Abstract
Objective
Cyproterone acetate (CPA) and spironolactone (SPL) are different antiandrogens in gender-affirming hormone therapy (GAHT) for transgender women. Few studies have evaluated their efficacy and user satisfaction, especially among East Asians. This study aimed to evaluate these aspects in Chinese transgender women.
Methods
Data were collected retrospectively from transgender women visiting the Peking University Third Hospital from 2012 to 2021. From 639 people identified as transgender women, 151 of them (80 using CPA and 71 using SPL, 16 to 40-year-old) under stable GAHT ≥6 months were enrolled. Total testosterone levels and visual analogue scale (VAS)-based satisfaction scores were evaluated.
Results
No difference was observed in age between the CPA and SPL groups (median [IQR], 22 [20–24] years and 23 [20–26] years, respectively). The duration of GAHT was longer in CPA group than in SPL group (18 [10–32] months vs. 12 [8–21] months, p = 0.009). Total testosterone levels were significantly lower with CPA treatment (25 mg/d) than with SPL treatment (100 mg/d) (median [IQR]: 0.7 [0.7–2.1] nmol/L vs. 13.0 [6.0–17.8] nmol/L, p < 0.001). The proportion of total testosterone levels reaching the recommended range was significantly higher in CPA group than in SPL group (75.0% vs. 11.3%, p < 0.001). VAS-based satisfaction scores for erection decreased and figure feminization were higher in CPA group than in SPL group, which remained unchanged after adjusting for age, treatment duration, estradiol dose, and comorbid mental disorders (p < 0.05). The prolactin levels were higher in CPA group than in SPL group (18.9 [11.8–28.1] ng/ml vs. 11.8 [7.9–18.4] ng/ml, p < 0.001). No severe safety events were reported in both groups.
Conclusion
In Chinese transgender women, CPA was more effective than SPL in lowering testosterone levels. Additionally, VAS scores indicated greater satisfaction with erection decreased and figure feminization using CPA compared to SPL.
Introduction
In a recent cross-sectional study among Chinese college students, the prevalence of self-identified transgender individuals was 2.6% (Wang et al., Citation2021). Yet transgender healthcare remains scarce in China. This is a problem given that gender-affirming hormone therapy (GAHT) has been found to be effective at alleviating gender dysphoria and improving mental health for transgender people (Baker et al., Citation2021; Hembree et al., Citation2017; T’Sjoen et al., Citation2019). GAHT in transgender women usually includes antiandrogen therapy and estrogen replacement. Cyproterone acetate (CPA) and spironolactone (SPL) are two commonly used antiandrogens for GAHT (Coleman et al., Citation2022; Hembree et al., Citation2017; Tangpricha & den Heijer, Citation2017). Some studies have suggested that CPA has a more pronounced effect on lowering testosterone levels than SPL (Burinkul et al., Citation2021; Sofer et al., Citation2020). Considering that CPA acts as an antiandrogen mainly by suppressing the synthesis of testosterone, while SPL works primarily by blocking the androgen receptor (Angus et al., Citation2019; Glintborg et al., Citation2021), the testosterone level may not be the only appropriate index for evaluating the efficacy of these two drugs. Moreover, user experience should be valued since the final goal of transgender medical care is to achieve self-acceptance and improve mental health.
Despite the fact that our hospital performed the first gender-affirming surgery in mainland China in 1983, there remains a shortage of gender clinics that offer transgender medical care, especially the GAHT. Prior to 2017, there were no domestic guidelines or government regulations on GAHT in China. Only a few doctors in China were willing to provide hormone prescriptions for transgender people, and they usually followed the hormone replacement regimens as recommended in clinical guidelines for other sex hormone deficiency disorders. Our previous research showed that in 2017, only 8.3% of hormone users obtained their prescriptions from domestic public hospitals, resulting in a widespread practice of self-medication (Liu et al., Citation2020). In addition, testosterone metabolism, body hair growth, and sebaceous gland production differ among ethnic groups (Gooren, Citation2014; Santner et al., Citation1998; van Houten & Gooren, Citation2000). However, most published guidelines or consensuses have been developed with white transgender populations (Gooren, Citation2014; Hembree et al., Citation2017; T’Sjoen et al., Citation2019), leading to a lack of understanding of GAHT efficacy and safety among East Asian transgender women. In 2017, following the update of the Management Regulation of Sex Reassignment Surgery by the National Health Commission of China, the first multidisciplinary gender clinic in China was established in our hospital. In order to evaluate the efficacy of antiandrogens in a user-centered way and to fill the knowledge gap regarding GAHT in transgender East Asian populations, we conducted a retrospective study on the biochemical efficacy, self-reported satisfaction, and safety of GAHT in Chinese transgender women visiting our gender clinic.
