.g. World Professional Association for Transgender Health, European Professional Association for Transgender Health, European Society for Sexual Medicine) and through the networks of the SSG members. Professional societies will be asked to distribute the recruitment invitation amongst their members. International healthcare centers known for providing the care as stated in the scope will be queried to share the invitation to participate to their healthcare professionals. Through the systematic reviews, authors of published studies will be identified, contacted and invited to participate.
Experts by lived experience will also be recruited internationally. This will be done by online promotion (e.g. social media, website) and by approaching support groups and international trans non-governmental organizations (e.g. Transgender Europe, International Lesbian, Gay, Bisexual, Trans and Intersex Association, Asia Pacific Transgender Network). Posters will be made and distributed to be displayed in waiting rooms of health care providing facilities to raise awareness and invite to participate. We invite experts by profession to also raise awareness among potential experts by experience in the consultation room.
Currently, no specific requirements or recommendations exist for a minimum or maximum number of participants to be included (Boulkedid et al., Citation2011). Theoretically, the more participants included should increase the validity and generalizability of the consensus reached (Murphy et al., Citation1998). For this project, we aim to have at least 20 participants in each stakeholder group from both the COS. Furthermore, the number of participants for each stakeholder group will not be limited.
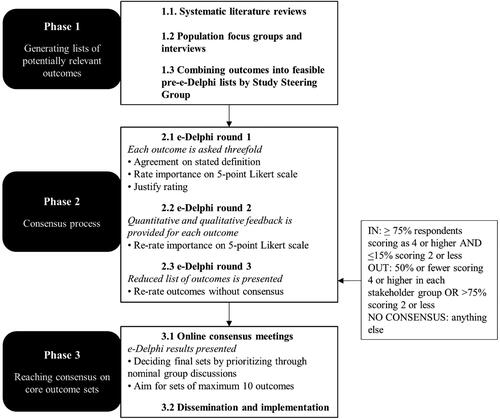
The GenderCOS project development process
An overview of the development process is provided in . It is identical for the development of both the feminizing and masculinizing gGAS COS, which will be run in parallel.
Phase 1: Generating lists of potentially relevant outcomes
Systematic literature reviews
A systematic review to obtain all outcomes that have been reported in clinical research related to feminizing gGAS has been published (Pidgeon et al., Citation2022). At the time of this manuscript submission a systematic review into masculinizing gGAS is being conducted. Both systematic reviews will adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline for systematic reviews (Dickson & Yeung, Citation2022). In these systematic reviews, the following data were manually extracted: author, year of publication, study design, population, number of patients, intervention, mean age, outcomes reported in design and results (including the definitions of outcomes if stated) and any descriptions of measurement instruments used (Williamson et al., Citation2017). The outcomes were classified using a taxonomy, which was specifically developed for outcomes in medical research (Dodd et al., Citation2018). This taxonomy was slightly modified for the systematic review for masculinizing gGAS, as ‘sexual outcomes’ was added to the outcome domain list.
Population focus groups and interviews
Prior to The GenderCOS project, a qualitative study was performed at the Department of Plastic, Reconstructive and Hand Surgery in Amsterdam UMC, the Netherlands to collect data for the development of decision aid for gGAS (here on referred to as “The GenderAID”) (Mokken. 2023). Focus groups and interviews were held with transgender and gender diverse individuals who had previously undergone gGAS. During these focus groups, transgender individuals were asked about their reasons for choosing certain surgical options, what they value in certain techniques and what outcomes they deem important and why. Since the research questions overlap significantly, the qualitative data from The GenderAID was repurposed for The GenderCOS project. The outcomes have been classified using the same modified taxonomy as described above (Dodd et al., Citation2018). The decision aid study received ethical approval from the Amsterdam UMC, location VUmc ethical board (Reference numbers: 2020.0653 and 2021.0026). The participants gave written consent for the data to be reused for other research purposes. In an overview of the focus groups and interviews can be found.
Table 3. Overview of qualitative data collection.
