Abstract
Bacterial endophytes were isolated from the leaves of Bougainvillea spectabilis Willd. and screened for their in vitro antagonistic activity against three important phytopathogenic fungi viz., Neocosmospora keratoplastica, Stemphylium vesicarium and Rhizoctonia solani. Among the 36 endophytic bacterial isolates screened, three isolates designated as BEB1, BEB5 and BEB6 inhibited the mycelial growth of all the three plant pathogenic fungi tested and recorded more than 10% growth inhibition. Scanning electron microscopic observations of hyphae of the pathogenic fungi at the periphery of the inhibition zone in the dual culture plate revealed morphological abnormalities such as shrinkage, pit formation and loss of turgidity. These isolates were identified as Bacillus subtilis (BEB1) and Bacillus cereus (BEB5 and BEB6) based on the MALDI Biotyper analysis. The Bacillus subtilis strain BEB1, which exhibited the highest level of antagonism, was tested for its sensitivity to cyanide and potential to degrade NaCN. The results revealed that this strain was capable of growing in a medium containing 5 mM of NaCN with a degradation efficiency of 53.8%. This endophyte is valuable for exploitation as a biocontrol agent to control diseases especially in the cyanogenic plants and bioremediation of cyanide from the industrial effluent.
Introduction
Preformed antimicrobial compounds, also known as ‘Phytoanticipins’, are present in a diverse group of plants (VanEtten et al. Citation1994; Pedras and Yaya Citation2015; Oros and Kallai Citation2019). These compounds include cyanogenic glycosides, saponins, glucosinolates and phenolics (Osbourn Citation1996). Phytoanticipins play an important role in the resistance of plants against pests and pathogens (Gleadow and Moller Citation2014; Piasecka et al. Citation2015). The cyanogenic glycosides are a group of amino acid- derived secondary metabolites that are present in the form of inactive precursors in plants and in response to pathogen infection or tissue damage these are activated to form hydrocyanic acid (HCN) by the hydrolytic enzymes of plants (Poulton Citation1990; Gleadow and Moller Citation2014). Over 2000 plant species such as cassava, sorghum, flax, rubber, lima bean, cocoyam, apple, apricot and white clover have been reported to contain cyanoglycosides (Poulton Citation1990; Francisco and Pinotti Citation2000; Vetter Citation2000; Gleadow and Moller Citation2014). The presence of HCN in the leaves of Bougainvillea spectabilis Willd. (Nyctaginaceae) has been reported (Chillawar Citation2017). Despite the toxicity of cyanogenic glycosides, many pathogens establish compatible relationship with these cyanogenic plants and cause diseases. It is well established that endophytic bacteria are commonly associated with higher plants (Liu et al. Citation2017) and these microorganisms may have potential to overcome the toxic effects of HCN. The aims of this research were to i) isolate and characterize endophytic bacteria from B. spectabilis, ii) evaluate their antagonistic activity against plant pathogenic fungi and iii) to test their sensitivity to cyanide.
Materials and methods
Plant material
Healthy leaves of Bougainvillea spectabilis Willd. were collected from the Botanic garden, Sultan Qaboos University, Muscat, Sultanate of Oman.
Fungal cultures
Three phytopathogenic fungal species viz., Neocosmospora keratoplastica (D. Geiser, O’Donnell, Short & Ning Zhang) Sand.-Den. & Crous (ID 2114), Stemphylium vesicarium (Wallr.) E.G. Simmons (ID 2392) and Rhizoctonia solani Kuhn (ID 2004) obtained from the Department of Crop Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University were used for the antifungal testing.