Methods
Participant selection
In this retrospective observational study, we screened individuals who identified as transgender women and visited the gender clinic at Peking University Third Hospital for gender-affirming medical care from January 1, 2012, to November 30, 2021. The inclusion criteria were as follows: 1) transgender women who had been evaluated and referred by mental health professionals; 2) those aged 16 to 40 years; 3) those under stable GAHT, including CPA or SPL, for ≥6 months; and 4) those who had not undergone orchiectomy. Cases were excluded if they 1) had sex development disorders; 2) concurrently used progesterone or other medications that might influence sex hormone levels; and 3) used parenteral estradiol. To reduce bias, we excluded those who took parenteral estrogen since most transgender women in our clinic received estrogen orally before 2021. This study was approved by the Ethics Committee of Peking University Third Hospital (M2021186). Informed consent was obtained from study participants. Given that it was a retrospective study, informed consent was approved for exemption if the participant could not be contacted. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Participant management
For drug-naïve transgender individuals, we adhered to the hormone management guidelines set forth by the World Professional Association for Transgender Health (WPATH) and the Endocrine Society (Coleman et al., Citation2022; Hembree et al., Citation2017). Due to the scarcity of CPA and the wide availability of SPL in China, we generally prioritized prescribing SPL over CPA, unless the transgender individual expressed a strong preference for CPA. Currently, the gonadotropin-releasing hormone analogue is costly and not covered by medical insurance, so it is rarely used as an antiandrogen for the transgender population in China. The initial prescription involves 2 mg/day of estradiol with either 80 mg/day of SPL or 25 mg/day of CPA orally. For transgender individuals who had self-initiated treatment before visiting us, we recorded their medication regimen and corresponding blood test results for data analysis. All the transgender visitors were recommended to monitor their hormone levels every three months and receive dose titration if necessary.
Data abstraction
Using a standardized data collection form, demographic data, mental health history, GAHT experience, and laboratory findings of the latest visit before November 30, 2021, were extracted from electronic medical records. Data collection took place from December 1, 2021, to January 30, 2022, and was independently verified by two investigators. All blood tests were conducted at the Peking University Third Hospital. Sex hormone assays were performed using the Siemens IMMULITE 2000 immunoassay system (Siemens Healthcare Diagnostics, Shanghai, China). The lower limit of detection and inter-assay coefficient of variation were as follows: 0.69 nmol/L (19.9 ng/dL) and 9.6% for total testosterone, 55.1 pmol/L (15.0 pg/mL) and 4.5% for estradiol, 0.5 ng/mL and 5.7% for prolactin, 0.1 mIU/mL and 4.9% for follicle-stimulating hormone (FSH), and 0.1 mIU/mL and 5.4% for luteinizing hormone (LH), respectively. The visual analogue scale (VAS) system has been widely used to evaluate people’s experiences and has been applied in recent studies of transgender health (Casado et al., Citation2017; Leyns et al., Citation2021; Meister et al., Citation2017). Since July 2019, the gender clinic at our institution has implemented the distribution of VAS-based GAHT satisfaction scores to transgender women as a means to incorporate self-reported outcomes of satisfaction with GAHT into transgender people’s care. For example, transgender women were encouraged to mark the point along the line that best represented their level of satisfaction regarding testicular atrophy, decrease in erections, reduction of facial/body hair, etc., under their current GAHT regimen (Supplementary Table 1). The satisfaction scores corresponding to the most recent follow-up were recorded.
Main outcomes
The primary outcomes of this study were the serum total testosterone level, the achievement of testosterone suppression to the normal range of cisgender women under antiandrogen treatment, and the self-rated satisfaction score of GAHT based on VAS. Additionally, safety events, including liver injury, hyperkalemia, thromboembolism, newly diagnosed tumors, hyperprolactinemia, and dyslipidemia, were recorded and analyzed. In our hospital, the upper limit of normal for alanine aminotransferase (ALT) in Chinese women was 40 U/L. Liver injury was defined as a new onset of elevated ALT ≥3 times the upper limit of normal (Aithal et al., Citation2011). Hyperprolactinemia was defined as a prolactin level >25 ng/mL.
Statistical analysis
Quantitative variables are shown as the median (interquartile range [IQR]). Categorical variables are shown as numbers (percentages). Laboratory results of total testosterone below the assay sensitivity were recorded as the lower limit of detection (0.69 nmol/L [19.9 ng/dL]) for calculation purposes. They were assigned as half of the lower limit values (0.35 nmol/L [10.1 ng/dL]) in the sensitivity analysis. Differences in demographic, clinical, and laboratory data between the two groups were analyzed by the Mann–Whitney U test, chi-square test, or Fisher’s exact test, as appropriate. To investigate factors related to whether testosterone levels could be suppressed to less than 1.74 nmol/L (50 ng/dL), the target recommended by the Endocrine Society guidelines (Hembree et al., Citation2017), logistic regression analysis was performed, including age, body mass index (BMI), antiandrogen type, antiandrogen access, antiandrogen therapy duration, and daily estradiol dose as variables. Comparison of VAS-based satisfaction scores with GAHT between the CPA and SPL groups was conducted using the Mann–Whitney U test. To account for the non-normally distributed satisfaction scores, we transformed them using the Blom rank-based normalization method. The difference in satisfaction scores between groups was then compared using analysis of covariance (ANCOVA), with age, duration of antiandrogen therapy, and daily estradiol dose as covariates, and comorbid mental disorders as a fixed factor. Statistical analysis was performed using SPSS Statistics 24.0 (IBM). Two-sided p values <0.05 were considered significant. Bonferroni correction was employed to control for Type I errors arising from multiple comparisons.
