?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Anhydrosafflor Yellow B (AHSYB), is an effective ingredient with a high content extracted from safflower. The effect of hydroxysafflor yellow A (HSYA), one of the main compounds in safflower, has been extensively investigated both in experimental study and in clinical practice. However, few studies of AHSYB on inflammation in rats with acute permanent cerebral ischemia was explored. In this study, three doses of AHSYB (1.75, 3.5, 7 mg/kg) were administered to rats. The results showed that AHSYB improved neurological deficit, reduced brain infarct volume, alleviated the brain inflammation and suppressed the elevation of both mRNA and protein levels of heat shock protein 60 (HSP60), toll-like receptor 4 (TLR-4), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). In addition, AHSYB treatment decreased NF-κB p65. All the above effects of AHSYB were more obvious at the dosage of 7 mg/kg. These findings suggest that AHSYB effectively alleviates brain injury followed by acute permanent cerebral ischemia in rats by anti-inflammatory mechanism.
Introduction
Acute ischemic stroke, accounting for more than 80% of all stroke, is a devastating neurological disorder with high morbidity and severe mortality (Mozaffarian et al. Citation2016; Sibani et al. Citation2019). Inflammation plays a very crucial role in the pathophysiological progress and endpoint outcome of ischemic stroke (Josef and Costantino Citation2016; Simone et al. Citation2017), since it can activate inflammatory response, featured by upregulation of proinflammatory cytokines, such as TNF-α and IL-6 (Minami et al. Citation2006). In consideration of the extreme importance of these proinflammatory cytokines for neuron dysfunction, even death in ischemic stroke (Griffiths et al. Citation2009), inhibiting inflammatory signal transduction might be a possible therapeutic strategy for this disease.
Safflower, as the dry flower of Carthamus tinctorius L, known as a blood stasis promoting herb in traditional Chinese medicine, has been extensively applied to treat several diseases such as trauma, cardiovascular disorders and postpartum illness for over 200 decades. The Safflower Injection, as a novel therapeutic drug, was approved for the treatment of cerebral ischemia by the State Food and Drug Administration of China in 2000. However, the responsible components for therapeutic effects of safflower were still not fully understood. Over 200 compounds were isolated from safflower, among which, the content of hydroxysafflor yellow A (HSYA) and AHSYB were higher (Li et al. Citation2011). There have been many researches on HSYA, but few studies on AHSYB, especially about the anti-infammatory effects of AHSYB on cerebral ischemia. Our research was designed to investigate whether AHSYB could alleviate brain injury followed by acute permanent cerebral ischemia in rats by anti-inflammatory mechanism.
Therefore, in this study, we aimed to examine: (1) whether AHSYB can alleviate rats brain injury triggered by acute permanent cerebral ischemia; (2) whether AHSYB has the anti-inflammatory effect and its underlying mechanism on rats brain impairment induced by acute permanent cerebral ischemia.
Materials and methods
Chemicals and reagents
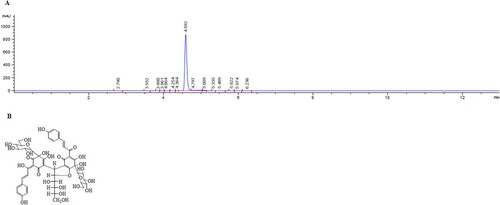
AHSYB (purity >97%, using HPLC as shown in Figure (A)) was purchased from Shanghai Standard Technology Co. Ltd (Shanghai, China). The chemical structure of AHSYB, with a molecular weight of 1044, is shown in Figure (B). The drug was dissolved in 0.9% saline and stored at the concentration of 14 mg/mL for cryopreservation, which could be thawed to the corresponding concentrations (7, 3.5 and 1.75 mg/mL). M-MLV Reverse Transcriptase, as well as Trizol Reagent, were obtained from Invitrogen Co. (Carlsbad, CA, USA). SYBR ® Premix Ex Taq™ (Perfect Real Time) kit was commercially bought from Takara Bio Inc. (Otsu, Shiga, Japan). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α and IL-6 were got from ExCell Biology Inc. (Shanghai, China). Antibodies against TLR-4, HSP60, β-actin, Lamin B1 and NF-κB p65 were purchased from Beyotime Institute of Biotechnology (Jiangsu, China).