Bacterial endophyte isolation
To isolate endophytic bacteria, the leaves were rinsed with distilled water and dried on sterile filter papers. The leaves were cut into small segments (0.5 cm) and surface sterilized with 1% NaOCl for 3 min, followed by washing with sterile distilled water three times, each for 1 min. The surface-sterilized plant tissues (1 g) were ground in 1 ml of sterile distilled water by using a sterilized mortar and pestle and 100 µl aliquots of the extracts were plated on Nutrient agar (NA) (Oxoid Ltd., Hampshire, UK) in Petri plates. The plates were incubated at 28 °C for 2 days. The efficiency of the surface sterilization was verified by plating 100 µl aliquots of the sterile distilled water used in the final rinse onto NA plates. The bacterial colonies appearing on the plates were purified by streak-plate technique and single colonies on the NA plates were selected and transferred to fresh NA medium.
Antagonistic potential of bacterial endophytes
The endophytic bacterial isolates were evaluated for their inhibitory effects on phytopathogeic fungi using a dual culture technique (Al-Hussini et al. Citation2019). Briefly, a 7-mm diameter mycelial plug of the test fungi taken from an actively growing culture was placed on one side of the Petri dish (9-mm diameter) containing PDA medium. The antagonist was streaked on the opposite side about 1 cm away from the edge of the Petri plate and perpendicular to the fungal disc. Three replications were maintained for each bacterial isolate. Petri dishes inoculated with fungal discs alone served as control. The plates were incubated at room temperature (25 ± 2°C) for 3–5 days or until the mycelial growth in the control completely covered the Petri plate. After incubation, the radial growth of the pathogens was recorded and percent growth inhibition was calculated (Shifa et al. Citation2015).
Scanning electron microscopy
To study the morphological changes in the hyphae of phytopathogenic fungi at the inhibition zone in dual culture plates, 0.5 cm pieces of agar medium containing mycelium were taken from the periphery and samples for the scanning electron microscopy were prepared as described by Goldstein et al. (Citation2003). The prepared samples were then examined with a JEOL JSM-7800F scanning electron microscope.
Identification of endophytic bacteria
The bacterial isolates showing antagonistic activity were identified using a MALDI Biotyper (Bruker Daltronics, MA, USA) with Bruker inbuilt MALDI-Biotyper database by following the manufacturer’s instructions. The score values <1.7 were considered unacceptable identification, scores between 1.70 and 1.99 were considered low-confidence identification and scores of ≥2.00 were considered high-confidence identification.
Cyanide degradation potential of endophytic bacteria
To study the effect of cyanide on the growth of endophytic bacteria, B. subtilis strain BEB1, which exhibited the highest level of antagonism, was grown in Nutrient broth containing different concentrations of NaCN (1, 5, 10, 15, 20 and 25 mM) for 72 h with shaking at 28°C. The absorbance of the cultures was measured at 660 nm using a spectrophotometer. The residual cyanide in the nutrient broth was determined as described by Kandasamy et al. (Citation2015) and the cyanide degradation efficiency (CDE) of the bacteria was calculated by using the formula: CDE (%) = IC-RC/IC × 100; where, IC = initial concentration of cyanide in the growth medium and RC = residual concentration of cyanide in the growth medium.
Statistical analysis
The data were subjected to analysis of variance using Minitab 17 (State College, PA, USA) and the treatment means were compared by least significant difference test (P ≤ 0.05).
Results and discussion
A total of 36 morphologically distinct endophytic bacterial isolates were obtained from the surface-sterilized leaf tissues of B. spectabilis. No bacterial colonies were observed from the distilled water collected from the last wash during surface sterilization procedure. Of the 36 endophytic bacterial isolates, five isolates designated as BEB1, BEB2, BEB3, BEB5 and BEB6 inhibited the mycelial growth of N. keratoplastica, S. vesicarium and R. solani in vitro (Table ). Among them, BEB1, BEB5 and BEB6 recorded more than 10% growth inhibition of all the three plant pathogenic fungi tested. The isolate BEB1 exhibited the highest antagonistic activity against all the three plant pathogenic fungi. The in vitro antagonistic activity of BEB1 against N. keratoplastica, R. solani and S. vesicarium is shown in Figure . The inhibition zone formation might be due to the release of various diffusible extracellular antifungal metabolites by these endophytes (Leifert et al. Citation1995; Fira et al. Citation2018). The varying degree of antagonism of the endophytic bacterial isolates against plant pathogenic fungi in the dual culture assay in the present study might be due to the type and level of production of antifungal metabolites by these bacterial endophytes.