Results
Characteristics of the participants in the CPA and SPL groups
A total of 639 people who identified as transgender women were screened. Of them, 151 individuals (80 in the CPA group and 71 in the SPL group) receiving continuous GAHT for at least six months were included in the final analysis (). The demographic and clinical characteristics of the participants are given in . No significant difference was found in age or BMI between the two groups. In the overall population, forty-five (29.8%) transgender women had comorbid mental disorders. Of all participants included, 58.9% (89/151) had already self-prescribed GAHTs before visiting us, with a significantly higher proportion in the CPA group than in the SPL group (83.8% vs. 31.0%, p < 0.001). As many as 56.3% of the transgender women in the CPA group reported obtaining medications through informal means (i.e. informal online e-commerce, private drug retailers, or close friends), while 87.3% of transgender women in the SPL group reported obtaining prescriptions from hospitals. The antiandrogen therapy duration was longer in the CPA group than in the SPL group (median [IQR] of 18 [10–32] months vs. 12 [8–21] months, p < 0.001). The median doses of antiandrogens were 25 [16–25] mg/d for CPA and 100 [80–120] mg/d for SPL. The oral estrogen used was estradiol valerate at a median dose of 2 [2–3] mg/d in both the CPA and SPL groups, respectively ().
Figure 1. Participant flow chart. VAS: visual analogue scale. a there is overlap in exclusion criteria for some individuals.
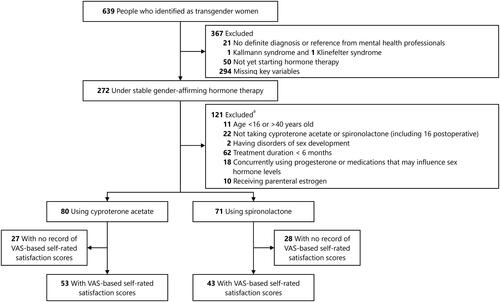
Table 1. Characteristics of participants.
The serum levels of sex hormones during GAHT
The serum total testosterone level in the CPA group was significantly lower than that in the SPL group (median [IQR] of 0.7 [0.7–2.1] nmol/L vs. 13.0 [6.0–17.8] nmol/L, p < 0.001). The proportion of transgender women with testosterone levels < 1.74 nmol/L (50 ng/dL) was significantly higher in the CPA group compared to the SPL group (75.0% vs. 11.3%, p < 0.001) (). In the sensitivity analysis, total testosterone levels below the assay sensitivity were assigned as half of the lower limit of detection. The results still demonstrated that people in the CPA group had significantly lower testosterone levels and a higher possibility of suppressing testosterone levels <1.74 nmol/L (50 ng/dL) than those in the SPL group (0.4 [0.4–2.1] nmol/L vs. 13.0 [6.0–17.8] nmol/L, 75.0% vs. 11.3%, both p < 0.001). Subsequently, we used age, BMI, antiandrogen type, antiandrogen access, self-initiation of treatment before consultation, antiandrogen therapy duration, and daily estradiol dose as variables for multivariable logistic regression analysis. The results showed that the antiandrogen type was the only independent factor associated with achieving testosterone suppression within the target range (odds ratio [OR] 34.60, 95% confidence interval [CI] 7.58–158.03, p < 0.001) (Supplementary Table 2). The levels of FSH and LH were lower in the CPA group compared to the SPL group (FSH: 0.6 [0.1–2.4] mIU/ml vs. 2.0 [1.2–3.2] mIU/ml, LH: 0.3 [0.1–1.7] mIU/ml vs. 4.3 [3.2–6.1] mIU/ml, both p < 0.001). Although the daily dose of administered estradiol was comparable, serum estradiol levels were lower in the CPA group than in the SPL group (140.8 [112.0–212.0] pmol/L vs. 194.5 [159.0–271.0] pmol/L, p < 0.001). The serum prolactin level was higher in the CPA group than in the SPL group (18.9 [11.8–28.1] ng/ml vs. 11.8 [7.9–18.4] ng/ml, p < 0.001) ().
Self-rated satisfaction scores during GAHT
Since July 2019, a total of 96 transgender women (53 in the CPA group and 43 in the SPL group) have reported their experiences with their current GAHT using satisfaction scores based on the 10-point VAS evaluation system (Supplementary Table 1). Internal consistency analysis revealed a Cronbach’s alpha coefficient of 0.726 for our VAS system. Transgender women in the CPA group reported higher satisfaction scores for erection decrease and figure feminization than those in the SPL group (all p < 0.05) (). After adjusting for age, antiandrogen therapy duration, and daily estradiol dose (Model 1) or age, antiandrogen therapy duration, daily estradiol dose, and comorbid mental disorders (Model 2) using ANCOVA, the differences in the Blom transformed VAS-based satisfaction scores between the two antiandrogen groups remained significant (p < 0.05). Moreover, the overall average satisfaction score was significantly higher in the CPA group than in the SPL group after these adjustments (p < 0.05) (). No significant differences were observed in other items between the two groups ().
Table 2. Visual analogue scale-based satisfaction scores for gender-affirming hormone therapy in Chinese transgender women.