Animal model and study design
The experimental procedures were approved by the Animal Ethics Committee of the Shanxi University of Chinese Medicine. Adult male Sprague–Dawley rats (weighing 220–240 g, aged 9–11weeks) were obtained from Chinese Academy of Military Medical Sciences (Grade SPF, CertiWcate No. SCXK (Jun) 2012-0004).
Rats were randomly divided into 5 groups (n = 18/group): control, model and AHSYB-treated groups (1.75, 3.5 and 7 mg/kg, respectively). The acute permanent cerebral ischemia was induced by middle cerebral artery occlusion (MCAO) in accordance with the modified monofilament method by Zea et al. (Citation1989). In brief, all the rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (350 mg/kg). Subsequently, a midline incision was made in the neck, the right internal carotid artery (ICA) as well as external carotid artery (ECA) were exposed and isolated. Afterwards, a nylon monofilament (0.24 mm of diameter and 40 mm of length) was inserted into the right ICA and ECA to occlude the right MCA. The rats in control group were taken the same surgical procedure without the monofilament insertion. Upon ischemia, AHSYB was immediately injected via sublingual vein, while the rats in model and control groups were treated with saline.
The neurological deficits of all rats were evaluated before MCAO and on the time-point of 24 h after MCAO in a blinded fashion according to the five-point scale system from Longa (Zea et al. Citation1989) by two investigators. The five-point scale system were defined as follows: being unable to walk spontaneously or in a comatose state, 4; falling to left, 3; circling to left, 2; being unable to extend left paw fully, 1; no neurological deficit, 0. The blood was collected from rat orbit under chloral hydrate anesthesia. Finally, the rats were sacrificed for the following assessments, six for 2,3,5-tri-phenyltetrazolium chloride (TTC) staining, six for histological assessment and immunohistochemistry (IHC) analysis and another six for reverse transcription-polymerase chain reaction (RT–PCR) analysis and Western Blot (WB) analysis.
TTC staining
All of the rats brains were in a coronal section cut into five 2-mm-thick slices and then stained in 1% TTC at 37°C for 30 min. The TTC-stained sections were photographed and measured by the system of Image Pro-Plus 6.0 in a blinded fashion. The brain infarct volumes in rats were showed as the ratio to the contralateral hemisphere volume. The percentage of infarct volume was calculated by the following equation: ([total contralateral hemispheric volume] − [total ipsilateral hemispheric stained volume]) / (total contralateral hemispheric volume) × 100%.
Histological assessment
The ischemic cerebral cortex specimens were fixed in 10% neutral formalin, embedded in paraffin and sectioned. The sections were stained with hematoxylin and eosin (HE) and Massons’s trichrome in line with conventional methods. The slides were evaluated under a light microscope (Nikon Eclipse 90i).
RT–PCR analysis
The ischemic cerebral cortex was prepared to analyze the expression of TLR-4, HSP60, TNF-α and IL-6 mRNA. Total RNA was isolated using TRIZOL reagent according to the manufacturer's instructions. RNA purity and concentration were assayed with Thermo Scientific NanoDrop 2000 (Wilmington, USA). The mRNAs were then reversetranscribed directly into cDNA using a RT–PCR kit. Target mRNA was quantified by real-time PCR using the SYBR® Premix Ex Taq™ kit on a Bio-Rad iCycler iQ5 Real-time Detection System. PCR amplification conditions were as follows: initial denaturation at 95°C for 15 s followed by 35 cycles of denaturation at 95°C for 5 s and annealing at 61°C for 15 s. The sequences of the respective sense and antisense primers were listed below (from 5′ to 3′): GACATCAAGAAGGTGGTGAAGC and TGTCATTGAGAGCAATGCCAGC for GAPDH; AGTTGAGGGGACTTTCCCAGGC and TCAACTCCCCTGAAAGGGTCCG for TLR-4; CCGCCCCGCAGAAATGCTTCGA and AGGCTCGAGCATCCGCACCAA for HSP60; GCCAATGGCATGGATCTCAA and ACTTGGGCAGGTTGACCTCA for TNF-α; GCTCTGGTCTTCTGGAGTTCC and GAGTTGGATGGTCTT GGTCCT for IL-6. Relative expression was quantified using 2ΔΔCt method by normalization to internal control, .