Combining data into feasible pre-Delphi lists
All extracted and classified outcomes will be used to generate two initial outcome lists. Both lists will undergo deduplication of exact matching outcomes. The remaining outcomes on each list will be further condensed based on similarities, rationalization of outcomes and removal of obsolete outcomes by the SSG. Furthermore, within the SSG there must be consensus reached on the (lay) definition of each exact outcome that will be used in the e-Delphi rounds. Also, the number of items considered will be determined for group review (Gargon et al., Citation2019). The final lists of outcomes will be translated following standardized cross-cultural translations—to guarantee linguistic appropriateness - into Dutch and Spanish and grouped by domain (Brookes et al., Citation2018).
Phase 2: Consensus process
Delphi method
Individuals who meet the inclusion criteria will be invited to participate in all three e-Delphi survey rounds via the e-mail address they registered with on The GenderCOS project website. All three rounds of e-Delphi surveys will be delivered through Survalyzer survey software (Survalyzer Survey Software). At the beginning of the e-Delphi survey, instructions will be given on how to fill in the surveys. Participants will be asked to complete a short demographic questionnaire () and provide informed e-Consent. They will also be presented with the option to be named as contributor in the publication regarding the COS concerned. Lastly, participants will be reminded that they are free to withdraw from the surveys at any time. Only the SMG will have access to the study data, which will be encrypted.
Table 4. Demographic data to be collected per stakeholder group.
The time period that the surveys are open for completion will be set at 6 wk. In between each round there will be a period of 10 wk for analysis of the completed surveys and compilation of data. After 2 wk, reminders will be sent weekly up to a maximum of 4 reminders.
In the first e-Delphi round the participant is, for each outcome, asked about the stated definition, to rate its importance on a 5-point Likert scale (e.g. 1 is not important, 2 is less important, 3 is neutral, 4 is important and 5 is very important) and to justify their rating. Descriptive statistics will be used to summarize the data. In the second round quantitative and qualitative feedback for each outcome from the first round is provided and participants are asked to rate the importance of the outcomes once more (Brookes et al., Citation2016; Fish et al., Citation2020; Khodyakov & Chen, Citation2020). Based on the consensus classification as described in , a reduced list of outcomes is presented in the third round and the participant is asked to give feedback on the list of excluded outcomes and to rate outcomes without consensus again. After completing all rounds, participants are asked whether they may be approached to partake in the consensus meeting (phase 3).
Table 5. Consensus classification.
Phase 3: Reaching consensus on core outcomes sets
Defining the final COS
The final COS for masculinizing and feminizing gGAS will be defined during two separate online consensus meetings (one for each COS). An equal, viable number of stakeholders from each stakeholder group will be invited to participate from those who indicated a willingness to partake in the meeting during the e-Delphi survey rounds. The meetings will be guided by an independent, trained facilitator, who is neither an expert by experience nor profession, but who has understanding of the methodology to reduce the risk of bias in the facilitation process (Harman et al., Citation2015; Williamson et al., Citation2017). The results from the final e-Delphi surveys will be presented during the meetings. Contingent upon this, retaining or removing outcomes from the final lists will be done by prioritization through discussion using a nominal group technique (Harvey & Holmes, Citation2012). Although there is no gold standard for the ideal number of outcomes within a COS, it is advised to generate a list of approximately 10 outcomes to maximize implementation and feasibility (Hughes et al., Citation2021; Maxwell et al., Citation2019). The COS will be complete once agreement is reached by all stakeholders on the final lists.
Dissemination and implementation
The two final COS will be disseminated as extensively and as efficiently as possible. We aim to publish both sets open-access in relevant and leading scientific journal(s). Furthermore, we will use the project website for online promotion, as well as other online outlets, such as social media. International patient organizations, healthcare professional organizations and other relevant societies will be approached and asked to cooperate in the dissemination. Also, we aim to present the final set during international scientific congresses. By including various key relevant international stakeholders in the development and by promoting the awareness of the COS we aim to maximize the uptake and thus implementation of the COS (Hughes et al., Citation2021).
Discussion
The development, dissemination and implementation of COS for gGAS will ensure that key relevant outcomes will be measured and reported in future research. This will enhance the comparison of reported outcomes between studies and facilitate guideline-development and evidence-based informed decision-making. Evaluation and consensus on how and when the core outcomes should be measured, will be the subject of an ensuing study. Through the use of the COS, we endeavor to improve the quality of clinical research. By developing the COS, an international network will be established, which can be leveraged for further collaboration within the field.