Safety events during GAHT in the two groups
Liver injury is a safety concern associated with GAHT. In this study, one (1.4%) transgender woman in the CPA group and one (1.5%) transgender woman in the SPL group developed liver injury during treatment. The person in the SPL group reporting liver injury had a comorbid nonalcoholic fatty liver disease. The person in the CPA group who reported liver injury had no prior history of liver disease, and liver enzyme levels returned to the normal range after discontinuation of the medication. None of the individuals had an elevated ALT level of more than 5 times the upper limit of the normal range, which is one of the clinical criteria for drug-induced liver injury (Aithal et al., Citation2011). There were 22 (28.6%) and 9 (13.4%) transgender women who developed hyperprolactinemia in the CPA and SPL groups, respectively, with a significant difference between the groups (p < 0.05) (). All transgender women with hyperprolactinemia in this study were asymptomatic, and most (93.5% in the CPA group and 97.0% in the SPL group) had prolactin levels within two times the upper limit of the normal range for cisgender women. No cases of acute kidney injury were reported in either group, and there was no significant difference in serum creatinine levels between the two groups (77.0 [72.0–86.0] umol/L vs. 79.5 [71.0–87.0] umol/L, p = 0.446). Although there were no cases of hyperkalemia in either group, the SPL group exhibited significantly higher blood potassium levels than the CPA group (4.1 [4.0–4.3] mmol/L vs. 4.0 [3.9–4.2] mmol/L, p < 0.05). Newly diagnosed breast nodules, classified as BI-RADS 2, were detected by ultrasound in one transgender woman in the SPL group. No other newly diagnosed tumors or thromboembolism events were observed ().
Table 3. Safety events during gender-affirming hormone therapy in Chinese transgender women.
Discussion
To our knowledge, this is the first report on the daily clinical practice of GAHT in transgender women from China. The findings of this study suggest that CPA was more effective than SPL in lowering serum total testosterone levels and achieved higher satisfaction with erection decrease and figure feminization in Chinese transgender women. No severe safety events were reported during GAHT.
CPA and SPL are both recommended as antiandrogens for transgender women (Coleman et al., Citation2022; Hembree et al., Citation2017), but there is limited data comparing these two drugs specifically in the East Asian population. Moreover, the availability and popularity of CPA and SPL are different in Europe and the United States, making direct comparisons challenging. A retrospective cross-sectional study showed that testosterone levels were lower in the CPA group (n = 21) than in the SPL group (n = 38) (Angus et al., Citation2019). In a recent pilot randomized controlled trial, after 12 wk of GAHT, a more pronounced decrease in testosterone levels was observed in the 41 participants with CPA than in the 52 participants with SPL (Burinkul et al., Citation2021). Our study, with a larger sample size of East Asian transgender women and a longer treatment duration, confirms the finding that CPA is more effective than SPL in reducing serum total testosterone levels. The difference in potency in suppressing androgen levels may be attributed to the different mechanisms of these two drugs. CPA directly inhibits testosterone production by acting on the hypothalamic-pituitary-gonadal axis (Angus et al., Citation2019; Glintborg et al., Citation2021). Consistently, our study showed that the serum levels of FSH and LH were greatly suppressed in the CPA group. In contrast, SPL primarily acts as an antiandrogen by blocking the binding of dihydrotestosterone to androgen receptors and partially by reducing testosterone biosynthesis (Angus et al., Citation2019). One study from the United States found that a quarter of transgender women treated with SPL failed to achieve any significant testosterone suppression (Liang et al., Citation2018). Additionally, in Burinkul et al.’s study, some transgender individuals experienced an increase in testosterone levels after receiving SPL (Burinkul et al., Citation2021). Hence, it is crucial to emphasize to hormone users that the primary goal of GAHT is to improve individual’s experiences rather than solely achieving a specific level of androgens. Especially for those taking SPL, the pharmacological mechanisms of SPL should be well elucidated to improve the user’s understanding and confidence in this regimen.
As discussed above, the primary goal of transgender medical care is to alleviate gender dysphoria and improve self-acceptance and overall well-being. Therefore, individual experiences should be well considered when evaluating the effectiveness of GAHT. However, few studies have reported on the personal experiences and feelings of transgender women regarding their use of antiandrogens. The VAS is a simple and versatile tool that can capture people’s self-reported experiences across various domains, such as pain and satiety. It is short and easy to apply in clinical settings without adding a significant burden to respondents or leading to careless responses due to boredom or fatigue. VAS has already been utilized in the transgender population for their perceptual assessment and satisfaction with voice therapy (Casado et al., Citation2017; Leyns et al., Citation2021; Meister et al., Citation2017). Since July 2019, our gender clinic has been utilizing VAS-based GAHT satisfaction scores to incorporate self-reported satisfaction with GAHT into transgender healthcare. The internal consistency analysis has shown the reliability of the VAS system among our participants. According to the VAS score, individuals using CPA reported better experiences in terms of erection reduction and figure feminization than SPL users. A recent randomized clinical trial reported no difference in participant-reported erection frequency between SPL and CPA treatments (Burinkul et al., Citation2021). Nevertheless, this trial was a short-term study of GAHT with a follow-up period of only 12 wk. Considering that most of the biological effects of GAHT typically manifest gradually after 3–6 months of treatment, a longer duration of treatment may be necessary to fully assess the efficacy of different hormone therapy strategies. Another point to be aware of is serum androgen levels, as some transgender women are very concerned about their testosterone levels. Here, our data showed that people using CPA achieved significantly lower testosterone levels than those using SPL. Hence, the dramatically visible decline of androgen levels by CPA treatment may have a positive psychological impact on transgender women, contributing to their overall satisfaction with GAHT.