IHC analysis
After being rehydrated in xylene and graded ethanol solutions, coronal slides of cerebral cortex were exposed to methanolhydrogen peroxide for 10 min, washed in phosphate buffered saline (PBS). The slides were blocked with serum for 20 min and incubated with primary antibodies against TLR-4 and HSP60 at 4°C overnight. After being washed three times with PBS, the slides were incubated with secondary antibodies at room temperature (RT) for 20 min. Slides were then washed with PBS and subsequently incubated with streptavidin–biotin–peroxidase complex for 10 min. Diaminobenzidine was then added for visualization and nuclei were counterstained with hematoxylin. Expression of TLR-4 and HSP60 were compared between groups by calculating the numbers of the positive stained cells (brown) from 10 random fields of each slide under light microscope (400 magnification).
WB analysis
The rat brains were shredded for NF-κB p65 level by WB. The protein was extracted using a Nuclear-Cytosol Extraction kit (Beyotime Institute of Biotechnology) in accordance with the manufacturer's instructions, and the content was determined using the BCA kit. β-actin, Lamin B1 and NF-κB p65 were assayed. The proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes by a semi-dry transfer method. After being blocked with 5% non-fatty milk in PBS, the membranes were incubated with the primary antibodies against β-actin, Lamin B1 and NF-κB p65 (1:1000 dilution in PBS) at 4°C overnight. After being washed three times with PBS-T (PBS + 0.1% Tween 20), the membranes were incubated with HRP-conjugated secondary antibodies (1:1000 dilution in PBS) at RT for 1 h. The specific protein bands were visualized by enhanced chemiluminescence (ECL) according to the manufacturer's instructions. The bands were then quantified by densitometric scanning.
ELISA
Inflammatory factors including TNF-α and IL-6 were detected by commercial ELISA kits. The experimental procedures were followed in accordance with the instructions designated by the manufacturer.
Statistical analysis
The results were analyzed by one-way analysis of variance (ANOVA) using GraphPad Prism 5 software. All of the data were shown as mean ± standard deviation (S.D). The p value of less than .05 was defined statistically significant.
Results
AHSYB improved the behavioral function in acute permanent cerebral ischemia rats
To explore the effect of AHSYB on neurological function followed by acute permanent cerebral ischemia, the neurological deficit score of rats after 24 h-ischemia were evaluated in accordance with the five-point scale system from Longa. As shown in Figure (A), neurological deficit score in the model group was significantly increased compared with sham group. AHSYB treatment at all three doses remarkably improved the neurological deficit of the MCAO rats, especially at the dosage of 7 mg/kg.
Figure 2. Effect of AHSYB on behavioral function (A) and brain infarct volume (B and C) in acute permanent cerebral ischemia rats. (A) Data are presented as mean ± S.D(n = 18), *p < .05, **p < .01 versus model group. (B) TTC staining. (C) Data are showed as mean ± S.D (n = 6),**p < .01, *** p < .001 versus model group.
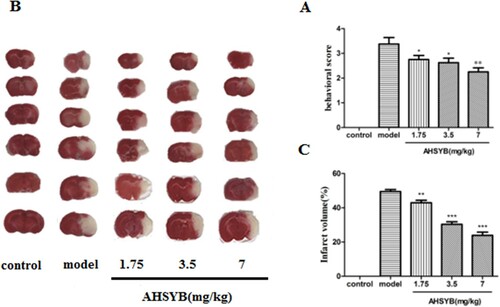
AHSYB decreased the brain infarct volume in acute permanent cerebral ischemia rats
Infarct volume of rats was measured by TTC staining after acute permanent cerebral ischemia. As displayed in Figure (B,C), no injured area was found in control group, while an extensive lesion was found in model group. However, compared with the model group, the cerebral infarct volumes in all three groups of AHSYB treatment were obviously decreased with a dose-dependent manner.