Figure 1. In vitro antagonistic activity of Bacillus subtilis BEB1 against Neocosmospora keratoplastica, Rhizoctonia solani and Stemphylium vesicarium. A) N. keratoplastica alone, B) N. keratoplastica + B. subtilis BEB1, C) R. solani alone, D) R. solani + B. subtilis BEB1, E) S. vesicarium alone, F) S. vesicarium + B. subtilis BEB1
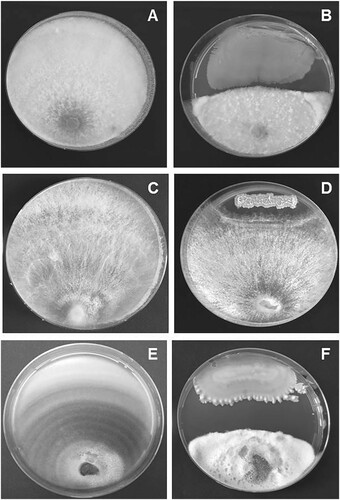
Table 1. In vitro antagonistic activity of endophytic bacteria isolated from Bougainvillea spectabilis.
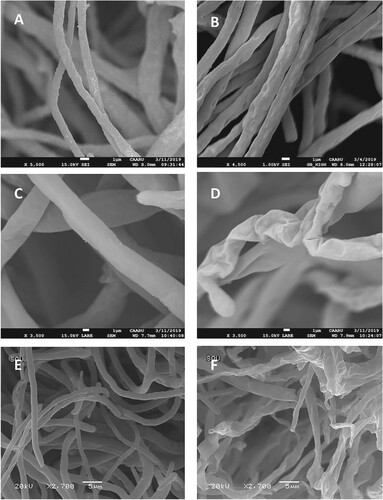
Based on the MALDI Biotyper analysis, the three bacterial isolates that showed more than 10% growth inhibition of plant pathogenic fungi were identified as Bacillus subtilis (BEB1) and Bacillus cereus (BEB5 and BEB6). These bacterial isolates had score values of >2.0. Scanning electron microscopic observations of hyphae of the pathogens at the margin of the inhibition zone in the dual culture plate revealed morphological abnormalities such as hyphal shrinkage, pit formation and loss of turgor in comparison to the control hyphae (Figure ). These results are in agreement with the findings of Hajlaoui et al. (Citation1992) who reported that the yeast like antagonistic fungus Sporothrix flocculosa caused complete plasmolysis of Sphaerotheca pannosa var. rosae, the rose powdery mildew fungus. The shrinkage of the hyphae indicates a possible loss of cytoplasmic contents (Garg et al. Citation2010). The observed loss of turgidity of hyphae of the pathogenic fungi in the inhibition zone suggests alterations in the permeability of the cell membrane (Halo et al. Citation2018). The hyphal malformations may be attributed to production of antibiotic substances by Bacillus sp. (Kotze et al. Citation2011).
Figure 2. Scanning electron micrographs showing morphological changes in the hyphae of Neocosmospora keratoplastica, Rhizoctonia solani and Stemphylium vesicarium due to antagonistic effect of Bacillus subtilis BEB1. A) N. keratoplastica alone, B) N. keratoplastica + B. subtilis BEB1, C) R. solani alone, D) R. solani + B. subtilis BEB1, E) S. vesicarium alone, F) S. vesicarium + B. subtilis BEB1.