In this study, we observed that the median serum estradiol levels in both groups were below the guidelines recommended range of 367–734 pmol/L (100–200 pg/mL) (Coleman et al., Citation2022; Hembree et al., Citation2017) at a median estradiol dose of 2 mg/d. Interestingly, although the GAHT experience scores were higher in the CPA group than in the SPL group, the serum estradiol levels of CPA users were actually significantly lower than those of SPL users in this Chinese population. The Australian position statement recommended maintaining serum estradiol levels at 250–600 pmol/L (68–163 pg/mL) during GAHT in transgender women based on data from local cross-sectional studies (Cheung et al., Citation2019). This suggests that further research is needed to clarify whether the currently recommended range is appropriate for all transgender women, considering the potential variations in ethnicity and other factors. In our study, we found that serum estradiol levels were much higher in the SPL group than in the CPA group, despite similar estradiol doses. One possible explanation for this discrepancy is that testosterone, which is not sufficiently reduced by SPL, can be converted to estradiol by aromatase in adipose tissue, liver and other tissues. Therefore, although SPL may not be as potent in suppressing androgen levels, it may be superior to CPA in maintaining estradiol levels in transgender women. However, future prospective clinical studies are needed to confirm this.
In addition to efficacy, safety is another essential concern in GAHT. Previous studies found that prolactin levels in transgender women using CPA are higher than those using other antiandrogens, such as SPL or GnRH analogues (Angus et al., Citation2019; Burinkul et al., Citation2021; Fung et al., Citation2016; Gava et al., Citation2020; Kuijpers et al., Citation2021). A recent systematic review of 17 studies reported that prolactin levels increased by over 100% with CPA and up to 45% with SPL among transgender women on estrogen therapy (Wilson et al., Citation2020). Similarly, our study showed that more individuals in the CPA group had mildly elevated prolactin levels compared to those in the SPL group. Although CPA is considered to cause a temporary increase in serum prolactin levels, with levels returning to normal after discontinuation of CPA and orchiectomy (Defreyne et al., Citation2017; Nota et al., Citation2017), the long-term effects of hyperprolactinemia in the transgender population are not well understood. Given that many transgender women may undergo antiandrogen treatment for years before gender-affirming genital surgery, it is rational to monitor serum prolactin levels during long-term GAHT with CPA. Recent studies comparing the effectiveness and safety of different doses of CPA have indicated that lower doses (10–20 mg/d) are equally effective in lowering testosterone levels compared to higher doses, with fewer side effects, including hyperprolactinemia (Even Zohar et al., Citation2021; Kuijpers et al., Citation2021). Our study also showed that the median prolactin level was close to the normal range when using a median dose of CPA of 25 mg/d. These findings indicate that prolactin levels might be positively associated with the dose of CPA. It is noteworthy that WPATH has already reduced the recommended dose of CPA to 10 mg/d in the latest version of standard care for transgender and gender diverse people (Coleman et al., Citation2022). Therefore, we suggest a low initial dose of CPA for Chinese or other East Asian transgender women. Additionally, gradual titration toward the lowest effective dose of CPA might be considered to ensure optimal antiandrogenic efficacy while maintaining a favorable safety profile.
In our study, we observed that 1.4% and 1.5% of the transgender women in the CPA and SPL groups, respectively, had ALT levels three times greater than the upper limits of the normal range. Previous studies have reported varying incidences of ALT or aspartate aminotransferase elevation after GAHT, ranging from 0% to 5.7% (Burinkul et al., Citation2021; Kuijpers et al., Citation2021; Meyer et al., Citation2020; Wierckx et al., Citation2014). Recently, a multicenter prospective study involving 889 transgender women reported a very low incidence of liver injury (≤0.1%) within 12 months of GAHT (Stangl et al., Citation2021). Another large longitudinal study also indicated that feminizing hormone therapy is unlikely to result in significant alterations in liver enzyme levels (Hashemi et al., Citation2021). These findings challenge the necessity for routine liver enzyme monitoring in transgender women. However, based on our results and the fact that many Chinese transgender women obtained hormone medications through informal channels, it may be prudent to continue liver enzyme monitoring during GAHT in China.
In this study, nearly 30% of transgender women reported previous mental disorders in their past medical history. This aligns with the findings of a recent systematic review, which reported a higher prevalence of mental health problems among Chinese transgender and gender nonconforming people compared to the general population in China (Huang et al., Citation2019; Lin et al., Citation2021). Formal gender-affirming treatment is scarce in China. In our previous cross-sectional survey conducted in 2017, we found that only 27.4% of Chinese transgender hormone users received hormone medications from formal medical sources. The difficulty in accessing hormone therapy was associated with increased rates of suicide ideation and self-injury (Liu et al., Citation2020). Similarly, in this study, many transgender women reported obtaining antiandrogens from informal channels, especially those using CPA, suggesting substantial risks. Therefore, policymakers should support and promote the development of transgender medicine in China to provide more access to formal medical resources and thus enhance the well-being of this population.