Authors’ contributions
PR and MV led the proposal and protocol development. All authors contributed to the study design and development of the protocol, and based on this PR, MV and MM drafted the manuscript. All authors revised the final manuscript and approved the submitted version.
Ethics approval and consent to participate
Ethical approval has been received from the Amsterdam UMC, location VUmc Ethical board Reference number: 2022.0102. All future participants of the study have to provide e-Consent before the start of the first e-Delphi survey round. This article does not contain any studies with human participants or animals performed by any of the authors.
List of Abbreviations
| COMET | = | Core Outcome Measurements in Effectiveness Trials |
| COS | = | Core Outcome Set(s) |
| COS-STAD | = | Core Outcome Set-STAndards for Development |
| COS-STAP | = | Core Outcome Set-STAndarised Protocol Items |
| gGAS | = | Genital Gender Affirming Surgery |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | = | International Prospective Register of Systematic Reviews |
| SMG | = | Study Management Group |
| SSG | = | Study Steering Group |
Supplemental Material
Download MS Word (21.1 KB)Supplemental Material
Download MS Word (15.9 KB)Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Al-Tamimi, M., Pigot, G. L., Elfering, L., Ozer, M., de Haseth, K., van de Grift, T. C., Mullender, M. G., Bouman, M. B., & Van der Sluis, W. B. (2020). Genital gender-affirming surgery in transgender men in The Netherlands from 1989 to 2018: The evolution of surgical care. Plastic and Reconstructive Surgery, 145(1), 153e–161e. https://doi.org/10.1097/PRS.0000000000006385
- Barone, M., Cogliandro, A., & Persichetti, P. (2018). Patient-reported outcome measures used in gender confirmation surgery: A systematic review. Plastic and Reconstructive Surgery, 142(6), 985e–986e. https://doi.org/10.1097/PRS.0000000000005035
- Boczar, D., Huayllani, M. T., Saleem, H. Y., Cinotto, G., Avila, F. R., Kassis, S., Lu, X., Rinker, B. D., & Forte, A. J. (2021). Surgical techniques of phalloplasty in transgender patients: A systematic review. Annals of Translational Medicine, 9(7), 607–607. https://doi.org/10.21037/atm-20-3527
- Boulkedid, R., Abdoul, H., Loustau, M., Sibony, O., & Alberti, C. (2011). Using and reporting the Delphi method for selecting healthcare quality indicators: A systematic review. PLoS One, 6(6), e20476. https://doi.org/10.1371/journal.pone.0020476
- Brookes, S. T., Chalmers, K. A., Avery, K. N. L., Coulman, K., & Blazeby, J. M. (2018). Impact of question order on prioritisation of outcomes in the development of a core outcome set: A randomised controlled trial. Trials, 19(1), 66. https://doi.org/10.1186/s13063-017-2405-6
- Brookes, S. T., Macefield, R. C., Williamson, P. R., McNair, A. G., Potter, S., Blencowe, N. S., Strong, S., & Blazeby, J. M. (2016). Three nested randomized controlled trials of peer-only or multiple stakeholder group feedback within Delphi surveys during core outcome and information set development. Trials, 17(1), 409. https://doi.org/10.1186/s13063-016-1479-x
- Castanon, C. D. G., Matic, S., Bizic, M., Stojanovic, B., Bencic, M., Grubor, N., Pusica, S., Korac, G., & Djordjevic, M. L. (2022). Laparoscopy assisted peritoneal pull-through vaginoplasty in transgender women. Urology, 166, 301–302. https://doi.org/10.1016/j.urology.2022.05.001
- Chaya, B. F., Berman, Z. P., Boczar, D., Trilles, J., Siringo, N. V., Diep, G. K., Rodriguez Colon, R., & Rodriguez, E. D. (2022). Gender affirmation surgery on the rise: Analysis of trends and outcomes. LGBT Health, 9(8), 582–588. https://doi.org/10.1089/lgbt.2021.0224