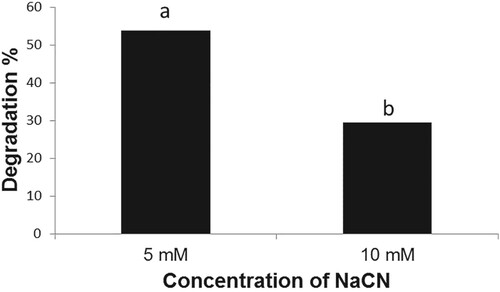
The B. subtilis strain BEB1, which exhibited the highest level of antagonism towards phytopathogenic fungi tested, was capable of growing in a medium containing NaCN (Figure ). The B. subtilis strain BEB1 caused 53.8 and 29.4% degradation of cyanide when grown in a medium containing NaCN at concentrations of 5 and 10 mM, respectively (Figure ). The utilization of cyanide as a nitrogen source by a few species of Pseudomonas for their growth has been reported (Harris and Knowles Citation1983; Rollinson et al. Citation1987; Luque-Almagro et al. Citation2016). The cyanide is converted into carbon dioxide and ammonia via an NADH-linked cyanide oxygenase system (Kunz et al. Citation1994; Luque-Almagro et al. Citation2005). The fungi, which infect cyanogenic plants, employ different mechanisms to tolerate HCN. For example, the ascomycete fungus Microcyclus ulei (P. Henn.) v. Arx, which infects rubber tree, employs cyanide-resistant respiration to tolerate HCN (Lieberei, Citation1988). Many fungal pathogens infecting cyanogenic plants are known to produce cyanide-detoxifying enzyme namely cyanide hydratase, which converts HCN into formamide (Fry and Millar Citation1972; Fry and Evans Citation1977; Wang et al. Citation1992). The production of cyanide hydratase by Gloeocercospora sorghi Bain & Edgerton ex Deighton, the sorghum pathogen (Wang et al. Citation1992) and Fusarium lateritium Nees, the sweet potato pathogen (Cluness et al. Citation1993) has been reported. The tolerance of B. subtilis strain BEB1 to cyanide in this study may be due to the production of enzymes that metabolize cyanide as well as due to its potential to form endospores (Earl et al. Citation2008).
Figure 3. Growth of Bacillus subtilis BEB1 at different concentrations of NaCN. The absorbance of the bacterial cultures was determined 72 h after inoculation. Values followed by different letters are significantly different according to LSD test (P < 0.05).
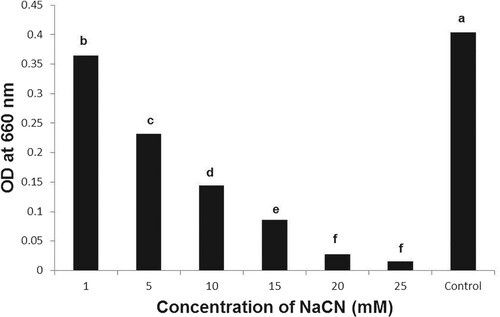
Figure 4. Degradation of NaCN by Bacillus subtilis BEB1. The cyanide content in the growth medium was determined 72 h after inoculation. Values followed by different letters are significantly different according to LSD test (P < 0.05).
Effluents from industries involved in metal plating, coal coking, coal gasification, ore leaching, aluminum electrolysis, manufacturing of pharmaceuticals, plastics and synthetic fibers contain large amounts of cyanides (Dumestre et al. Citation1997). To minimize health and environmental risks, the cyanide content in these effluents has to be reduced to very low levels (0.1 mg of CN per liter) before discharging (Smith and Mudder Citation1991). A wide range of microorganisms is known to metabolize cyanides (Harris et al. Citation1987; Mirizadeh et al. Citation2014). Treatment of industrial effluents with such biological agents may be an alternative and environmentally friendly method to degrade cyanide. Kandasamy et al. (Citation2015) isolated cyanide tolerant strains of Bacillus pumilus, Bacillus cereus and Pseudomonas putida from cassava factory wastewater and demonstrated that B. pumilus and P. putida could remove 63 and 61 per cent of cyanide at 5 mM. Razanamahandry et al. (Citation2019) reported that the bacteria isolated from mining wastewater and thiocyanate containing wastewater showed biodegradation of free cyanide.