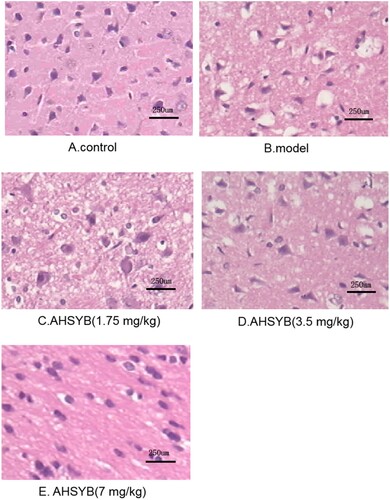
AHSYB alleviated the pathological impairment followed by acute permanent cerebral ischemia in rats
As shown in Figure (A), cerebral cortex from the control group showed normal structure without pathological changes. On the contrary, ischemic cerebral cortex from the model group exhibited markedly destructive characteristics: reduced neurons, shrunken cell bodies and infiltration of multiple inflammatory cells (Figure (B)). AHSYB treatment significantly attenuated the above pathological changes (Figure (C–E)) and the effect of AHSYB was most significant at the dosage of 7 mg/kg (Figure (E)).
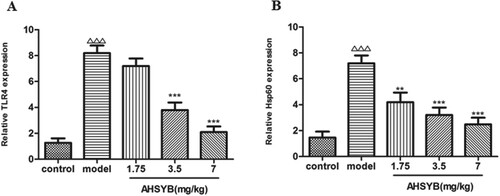
AHSYB decreased the mRNA expressions of TLR-4 and HSP60 in cerebral cortex of rats with acute permanent cerebral ischemia
RT–PCR was performed to measure the roles of AHSYB on mRNA expressions of TLR-4 and HSP60 after acute permanent cerebral ischemia. As shown in Figure , TLR-4 and HSP60 mRNA increased significantly after acute permanent cerebral ischemia and these changes were attenuated by AHSYB treatment. The effect was the most significant with AHSYB treatment at the dosage of 7 mg/kg.
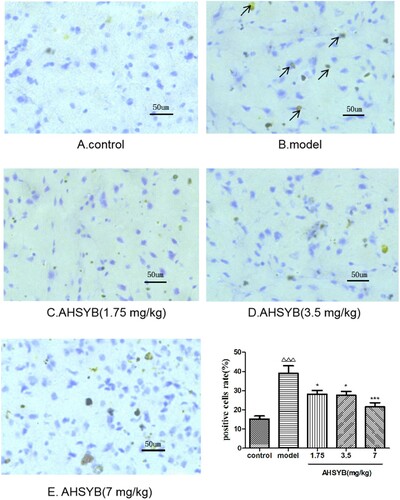
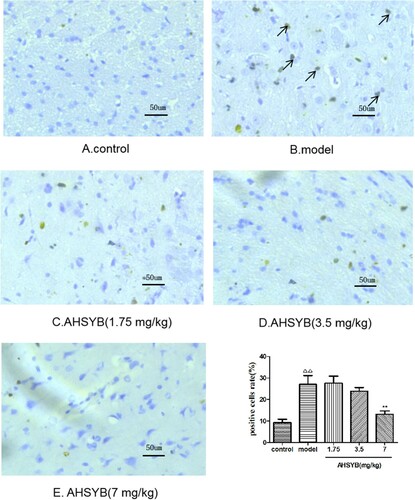
AHSYB suppressed TLR-4 protein expression in cerebral cortex of rats with acute permanent cerebral ischemia
After acute permanent cerebral ischemia, more TLR-4 was expressed. Positive staining for TLR-4 was brown. As shown in Figure , the TLR-4 positive cells were limited in the rats of control group (Figure (A)), which significantly increased in model group (Figure (B)). However, AHSYB treatment resulted in the decrease of TLR-4 positive cells (Figure (C–E)), which was obviously at the dosage of 7 mg/kg (Figure (E)).