- Chen, M. L., Reyblat, P., Poh, M. M., & Chi, A. C. (2019). Overview of surgical techniques in gender-affirming genital surgery. Translational Andrology and Urology, 8(3), 191–208. https://doi.org/10.21037/tau.2019.06.19
- Coleman, E., Radix, A. E., Bouman, W. P., Brown, G. R., de Vries, A. L. C., Deutsch, M. B., Ettner, R., Fraser, L., Goodman, M., Green, J., Hancock, A. B., Johnson, T. W., Karasic, D. H., Knudson, G. A., Leibowitz, S. F., Meyer-Bahlburg, H. F. L., Monstrey, S. J., Motmans, J., Nahata, L., … Arcelus, J. (2022). Standards of care for the health of transgender and gender diverse people, version 8. International Journal of Transgender Health, 23(Suppl 1), S1–S259. https://doi.org/10.1080/26895269.2022.2100644
- Core Outcome Measures in Effectiveness Trials (COMET) database: Registration study number 2064. https://www.comet-initiative.org/Studies/Details/2064
- Core Outcome Measures in Effectiveness Trials (COMET) database: Registration study number 2067. https://www.comet-initiative.org/Studies/Details/2067
- Dickson, K., & Yeung, C. A. (2022). PRISMA 2020 updated guideline. British Dental Journal, 232(11), 760–761. https://doi.org/10.1038/s41415-022-4359-7
- Djordjevic, M. L., Stojanovic, B., & Bizic, M. (2019). Metoidioplasty: Techniques and outcomes. Translational Andrology and Urology, 8(3), 248–253. https://doi.org/10.21037/tau.2019.06.12
- Dodd, S., Clarke, M., Becker, L., Mavergames, C., Fish, R., & Williamson, P. R. (2018). A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. Journal of Clinical Epidemiology, 96, 84–92. https://doi.org/10.1016/j.jclinepi.2017.12.020
- Fish, R., MacLennan, S., Alkhaffaf, B., & Williamson, P. R. (2020). Vicarious thinking" was a key driver of score change in Delphi surveys for COS development and is facilitated by feedback of results. Journal of Clinical Epidemiology, 128, 118–129. https://doi.org/10.1016/j.jclinepi.2020.09.028
- Frey, J. D., Poudrier, G., Chiodo, M. V., & Hazen, A. (2017). An update on genital reconstruction options for the female-to-male transgender patient: A review of the literature. Plastic and Reconstructive Surgery, 139(3), 728–737. https://doi.org/10.1097/PRS.0000000000003062
- Frey, J. D., Poudrier, G., Thomson, J. E., & Hazen, A. (2017). A historical review of gender-affirming medicine: Focus on genital reconstruction surgery. The Journal of Sexual Medicine, 14(8), 991–1002. https://doi.org/10.1016/j.jsxm.2017.06.007
- Gargon, E., Crew, R., Burnside, G., & Williamson, P. R. (2019). Higher number of items associated with significantly lower response rates in COS Delphi surveys. Journal of Clinical Epidemiology, 108, 110–120. https://doi.org/10.1016/j.jclinepi.2018.12.010
- Hadj-Moussa, M., Ohl, D. A., & Kuzon, W. M.Jr. (2018). Feminizing genital gender-confirmation surgery. Sexual Medicine Reviews, 6(3), 457–468 e452. https://doi.org/10.1016/j.sxmr.2017.11.005
- Harman, N. L., Bruce, I. A., Kirkham, J. J., Tierney, S., Callery, P., O’Brien, K., Bennett, A. M., Chorbachi, R., Hall, P. N., Harding-Bell, A., Parfect, V. H., Rumsey, N., Sell, D., Sharma, R., & Williamson, P. R. (2015). The importance of integration of stakeholder views in core outcome set development: Otitis media with effusion in children with cleft palate. PloS One, 10(6), e0129514. https://doi.org/10.1371/journal.pone.0129514