Bacillus spp. are extensively used as plant growth-promoting bacteria because of their ability to suppress plant pathogens and augment plant health (Jayaraj et al. Citation2005; Hu et al. Citation2014). Bacillus species are known to produce antibiotics such as mycobacillins, iturins, bacillomycins, surfactins, mycosubtilins, fungistatins, subsporins and fengycins (Fira et al. Citation2018). The Food and Drug Administration (FDA) of the United States has approved the ‘generally regarded as safe’ (GRAS) status to B. subtilis (Harwood and Wipat Citation1996). Hence, the endophyte B. subtilis BEB1, which showed high levels of antagonistic activity against plant pathogenic fungi and cyanide detoxification potential is valuable for exploitation as a biocontrol agent to control diseases especially in the cyanogenic plants and bioremediation of cyanide from the industrial waste effluents.
Acknowledgements
The authors thank the Central Analytical and Applied Research Unit (CAARU) and the College of Medicine, Sultan Qaboos University for MALDI-Biotyper analysis and Scanning Electron Microscope imaging, respectively.
Disclosure statement
The authors declare that they have no conflict of interest.
Authors’ contributions
RV, AMA designed the study, BASA, SSA, ZMA, IHA conducted lab experiments, RV, AMA supervised the research project, BASA, RV, AMA wrote the manuscript.
Compliance with ethical standards
Ethical approval
This article is original and not published elsewhere. All authors discussed the results, read and approved the final manuscript. The authors confirm that there are no ethical issues in publication of the manuscript.
Additional information
Funding
References
- Al-Hussini HS, Al-Rawahi AY, Al-Marhoon AA, Al-Abri SA, Al-Mahmooli IH, Al- Sadi AM, Velazhahan R. 2019. Biological control of damping-off of tomato caused by Pythium aphanidermatum by using native antagonistic rhizobacteria isolated from Omani soil. J Plant Pathol. 101:315–322. doi: https://doi.org/10.1007/s42161-018-0184-x
- Chillawar R. 2017. Quantitative estimation of hydrocyanic acid (HCN) in some cyanogenic plants with the help of microbial glycosidase enzyme. Int J Appl Res. 3:265–266.
- Cluness MJ, Turner PD, Clements E, Brown DT, O’Reilly C. 1993. Purification and properties of cyanide hydratase from Fusarium lateritium and analysis of the corresponding chy1 gene. J Gen Microbiol. 139:1807–1815. doi: https://doi.org/10.1099/00221287-139-8-1807
- Dumestre A, Chone T, Portal JM, Gerard M, Berthelin J. 1997. Cyanide degradation under alkaline conditions by a strain of Fusarium solani isolated from contaminated soils. Appl Environ Microbiol. 63:2729–2734. doi: https://doi.org/10.1128/AEM.63.7.2729-2734.1997
- Earl AM, Losick R, Kolter R. 2008. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 16:269–275. doi: https://doi.org/10.1016/j.tim.2008.03.004
- Fira D, Dimkic I, Beric T, Lozo J, Stankovic S. 2018. Biological control of plant pathogens by Bacillus species. J Biotechnol. 285:44–55. doi: https://doi.org/10.1016/j.jbiotec.2018.07.044
- Francisco IA, Pinotti MHP. 2000. Cyanogenic glycosides in plants. Braz Arch Biol Technol. 43:487–492. doi: https://doi.org/10.1590/S1516-89132000000500007
- Fry WE, Evans PH. 1977. Association of formamide hydro-lyase with fungal pathogenicity to cyanogenic plants. Phytopathology. 77:1001–1006. doi: https://doi.org/10.1094/Phyto-67-1001
- Fry WE, Millar RL. 1972. Cyanide degradation by an enzyme from Stemphylium loti. Arch Biochem Biophys. 151:468–474. doi: https://doi.org/10.1016/0003-9861(72)90523-1
- Garg H, Li H, Sivasithamparam K, Kuo J, Barbetti MJ. 2010. The infection processes of Sclerotinia sclerotiorum in cotyledon tissue of a resistant and a susceptible genotype of Brassica napus. Ann Bot. 106:897–908. doi: https://doi.org/10.1093/aob/mcq196
- Gleadow RM, Moller BL. 2014. Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annu Rev Plant Biol. 65:155–185. doi: https://doi.org/10.1146/annurev-arplant-050213-040027
- Goldstein J, Newbury DE, Joy DC, Lyman CE, Echlin P, Lifshin E, Sawyer L, Michael JR. 2003. Scanning electron microscopy and X-Ray Microanalysis, 3rd Edition. US: Springer. p. 689.