AHSYB suppressed HSP60 protein expression in cerebral cortex of rats with acute permanent cerebral ischemia
After acute permanent cerebral ischemia, more HSP60 was expressed. Positive staining for HSP60 was brown. As shown in Figure , the HSP60 positive cells were rare in the rats of control group (Figure (A)), which significantly increased in model group (Figure (B)). Moreover, AHSYB administration decreased the HSP60 positive cells (Figure (C–E), which was obvious at the dosage of 7 mg/kg (Figure (E)).
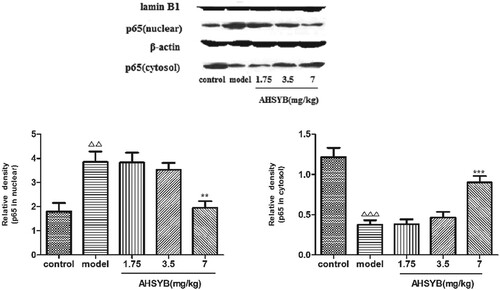
AHSYB suppressed NF-κB p65 translocation in cerebral cortex of rats with acute permanent cerebral ischemia
NF-κB, as a critical transcription factor, modulates multiple gene expression, including chemokines, cytokines as well as growth factors. WB assay was conducted to assess nuclear translocation of NF-κB p65. As displayed in Figure , after acute permanent cerebral ischemia, p65 level in cytosol was significantly reduced, while that in nucleus was conversely increased compared with control group. However, in AHSYB groups, the level of cytosolic p65 were elevated and nucleic p65 were decreased compared with the model group, which was most significant in the AHSYB group with highest dose. The results indicate that the nuclear translocation of p65 protein induced by acute permanent cerebral ischemia can be attenuated by AHSYB treatment in rats.
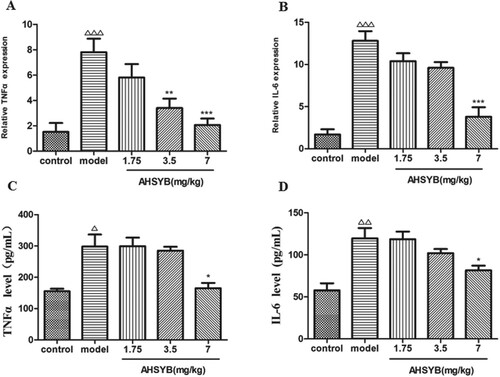
AHSYB decreased the mRNA expression and protein level of cytokines TNF-α and IL-6 in cerebral cortex and sera of rats with acute permanent cerebral ischemia
RT–PCR was performed to measure the roles of AHSYB on mRNA expression of inflammatory factors including TNF-α and IL-6 after acute permanent cerebral ischemia. As shown in Figure (A,B), TNF-α and IL-6 mRNA in brain increased significantly after acute permanent cerebral ischemia and these changes were attenuated by AHSYB treatment. The effect was the most significant with AHSYB treatment at the dosage of 7 mg/kg. As shown in Figure (C,D), TNF-α and IL-6 protein levels in the rats sera were obviously enhanced in model group than in control group and AHSYB treatment with the dosage of 7 mg/kg suppressed the increase.
Figure 8. Effect of AHSYB on the mRNA expression (A and B) and protein level (C and D) of cytokines TNF-α and IL-6 in cerebral cortex of rats with acute permanent cerebral ischemia. (A and B) Data are presented as mean ± S.D (n = 6), ΔΔΔp < .001 versus control group, ** p < .01, ***p < .001 versus model group. (C and D) Data are presented as mean ± S.D. (n = 6), Δp < .05, ΔΔp < .01 versus control group, *p < .05 versus model group.