- Harvey, N., & Holmes, C. A. (2012). Nominal group technique: An effective method for obtaining group consensus. International Journal of Nursing Practice, 18(2), 188–194. https://doi.org/10.1111/j.1440-172X.2012.02017.x
- Horbach, S. E., Bouman, M. B., Smit, J. M., Ozer, M., Buncamper, M. E., & Mullender, M. G. (2015). Outcome of vaginoplasty in male-to-female transgenders: A systematic review of surgical techniques. The Journal of Sexual Medicine, 12(6), 1499–1512. https://doi.org/10.1111/jsm.12868
- Hughes, K. L., Clarke, M., & Williamson, P. R. (2021). A systematic review finds Core Outcome Set uptake varies widely across different areas of health. Journal of Clinical Epidemiology, 129, 114–123. https://doi.org/10.1016/j.jclinepi.2020.09.029
- Javier, C., Crimston, C. R., & Barlow, F. K. (2022). Surgical satisfaction and quality of life outcomes reported by transgender men and women at least one year post gender-affirming surgery: A systematic literature review. International Journal of Transgender Health, 23(3), 255–273. https://doi.org/10.1080/26895269.2022.2038334
- Jiang, D., Witten, J., Berli, J., & Dugi, D.3rd (2018). Does Depth matter? Factors affecting choice of vulvoplasty over vaginoplasty as gender-affirming genital surgery for transgender women. The Journal of Sexual Medicine, 15(6), 902–906. https://doi.org/10.1016/j.jsxm.2018.03.085
- Khodyakov, D., & Chen, C. (2020). Response changes in Delphi processes: Why is it important to provide high-quality feedback to Delphi participants? Journal of Clinical Epidemiology, 125, 160–161. https://doi.org/10.1016/j.jclinepi.2020.04.029
- Kirkham, J. J., Gorst, S., Altman, D. G., Blazeby, J. M., Clarke, M., Tunis, S., Williamson, P. R., & Group, C.-S., for the COS-STAP Group. (2019). Core Outcome Set-STAndardised protocol items: The COS-STAP statement. Trials, 20(1), 116. https://doi.org/10.1186/s13063-019-3230-x
- Li, J. S., Crane, C. N., & Santucci, R. A. (2021). Vaginoplasty tips and tricks. International Brazil Journal of Urolgy, 47(2), 263–273. https://doi.org/10.1590/S1677-5538.IBJU.2020.0338
- Li, V. Y., Demzik, A., Snyder, L., Ogunleye, A. A., Wang, A., & Figler, B. D. (2022). Genital gender affirming surgery. The American Surgeon, 88(12), 2817–2822. https://doi.org/10.1177/00031348221109479
- Maxwell, L. J., Beaton, D. E., Shea, B. J., Wells, G. A., Boers, M., Grosskleg, S., Bingham, C. O., 3rd, Conaghan, P. G., D’Agostino, M. A., de Wit, M., Gossec, L., March, L., Simon, L. S., Singh, J. A., Strand, V., & Tugwell, P. (2019). Core Domain Set selection according to OMERACT Filter 2.1: The OMERACT methodology. The Journal of Rheumatology, 46(8), 1014–1020. https://doi.org/10.3899/jrheum.181097
- Mokken, S. E. (2023). The GenderAID: A decision aid for genital gender surgery. https://genderaid.org/en
- Morrison, S. D., Morris, M. P., Mokken, S. E., Buncamper, M. E., & Ozer, M. (2022). Technical refinements to extended metoidioplasty without urethral lengthening: surgical technique. Plastic and Reconstructive Surgery. Global Open, 10(2), e4101. https://doi.org/10.1097/GOX.0000000000004101
- Murphy, M. K., Black, N. A., Lamping, D. L., McKee, C. M., Sanderson, C. F., Askham, J., & Marteau, T. (1998). Consensus development methods, and their use in clinical guideline development.Health Technology Assessment, 2(3), i–iv, 1–88. https://www.ncbi.nlm.nih.gov/pubmed/9561895 https://doi.org/10.3310/hta2030
- Oles, N., Darrach, H., Landford, W., Garza, M., Twose, C., Park, C. S., Tran, P., Schechter, L. S., Lau, B., & Coon, D. (2022). Gender affirming surgery: A comprehensive, systematic review of all peer-reviewed literature and methods of assessing patient-centered outcomes (part 2: genital reconstruction). Annals of Surgery, 275(1), e67–e74. https://doi.org/10.1097/SLA.0000000000004717