- Hajlaoui MR, Benhamou N, Belanger NR. 1992. Cytochemical study of the antagonistic activity of Sporothrix flocculosa on rose powdery mildew, Sphaerotheca pannosa var. rosae. Phytopathology. 82:583–589. doi: https://doi.org/10.1094/Phyto-82-583
- Halo BA, Al-Yahyai RA, Al-Sadi AM. 2018. Aspergillus terreus inhibits growth and induces morphological abnormalities in Pythium aphanidermatum and suppresses Pythium-induced damping-off of cucumber. Front Microbiol. 9:95. doi: https://doi.org/10.3389/fmicb.2018.00095
- Harris RE, Bunch AW, Knowles CJ. 1987. Microbial cyanide and nitrile metabolism. Sci Prog. 71:293–304.
- Harris RE, Knowles CJ. 1983. Isolation and growth of a Pseudomonas species that utilizes cyanide as a source of nitrogen. J Gen Microbiol. 129:1005–1011.
- Harwood CR, Wipat A. 1996. Sequencing and functional analysis of the genome of Bacillus subtilis strain 168. FEBS Lett. 389:84–87. doi: https://doi.org/10.1016/0014-5793(96)00524-8
- Hu X, Roberts DP, Xie L, Maul JE, Yu C, Li Y, Jiang M, Liao X, Che Z, Liao X. 2014. Formulation of Bacillus subtilis BY-2 suppresses Sclerotinia sclerotiorum on oilseed rape in the field. Biol Control. 70:54–64. doi: https://doi.org/10.1016/j.biocontrol.2013.12.005
- Jayaraj J, Radhakrishnan NV, Kannan R, Sakthivel K, Suganya D, Venkatesan S, Velazhahan R. 2005. Development of new formulations of Bacillus subtilis for management of tomato damping-off caused by Pythium aphanidermatum. Biocontrol Sci Technol. 15:55–65. doi: https://doi.org/10.1080/09583150400015920
- Kandasamy S, Dananjeyan B, Krishnamurthy K, Benckiser G. 2015. Aerobic cyanide degradation by bacterial isolates from cassava factory wastewater. Braz J Microbiol. 46:659–666. doi: https://doi.org/10.1590/S1517-838246320130516
- Kotze C, Van Niekerk J, Mostert L, Halleen F, Fourie P. 2011. Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathol Mediterr. 50:S247–S263.