Discussion
Nowadays, ischemic stroke has become a serious public health threat in developing countries. Many mechanisms contributes to the injury induced by cerebral ischemia, such as free radical injury, inflammatory response, calcium overload and others (Famakin Citation2014). Among these mechanisms, inflammatory response is confirmed to be the leading cause (Petrovic-Djergovic et al. Citation2016). Within several minutes after cerebral ischemia, the proinflammatory responses are initiated and then cause tissue injury, inappropriate cellular repairment and dysfunction, so suppressing the initial inflammatory response might be a potent treatment for acute ischemic stroke (Fisher and Saver Citation2015). Some traditional Chinese medical herbs have attracted popular attention for their treatment of ischemic stroke.
Because of its minor side effects and satisfactory curative effects demonstrated by lots of clinical studies, the safflower injection becomes more and more popular in the clinical use (Jiang and Li Citation2017). Previous researches confirmed that HSYA was the main effective substance. However, in more and more recent studies researchers found that in addition to HSYA, AHSYB is also the main bioactive ingredient (Li et al. Citation2011; Qu et al. Citation2016). Some researches found that HSYA could attenuate ischemia cerebral injury by anti-inflammatory mechanism (Jin et al. Citation2013; Sun et al. Citation2012; Song et al. Citation2013; Chen et al. Citation2019). However, there has been little study about the anti-inflammatory effect of AHSYB on brain injury induced by acute permanent cerebral ischemia. Thus, our study was designed to explore the anti-inflammatory mechanism of AHSYB on brain injury followed by acute permanent cerebral ischemia in rats.
In this study, we performed MCAO on rat to mimic acute permanent cerebral ischemia in human. The results of neurological deficit scores and infarct volume showed that the model used here was successful. AHSYB treatment obviously reduced the neurological deficit scores and the infarct volume in a dose-dependent manner. HE staining was always used to assess the brain pathologic changes. In our study, we found AHSYB administration significantly ameliorated the related pathological changes. These results indicated that AHSYB had protective effects against brain injury induced by acute permanent cerebral ischemia.
Toll-like receptors (TLRs), a transmembrane pattern-recognition receptor family, are critically involved in the induction and modulation of inflammatory/immune responses. TLR-4 has been confirmed to participate in certain pathological conditions, including cerebral ischemia injury, cardiac diseases and sepsis. Various researches have shown the activation of TLR-4-mediated signaling after ischemia contributes to enhanced inflammatory responses as well as subsequent brain damage (Hua et al. Citation2009; Buchanan et al. Citation2010; Hua et al. Citation2015; Mahban et al. Citation2017). TLR-4, as an essential receptor of innate immune, contains multiple ligands including heat shock proteins, bacterial muramic acid, lipopolysaccharide and flagella (Triantafilou and Triantafilou Citation2004; Tsan and Gao Citation2004). Among them, HSP60 is an endogenous ligand of TLR-4 (Lehnardt Citation2008; Li Citation2011). In addition, HSP60 release in response to central nervous system (CNS) damage has been reported, followed by subsequent activation of innate immunity in a TLR-4-dependent pattern (Lehnardt Citation2008). In this study, both mRNA and protein expression of TLR-4 and HSP60 were up-regulated following acute permanent cerebral ischemia, which could be suppressed by AHSYB.
When TLR-4 is exposed to its ligands, NF-κB can be activated. NF-κB, a vital transcription factor, can modulate not only gene expression involved in psychologically immunological responses under resting state, but also gene expression of multiple inflammatory cytokines in the case of inflammation (Jordi et al. Citation2008; Durand and Baldwin Citation2017). The translocation of NF-κ B p65 into nuclei indicates the activation of NF-κB. Accumulating evidences have shown that activated NF-κB contributes to brain ischemia injury (Zhang et al. Citation2008). Here, acute permanent cerebral ischemia induced the nuclear translation of NF-κB p65 in brain. However, AHSYB treatments obviously down-regulated the nuclear translation of NF-κB p65. The result suggested that AHSYB could inhibit NF-κB p65 activation.
The activation of NF-κB p65 can subsequently lead to related cytokines secretion. Proinflammatory cytokines, such as TNF-α and IL-6, are generally increased during the early phase of inflammation in acute permanent cerebral ischemia (Zhang et al. Citation2008). Persistent increased levels of these proinflammatory cytokines have a correlation with worse outcome of acute permanent cerebral ischemia (Zhang et al. Citation2008). The results showed both mRNA and protein expression of TNF-α and IL-6 were increased following acute permanent cerebral ischemia, which were effectively inhibited by AHSYB administration.