To our knowledge, this is the first study to report the routine clinical practice of GAHT in transgender women from China. Our study provides valuable data on medications, laboratory findings, and hormone users’ feedback. Most of the published guidelines and consensuses on GAHT have been developed based on studies involving predominantly white transgender individuals, and it is recommended that ethnic differences warrant tailoring of treatment (Hembree et al., Citation2017). Our report has filled the gap in evidence for studies of GAHT in East Asian transgender women. In addition, we introduced the use of a VAS-based self-rated satisfaction score for GAHT. This simple and quick evaluation system allows hormone users and healthcare providers to easily understand the experiences and needs of individuals during GAHT. The use of this system can be instructive for developing drug regimens in an individualized way where the experiences of hormone users are valued.
Study limitations
Our study has several limitations. First, this was a retrospective study, and many participants had already taken self-prescribed GAHT before visiting our clinic. It was impossible to obtain their baseline characteristics to conduct a longitudinal analysis. Recently, a prospective cohort study of GAHT (NCT05318755) was launched at our center, which will provide more robust data to evaluate the efficacy and safety of GAHT in the East Asian transgender population. Second, due to the short supply of CPA in China, we usually prioritized SPL over CPA when prescribing. This nonrandomized selection of CPA or SPL could be influenced by individual preferences and drug availability, potentially introducing bias. However, the main results remained consistent after adjusting for potential confounding factors in the sensitivity analysis. Third, the availability of CPA from reliable pharmaceutical companies is quite limited in China, leading many transgender women to purchase CPA from informal channels. Notably, this choice of obtaining CPA from informal channels could be considered an active decision driven by personal preference. This proactive approach to selecting CPA as a treatment option might have positively influenced their satisfaction with the therapy. Besides, we emphasize that obtaining hormone medications from informal channels carries inherent risks. The effectiveness and safety of medications obtained through such channels cannot be absolutely guaranteed. Therefore, we strongly discourage acquiring hormone therapies from informal sources and call for improved access to hormone medications for transgender people in China. Fourth, the dose of SPL in our study population was relatively low. Whether a higher dose of SPL could provide a more potent effect on reducing androgen levels in the East Asian population is unknown. Fifth, it is crucial to acknowledge that expectations and preferences regarding the outcomes of GAHT may be highly personalized. While the VAS scores employed in this study provide a general assessment, they may not fully capture the diverse medical needs and desires of transgender individuals. Sixth, the antiandrogen therapy duration was longer in the CPA group than in the SPL group, which may introduce bias into the results. Finally, it is worth noting that mass spectrometry is not available for routine clinical practice in our hospital. Steroid hormone measurements were performed using electrochemiluminescence immunoassay instead of the gold standard of mass spectrometry. Nevertheless, a previous study demonstrated a strong linear correlation in total testosterone values between the results obtained from our immunoassay platform and the liquid chromatography-tandem mass spectrometry platform, with a correlation coefficient of 0.988 (Xu et al., Citation2020).
Conclusion
In conclusion, this retrospective study of GAHT showed that CPA had a more pronounced effect on lowering serum total testosterone levels than SPL and could achieve better satisfaction in terms of erection decrease and figure feminization in Chinese transgender women. No severe safety events were reported during GAHT. Well-designed clinical studies are still needed to further evaluate the efficacy and safety of different GAHT regimens in the Chinese transgender population.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consents were obtained from study participants, except in cases where contact could not be established due to the retrospective nature of the study.
Supplemental Material
Download MS Word (38 KB)Acknowledgments
We thank Prof. Lin Zeng from the Clinical Epidemiology Research Centre at Peking University Third Hospital for statistical assistance. We appreciate Prof. Runsen Chen from the Vanke School of Public Health at Tsinghua University for his guidance in writing this article.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Aithal, G. P., Watkins, P. B., Andrade, R. J., Larrey, D., Molokhia, M., Takikawa, H., Hunt, C. M., Wilke, R. A., Avigan, M., Kaplowitz, N., Bjornsson, E., & Daly, A. K. (2011). Case definition and phenotype standardization in drug-induced liver injury. Clinical Pharmacology and Therapeutics, 89(6), 806–815. https://doi.org/10.1038/clpt.2011.58