- Pariser, J. J., & Kim, N. (2019). Transgender vaginoplasty: Techniques and outcomes. Translational Andrology and Urology, 8(3), 241–247. https://doi.org/10.21037/tau.2019.06.03
- Pidgeon, T. E., Franchi, T., Lo, A. C. Q., Mathew, G., Shah, H. V., Iakovou, D., Borrelli, M. R., Sohrabi, C., & Rashid, T. (2022). Outcome measures reported following feminizing genital gender affirmation surgery for transgender women and gender diverse individuals: A systematic review. International Journal of Transgender Health, 24(2), 149–173. https://doi.org/10.1080/26895269.2022.2147117
- Roijer, P. J., Vallinga, M. S. (2022). Core outcome sets genital gender surgery. www.gendercos.org
- Selvaggi, G., & Bellringer, J. (2011). Gender reassignment surgery: An overview. Nature Reviews. Urology, 8(5), 274–282. https://doi.org/10.1038/nrurol.2011.46
- Selvaggi, G., Hoebeke, P., Ceulemans, P., Hamdi, M., Van Landuyt, K., Blondeel, P., De Cuypere, G., & Monstrey, S. (2009). Scrotal reconstruction in female-to-male transsexuals: A novel scrotoplasty. Plastic and Reconstructive Surgery, 123(6), 1710–1718. https://doi.org/10.1097/PRS.0b013e3181a659fe
- Sommeling, C. E., De Wolf, E. J., Salim, A., Monstrey, S., Opsomer, D., Claes, K., & D’Arpa, S. (2018). A new technique for coronaplasty in penile reconstruction. The Journal of Sexual Medicine, 15(6), 920–923. https://doi.org/10.1016/j.jsxm.2018.01.024
- Survalyzer Survey software. https://survalyzer.com/
- van de Grift, T. C., Elaut, E., Cerwenka, S. C., Cohen-Kettenis, P. T., & Kreukels, B. P. C. (2018). Surgical satisfaction, quality of life, and their association after gender-affirming surgery: A follow-up study. Journal of Sex & Marital Therapy, 44(2), 138–148. https://doi.org/10.1080/0092623X.2017.1326190
- van der Sluis, W. B., Schafer, T., Nijhuis, T. H. J., & Bouman, M. B. (2022). Genital gender-affirming surgery for transgender women. Best Practice & Research. Clinical Obstetrics & Gynaecology, 86, 102297. https://doi.org/10.1016/j.bpobgyn.2022.102297
- Wang, A. M. Q., Tsang, V., Mankowski, P., Demsey, D., Kavanagh, A., & Genoway, K. (2022). Outcomes following gender affirming phalloplasty: A systematic review and meta-analysis. Sexual Medicine Reviews, 10(4), 499–512. https://doi.org/10.1016/j.sxmr.2022.03.002
- Wiepjes, C. M., Nota, N. M., de Blok, C. J. M., Klaver, M., de Vries, A. L. C., Wensing-Kruger, S. A., de Jongh, R. T., Bouman, M. B., Steensma, T. D., Cohen-Kettenis, P., Gooren, L. J. G., Kreukels, B. P. C., & den Heijer, M. (2018). The Amsterdam Cohort of Gender Dysphoria Study (1972-2015): Trends in prevalence, treatment, and regrets. The Journal of Sexual Medicine, 15(4), 582–590. https://doi.org/10.1016/j.jsxm.2018.01.016
- Williamson, P. R., Altman, D. G., Bagley, H., Barnes, K. L., Blazeby, J. M., Brookes, S. T., Clarke, M., Gargon, E., Gorst, S., Harman, N., Kirkham, J. J., McNair, A., Prinsen, C. A. C., Schmitt, J., Terwee, C. B., & Young, B. (2017). The COMET handbook: Version 1.0. Trials, 18(S3), 280. https://doi.org/10.1186/s13063-017-1978-4
- Wroblewski, P., Gustafsson, J., & Selvaggi, G. (2013). Sex reassignment surgery for transsexuals. Current Opinion in Endocrinology, Diabetes, and Obesity, 20(6), 570–574. https://doi.org/10.1097/01.med.0000436190.80104.56
Appendix
Appendix 1. Core Outcome Set-STAndardised Protocol Items: the COS-STAP Statement checklist.