In the present study, we found that AHSYB decreased the behavior score, infarct volume and ameliorated brain pathologic changes in rats induced by acute permanent cerebral ischemia. Moreover, AHSYB had anti-inflammatory effect on brain followed by acute permanent cerebral ischemia in rats by attenuating the elevated mRNA and protein levels of TLR-4 and HSP60, inhibiting NF-κB activation and reducing the augmented expression of proinflammatory cytokines.
Conclusion
All of the results strongly indicated that AHSYB alleviated brain injury followed by acute permanent cerebral ischemia in rats by anti-inflammatory mechanism. The study provided some scientific evidences for the cerebral protection effects of AHSYB, indicating that AHSYB might be a potentially novel therapeutic agent for the inflammatory disorders, including acute ischemic stroke.
Acknowledgements
This work was sponsored by Shanxi Applied Basic Research Project (No. 201901D211538), Science and Technology Innovation Project of Colleges and Universities in Shanxi Province (No. 2019L0734), Open Fund of Shanxi Key Laboratory of Inflammatory Neurodegenerative Diseases in Shanxi Datong University (No. KF2019002), Scientific Research Program of Traditional Chinese Medicine Administration in Shanxi Province (No. 2016ZYYZ02) and Astragalus Resource Industrialization and Industrial Internationalization Cooperative Innovation Center Project of Shanxi Province (No. HQXTCXZX2016-028).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Buchanan MM, Hutchinson M, Watkins LR, Yin H. 2010. Toll-like receptor 4 in CNS pathologies. J Neurochem. 114:13–27.
- Chen S, Sun M, Zhao XH, Yang ZF, Liu WX, Cao JY. 2019. Neuroprotection of hydroxysafflor yellow A in experimental cerebral ischemia/reperfusion injury via metabolic inhibition of phenylalanine and mitochondrial biogenesis. Mol Med Rep. 19:3009–3020.
- Durand JK, Baldwin AS. 2017. Targeting IKK and NF-κB for therapy. Adv Protein Chem Struct Biol. 107:77–115. doi: https://doi.org/10.1016/bs.apcsb.2016.11.006
- Famakin BM. 2014. The immune response to acute focal cerebral ischemia and associated post-stroke immunodepression: a focused review. Aging Dis. 5:307–326.
- Fisher M, Saver JL. 2015. Future directions of acute ischaemic stroke therapy. Lancet Neurol. 14:758–767. doi: https://doi.org/10.1016/S1474-4422(15)00054-X
- Griffiths MR, Gasque P, Neal JW. 2009. The multiple roles of the innate immune AHSYB stem in the regulation of apoptosis and inflammation in the brain. J Neuropathol Exp Neurol. 68:217–226. doi: https://doi.org/10.1097/NEN.0b013e3181996688
- Hua F, Ma J, Ha T, Kelley JL, Kao RL, Schweitzer JB. 2009. Differential roles of TLR2 and TLR-4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. 1262:100–108. doi: https://doi.org/10.1016/j.brainres.2009.01.018
- Hua F, Tang HL, Wang J, Megan CP, Hua XD, Donald GS. 2015. TAK-242, an antagonist for toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cerebral Blood Flow Metab. 2:1–7.
- Jiang W, Li GL. 2017. Neurology, clinical study on Safflower injection combined with cilostazol in treatment of acute ischemic cerebral infarction. Drugs and Clinic. 32:617–620.