- Angus, L., Leemaqz, S., Ooi, O., Cundill, P., Silberstein, N., Locke, P., Zajac, J. D., & Cheung, A. S. (2019). Cyproterone acetate or spironolactone in lowering testosterone concentrations for transgender individuals receiving oestradiol therapy. Endocrine Connections, 8(7), 935–940. https://doi.org/10.1530/EC-19-0272
- Baker, K. E., Wilson, L. M., Sharma, R., Dukhanin, V., McArthur, K., & Robinson, K. A. (2021). Hormone therapy, mental health, and quality of life among transgender people: A systematic review. Journal of the Endocrine Society, 5(4), bvab011. https://doi.org/10.1210/jendso/bvab011
- Burinkul, S., Panyakhamlerd, K., Suwan, A., Tuntiviriyapun, P., & Wainipitapong, S. (2021). Anti-androgenic effects comparison between cyproterone acetate and spironolactone in transgender women: A randomized controlled trial. The Journal of Sexual Medicine, 18(7), 1299–1307. https://doi.org/10.1016/j.jsxm.2021.05.003
- Casado, J. C., Rodríguez-Parra, M. J., & Adrián, J. A. (2017). Voice feminization in male-to-female transgendered clients after Wendler’s glottoplasty with vs. without voice therapy support. European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies : Affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery, 274(4), 2049–2058. https://doi.org/10.1007/s00405-016-4420-8
- Cheung, A. S., Wynne, K., Erasmus, J., Murray, S., & Zajac, J. D. (2019). Position statement on the hormonal management of adult transgender and gender diverse individuals. The Medical Journal of Australia, 211(3), 127–133. https://doi.org/10.5694/mja2.50259
- Coleman, E., Radix, A. E., Bouman, W. P., Brown, G. R., de Vries, A. L. C., Deutsch, M. B., Ettner, R., Fraser, L., Goodman, M., Green, J., Hancock, A. B., Johnson, T. W., Karasic, D. H., Knudson, G. A., Leibowitz, S. F., Meyer-Bahlburg, H. F. L., Monstrey, S. J., Motmans, J., Nahata, L., … Arcelus, J. (2022). Standards of care for the health of transgender and gender diverse people, Version 8. International Journal of Transgender Health, 23(Suppl 1), S1–S259. https://doi.org/10.1080/26895269.2022.2100644
- Defreyne, J., Nota, N., Pereira, C., Schreiner, T., Fisher, A. D., den Heijer, M., & T’Sjoen, G. (2017). Transient elevated serum prolactin in trans women is caused by cyproterone acetate treatment. LGBT Health, 4(5), 328–336. https://doi.org/10.1089/lgbt.2016.0190
- Even Zohar, N., Sofer, Y., Yaish, I., Serebro, M., Tordjman, K., & Greenman, Y. (2021). Low-dose cyproterone acetate treatment for transgender women. The Journal of Sexual Medicine, 18(7), 1292–1298. https://doi.org/10.1016/j.jsxm.2021.04.008
- Fung, R., Hellstern-Layefsky, M., Tastenhoye, C., Lega, I., & Steele, L. (2016). Differential effects of cyproterone acetate vs spironolactone on serum high-density lipoprotein and prolactin concentrations in the hormonal treatment of transgender women. The Journal of Sexual Medicine, 13(11), 1765–1772. https://doi.org/10.1016/j.jsxm.2016.09.012
- Gava, G., Mancini, I., Alvisi, S., Seracchioli, R., & Meriggiola, M. C. (2020). A comparison of 5-year administration of cyproterone acetate or leuprolide acetate in combination with estradiol in transwomen. European Journal of Endocrinology, 183(6), 561–569. https://doi.org/10.1530/EJE-20-0370
- Glintborg, D., T’Sjoen, G., Ravn, P., & Andersen, M. S. (2021). Management of endocrine disease: Optimal feminizing hormone treatment in transgender people. European Journal of Endocrinology, 185(2), R49–R63. https://doi.org/10.1530/EJE-21-0059
- Gooren, L. J. (2014). Should cross-sex hormone treatment of transsexual subjects vary with ethnic group? Asian Journal of Andrology, 16(6), 809–810. https://doi.org/10.4103/1008-682X.133972
- Hashemi, L., Zhang, Q., Getahun, D., Jasuja, G. K., McCracken, C., Pisegna, J., Roblin, D., Silverberg, M. J., Tangpricha, V., Vupputuri, S., & Goodman, M. (2021). Longitudinal changes in liver enzyme levels among transgender people receiving gender affirming hormone therapy. The Journal of Sexual Medicine, 18(9), 1662–1675. https://doi.org/10.1016/j.jsxm.2021.06.011
- Hembree, W. C., Cohen-Kettenis, P. T., Gooren, L., Hannema, S. E., Meyer, W. J., Murad, M. H., Rosenthal, S. M., Safer, J. D., Tangpricha, V., & T’Sjoen, G. G. (2017). Endocrine treatment of gender-dysphoric/gender-incongruent persons: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism, 102(11), 3869–3903. https://doi.org/10.1210/jc.2017-01658
- Huang, Y., Wang, Y., Wang, H., Liu, Z., Yu, X., Yan, J., Yu, Y., Kou, C., Xu, X., Lu, J., Wang, Z., He, S., Xu, Y., He, Y., Li, T., Guo, W., Tian, H., Xu, G., Xu, X., … Wu, Y. (2019). Prevalence of mental disorders in China: A cross-sectional epidemiological study. The Lancet. Psychiatry, 6(3), 211–224. https://doi.org/10.1016/S2215-0366(18)30511-X
- Kuijpers, S. M. E., Wiepjes, C. M., Conemans, E. B., Fisher, A. D., T’Sjoen, G., & den Heijer, M. (2021). Toward a lowest effective dose of cyproterone acetate in trans women: Results from the ENIGI study. The Journal of Clinical Endocrinology and Metabolism, 106(10), e3936–e3945. https://doi.org/10.1210/clinem/dgab427