- Kunz DA, Wang CS, Chan JL. 1994. Alternative routes of enzymic cyanide metabolism in Pseudomonas fluorescens NCIMB 11764. Microbiology. 140:1705–1712. doi: https://doi.org/10.1099/13500872-140-7-1705
- Leifert C, Li H, Chidburee S, Hampson S, Workman S, Sigee D, Epton HA, Harbour A. 1995. Antibiotic production and biocontrol activity by Bacillus subtilis CL27 and Bacillus pumilus CL45. J Appl Bacteriol. 78:97–108. doi: https://doi.org/10.1111/j.1365-2672.1995.tb02829.x
- Lieberei R. 1988. Relationship of cyanogenic capacity of the rubber tree Hevea brasiliensis to susceptibility to Microcyclus ulei, the agent causing South American leaf blight. J Phytopathol. 122:54–67. doi: https://doi.org/10.1111/j.1439-0434.1988.tb00990.x
- Liu H, Carvalhais LC, Crawford M, Singh E, Dennis PG, Pieterse CMJ, Schenk PM. 2017. Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front Microbiol. 8:2552. doi: https://doi.org/10.3389/fmicb.2017.02552
- Luque-Almagro VM, Huertas MJ, Martinez-Luque M, Moreno-Vivian C, Roldan MD, Garcia-Gil LJ, Castillo F, Blasco R. 2005. Bacterial degradation of cyanide and its metal complexes under alkaline conditions. Appl Environ Microbiol. 71:940–947. doi: https://doi.org/10.1128/AEM.71.2.940-947.2005
- Luque-Almagro VM, Moreno-Vivian C, Roldan MD. 2016. Biodegradation of cyanide wastes from mining and jewellery industries. Curr Opin Biotechnol. 38:9–13. doi: https://doi.org/10.1016/j.copbio.2015.12.004
- Mirizadeh S, Yaghmaei S, Nejad ZG. 2014. Biodegradation of cyanide by a new isolated strain under alkaline conditions and optimization by response surface methodology (RSM). J Env Health Sci Eng. 12:85. doi: https://doi.org/10.1186/2052-336X-12-85
- Oros G, Kallai Z. 2019. Phytoanticipins: The Constitutive Defense compounds as potential Botanical Fungicides. In: JogaiahS, AbdelrahmanM, editors. Bioactive molecules in plant defense. Cham: Springer; p. 179–229.
- Osbourn AE. 1996. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell. 8:1821–1831. doi: https://doi.org/10.2307/3870232
- Pedras MSC, Yaya EE. 2015. Plant chemical defenses: are all constitutive antimicrobial metabolites phytoanticipins? Nat Prod Commun. 10:209–218.
- Piasecka A, Jedrzejczak-Rey N, Bednarek P. 2015. Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol. 206:948–964. doi: https://doi.org/10.1111/nph.13325
- Poulton JE. 1990. Cyanogenesis in plants. Plant Physiol. 94:401–405. doi: https://doi.org/10.1104/pp.94.2.401
- Razanamahandry LC, Onwordi CT, Saban W, Bashir AKH, Mekuto L, Malenga E, Manikandan E, Fosso-Kankeu E, Maaza M, Ntwampe SKO. 2019. Performance of various cyanide degrading bacteria on the biodegradation of free cyanide in water. J Hazard Mater. 380:120900. doi: https://doi.org/10.1016/j.jhazmat.2019.120900
- Rollinson G, Jones R, Meadows MP, Harris RE, Knowles CJ. 1987. The growth of a cyanide-utilizing strain of Pseudomonas fluorescens in liquid culture on nickel cyanide as a source of nitrogen. FEMS Microbiol Lett. 40:199–205. doi: https://doi.org/10.1111/j.1574-6968.1987.tb02025.x
- Shifa H, Gopalakrishnan C, Velazhahan R. 2015. Efficacy of Bacillus subtilis G1 in suppression of stem rot caused by Sclerotium rolfsii and growth promotion of groundnut. Int J Agric Environ Biotechnol. 8:91–98.
- Smith A, Mudder TI. 1991. The chemistry and treatment of cyanidation wastes. London, United Kingdom: Mining Journal Books Ltd.
- VanEtten HD, Mansfield JW, Bailey JA, Farmer EE. 1994. Two classes of plant antibiotics: Phytoalexins versus “phytoanticipins.”. Plant Cell. 6:1191–1192. doi: https://doi.org/10.2307/3869817
- Vetter J. 2000. Plant cyanogenic glycosides. Toxicon. 38:11–36. doi: https://doi.org/10.1016/S0041-0101(99)00128-2
- Wang P, Matthews DE, VanEtten HD. 1992. Purification and characterization of cyanide hydratase from the phytopathogenic fungus Gloeocercospora sorghi. Arch Biochem Biophys. 298:569–575. doi: https://doi.org/10.1016/0003-9861(92)90451-2