- Jin M, Sun CY, Pei CQ, Wang L, Zhang PC. 2013. Effect of safflor yellow injection on inhibiting lipopolysaccharide induced pulmonary infammatory injury in mice. Chin J Integrat Med. 19:836–843. doi: https://doi.org/10.1007/s11655-012-1151-6
- Jordi R, Monica G, Christian S, Katerina A, Annelies SZ, Victor N, Randall SJ, Gabriel GH, Karin M. 2008. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 453:807–811. doi: https://doi.org/10.1038/nature06905
- Josef A, Costantino I. 2016. Inflammation and stroke: an overview. Neurotherapeutics. 13:661–670. doi: https://doi.org/10.1007/s13311-016-0483-x
- Lehnardt S. 2008. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 28:2320–2331. doi: https://doi.org/10.1523/JNEUROSCI.4760-07.2008
- Li Y. 2011. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and toll-like receptor 4. J Biol Chem. 286:31308–31319. doi: https://doi.org/10.1074/jbc.M111.246124
- Li F, Run P, Zhao HY, Liu X, Ma C, Wang BR. 2011. Stability and degradation of hydroxysafflor yellow A and anhydrosafflor yellow B in the safflower injection studied by HPLC-DAD-ESI-MS. J Chin Pharmaceut Sci. 20:47–56.
- Mahban R, Faheem M, Shermineh M, Kamal N, Mohammad A, Nady B, Seyed MN, Seyed FN. 2017. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. 36:11–19. doi: https://doi.org/10.1016/j.arr.2017.02.004
- Minami M, Katayama T, Satoh M. 2006. Brain cytokines and chemokines: roles in ischemic injury and pain. J Pharmacol Sci. 100:461–470. doi: https://doi.org/10.1254/jphs.CRJ06005X
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ. 2016. Executive summary: heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 133:447–454. doi: https://doi.org/10.1161/CIR.0000000000000366
- Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. 2016. Inflammatory disequilibrium in stroke. Circ Res. 119:142–158. doi: https://doi.org/10.1161/CIRCRESAHA.116.308022
- Qu C, Wang LY, Jin WT. 2016. Comparative analysis of the effects of hydroxysafor yellow A and anhydrosafor yellow B in safflower series of herb pairs using prep-HPLC and a selective knock-out approach. Molecules. 21:1480. doi: https://doi.org/10.3390/molecules21111480
- Sibani S, Dipankar C, Arijit B, Mrinal KG. 2019. Cerebral ischemic stroke: cellular fate and therapeutic opportunities. Front Biosci. 24:435–450. doi: https://doi.org/10.2741/4727
- Simone V, Arturo C, Marco A, Domenico C. 2017. Postischemic inflammation in acute stroke. J Clin Neurol. 13:1–9. doi: https://doi.org/10.3988/jcn.2017.13.1.1
- Song LJ, Zhu Y, Jin M, Zang BX. 2013. Hydroxysafflflor yellow A inhibits lipopolysaccharide-induced inflflammatory signal transduction in human alveolar epithelial A549 cells. Fitoterapia. 84:107–114. doi: https://doi.org/10.1016/j.fitote.2012.11.004
- Sun L, Yang L, Xu YW, Liang H, Han J, Zhao RJ. 2012. Neuroprotection of hydroxysafflor yellow A in the transient focal ischemia: Inhibition of protein oxidation/nitration, 12/15-lipoxygenase and blood-brain barrier disruption. Brain Res. 1473:227–235. doi: https://doi.org/10.1016/j.brainres.2012.07.047
- Triantafilou M, Triantafilou K. 2004. Heat-shock protein 70 and heat-shock protein 90 associate with toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans. 32:636–639. doi: https://doi.org/10.1042/BST0320636
- Tsan MF, Gao B. 2004. Endogenous ligands of toll-like receptors. J Leukoc Biol. 76:514–519. doi: https://doi.org/10.1189/jlb.0304127
- Zea LE, Weinstein PR, Carlson S, Cummins R. 1989. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20:84–91. doi: https://doi.org/10.1161/01.STR.20.1.84
- Zhang HL, Gu ZL, Savitz SI, Han F, Fukunaga K, Qin ZH. 2008. Neuroprotective effects of prostaglandin A1 in rat models of permanent focal cerebral ischemia are associated with nuclear factor-κB inhibition and peroxisome proliferator activated receptor-up-regulation. J Neurosci Res. 86:1132–1141. doi: https://doi.org/10.1002/jnr.21569