- Leyns, C., Corthals, P., Cosyns, M., Papeleu, T., Van Borsel, J., Morsomme, D., T’Sjoen, G., & D’Haeseleer, E. (2021). Acoustic and perceptual effects of articulation exercises in transgender women. Journal of Voice: Official Journal of the Voice Foundation, 38(1), 246.e15–246.e25. https://doi.org/10.1016/j.jvoice.2021.06.033
- Liang, J. J., Jolly, D., Chan, K. J., & Safer, J. D. (2018). Testosterone levels achieved by medically treated transgender women in a United States endocrinology clinic cohort. Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists, 24(2), 135–142. https://doi.org/10.4158/EP-2017-0116
- Lin, Y., Xie, H., Huang, Z., Zhang, Q., Wilson, A., Hou, J., Zhao, X., Wang, Y., Pan, B., Liu, Y., Han, M., & Chen, R. (2021). The mental health of transgender and gender non-conforming people in China: A systematic review. The Lancet Public Health, 6(12), e954–e969. https://doi.org/10.1016/S2468-2667(21)00236-X
- Liu, Y., Xin, Y., Qi, J., Wang, H., Hong, T., Yang, X., Li, B., Chang, X., Knudson, G. F., Zhao, Z., & Pan, B. (2020). The desire and status of gender-affirming hormone therapy and surgery in transgender men and women in China: A national population study. The Journal of Sexual Medicine, 17(11), 2291–2298. https://doi.org/10.1016/j.jsxm.2020.07.081
- Meister, J., Hagen, R., Shehata-Dieler, W., Kühn, H., Kraus, F., & Kleinsasser, N. (2017). Pitch elevation in male-to-female transgender persons-the Wurzburg approach. Journal of Voice, 31(2), e7–e15. https://doi.org/10.1016/j.jvoice.2016.07.018
- Meyer, G., Mayer, M., Mondorf, A., Flügel, A. K., Herrmann, E., & Bojunga, J. (2020). Safety and rapid efficacy of guideline-based gender-affirming hormone therapy: An analysis of 388 individuals diagnosed with gender dysphoria. European Journal of Endocrinology, 182(2), 149–156. https://doi.org/10.1530/EJE-19-0463
- Nota, N. M., Dekker, M., Klaver, M., Wiepjes, C. M., van Trotsenburg, M. A., Heijboer, A. C., & den Heijer, M. (2017). Prolactin levels during short- and long-term cross-sex hormone treatment: An observational study in transgender persons. Andrologia, 49(6), e12666. https://doi.org/10.1111/and.12666
- Santner, S. J., Albertson, B., Zhang, G. Y., Zhang, G. H., Santulli, M., Wang, C., Demers, L. M., Shackleton, C., & Santen, R. J. (1998). Comparative rates of androgen production and metabolism in Caucasian and Chinese subjects. The Journal of Clinical Endocrinology and Metabolism, 83(6), 2104–2109. https://doi.org/10.1210/jcem.83.6.4898
- Sofer, Y., Yaish, I., Yaron, M., Bach, M. Y., Stern, N., & Greenman, Y. (2020). Differential endocrine and metabolic effects of testosterone suppressive agents in transgender women. Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists, 26(8), 883–890. https://doi.org/10.4158/EP-2020-0032
- Stangl, T. A., Wiepjes, C. M., Defreyne, J., Conemans, E., Fisher, A D., Schreiner, T., T’Sjoen, G., & den Heijer, M. (2021). Is there a need for liver enzyme monitoring in people using gender-affirming hormone therapy? European Journal of Endocrinology, 184(4), 513–520. https://doi.org/10.1530/EJE-20-1064
- T’Sjoen, G., Arcelus, J., Gooren, L., Klink, D. T., & Tangpricha, V. (2019). Endocrinology of transgender medicine. Endocrine Reviews, 40(1), 97–117. https://doi.org/10.1210/er.2018-00011
- Tangpricha, V., & den Heijer, M. (2017). Oestrogen and anti-androgen therapy for transgender women. The Lancet. Diabetes & Endocrinology, 5(4), 291–300. https://doi.org/10.1016/S2213-8587(16)30319-9
- van Houten, M. E., & Gooren, L. J. (2000). Differences in reproductive endocrinology between Asian men and Caucasian men–a literature review. Asian Journal of Andrology, 2(1), 13–20. https://www.ncbi.nlm.nih.gov/pubmed/11228931
- Wang, Y., Feng, Y., Su, D., Wilson, A., Pan, B., Liu, Y., Wang, N., Guo, B., Han, M., Zucker, K. J., & Chen, R. (2021). Validation of the Chinese version of the gender identity/gender dysphoria questionnaire for adolescents and adults. The Journal of Sexual Medicine, 18(9), 1632–1640. https://doi.org/10.1016/j.jsxm.2021.05.007
- Wierckx, K., Van Caenegem, E., Schreiner, T., Haraldsen, I., Fisher, A. D., Toye, K., Kaufman, J. M., & T’Sjoen, G. (2014). Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: Results from the European network for the investigation of gender incongruence. The Journal of Sexual Medicine, 11(8), 1999–2011. https://doi.org/10.1111/jsm.12571
- Wilson, L. M., Baker, K. E., Sharma, R., Dukhanin, V., McArthur, K., & Robinson, K. A. (2020). Effects of antiandrogens on prolactin levels among transgender women on estrogen therapy: A systematic review. International Journal of Transgender Health, 21(4), 391–402. https://doi.org/10.1080/15532739.2020.1819505
- Xu, H. Y., Jiang, H., Feng, G. S., Feng, Y., Han, Y., Tang, W. H., Zhang, H. X., Chen, F. H., Zhang, H. X., Liu, D. F., Li, R., & Qiao, J. (2020). Establishing the lower limits of total serum testosterone among Chinese proven fertile men who received treatment of assisted reproductive technology. Asian Journal of Andrology, 22(4), 396–400. https://doi.org/10.4103/aja.aja_100_19
