Abstract
Tumor necrosis factor-α induced protein 8 like 2 (TIPE2) is one of the newly discovered negative regulators for body’s immune balance. The present study aimed to investigate the effect of TIPE2 gene-modified human amniotic mesenchymal stem cells (hAD-MSCs) on immune tolerance. In this study, the TIPE2 over-expressed and the non-transfected hAD-MSCs were severally co-cultured with injured cardiomyocytes. Cell cycle and apoptosis were detected by flow cytometry. Cell viability was measured by MTT, and expressions of immune-related factors were detected by qRT-PCR and western blot. When compared with the empty vector-transfected hAD-MSCs, the TIPE2-overexpression hAD-MSCs co-cultured with injured cardiomyocytes show accelerated cell viability and declined apoptosis. After TIPE2 over-expression, the mRNA and protein levels of p38, extracellular signal-regulated kinases (ERK) and interferon-γ (INF-γ) notably decreased, whereas those of transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) increased in a converse trend. This study suggested that TIPE2 may enhance the cellular immune tolerance in co-culture systems of hAD-MSCs and injured cardiomyocyte, providing a theoretical basis for the allogeneic heart transplantation.
Introduction
Heart transplantation is the only method to cure end-stage heart disease. Immunosuppression and myocardial ischemia-reperfusion are the major obstacles for heart transplantation. Inducing a donor-specific immune tolerance is the best way to alleviate the rejection. Most of the acute immune rejections can be efficiently suppressed through immunosuppression such as calcineurin inhibitors, cyclosporin A and everolimus, but the immune tolerance induction of graft after drug withdrawal cannot be maintained in that way (Shah et al. Citation2019). Regulatory T cells, costimulatory signals and exosomes are used for immune induction in clinical heart transplantation (Madsen Citation2017). The antilymphocyte globulin, antithymocyte globulin, and IL-2 receptor antagonist are the main drug for immunoinduction therapy, while immunoinduction therapy does not benefit all recipients (Yusen et al. Citation2016). Thus, the clinical focus turned to develop a drug for continuous maintenance immunosuppression. T-helper1 (Thl) cytokines are related to transplant rejection, while Th2 cytokines are related to the formation of immune tolerance after transplantation. And immune tolerance maintaining is established on the base of up-regulated Th2 cells with the down-regulation of Th1 (D. X. Li et al. Citation2014). In the rat model of heart transplantation, when compared with the control they found that the immune-tolerance group revealed the up-regulated Th2 cytokines IL-4 and IL-10 and the down-regulated Th1 cytokines INF-γ and IL-2, but the condition of rejection group was opposite (Bao et al. Citation2015).
Mesenchymal stem cells (MSCs) are more than a kind of adult stem cells of high self-replication, multi-directional differentiation, cell implantation promotion and hematopoietic support but also strongly plastic and immunomodulatory, which can inhibit immune allo-response, thus inducing transplantation tolerance and a life-long immune suppression (Chamberlain et al. Citation2007; Podesta et al. Citation2019). The immunoregulatory properties of MSC are comprised of inhibition to T cell proliferation, and regulation to innate immune cell populations (monocytes, macrophages, dendritic cells, neutrophils, natural killer cells, and mast cells) and adaptive immunity (Th1, B lymphocyte, and cytotoxic T lymphocyte) (Kariminekoo et al. Citation2016; Podesta et al. Citation2019). Immunoregulatory function of MSCs is also mediated by the secretion of immunosuppressive factors such as 2, 3-diioxygenase, Prostaglandin E2, IL-10, TGF-β, and induction of regulatory T cell (Treg) (Soleymaninejadian et al. Citation2012). Tregs play a key role in the immune tolerance of heart transplantation, and it induces immune tolerance by secreting cytokines TGF-β/IL-10 and inhibiting IL-2/INF-γ transcription (Klaeske et al. Citation2020). The TGF-β-induced Treg cells migration to transplanted heart surface enables to prolong the life-span of allograft survival in mice (Ozdemir et al. Citation2006). Plus, hAD-MSCs also manifest immunosuppressive properties (Alikarami et al. Citation2015). And particularly, it is more remarkable to be observed in the plasticity study of hAD-MSCs (Valarmathi et al. Citation2018).
TIPE2 belongs to tumor necrosis factor alpha-inducible protein 8 (TNFAIP8) family and is one of the newly discovered negative regulators of body’s immune balance (Sun et al. Citation2008; Oho et al. Citation2016). And it is an independent gene discovered in 2002 (Strausberg et al. Citation2002). Human and mouse TIPE2 genes are located on chromosome 1 (1q21.2-1q21.3) and chromosome 3 (3f1-3f3) (Strausberg et al. Citation2002). TIPE2 regulates innate and adaptive immunity to maintain immune tolerance by inhibiting the activation pathways of Toll-like receptor and T-cell receptor (Sun et al. Citation2008). TIPE2 also involves in the inhibitions of multiple signaling pathways, such as the NF-κB, AKT and ERK1/2 signaling, and the phosphorylation of JNK and p38 (Y. Zhu et al. Citation2016; R. Liu et al. Citation2017). Cell surface molecules as well as the cytokines (IL-10, TGF-β) dropped markedly when TIPE2 was silenced by siRNA (Luan et al. Citation2011). So far, there are few reports on the relationship between allogeneic organ transplantation and TIPE2 expression. TIPE2 may be recognized as one of diagnosis molecular markers in patients of renal allograft rejection (Jia et al. Citation2013). The TIPE2 level in peripheral blood mononuclear cells of the patients with acute rejection due to kidney transplant was lessened, moreover, that of the allograft tissue of chronic rejection was even lower (Jia et al. Citation2013). The overexpression of TIPE2 prolongs the survival of rat allogeneic heart allografts and the expression of TIPE2 in the treatment of immunosuppressive agents (FK506) group higher than in control group (Youbin et al. Citation2015).
These data indicate that TIPE2 may play an irreplaceable regulatory role in heart transplantation. Despite some research foundation, there are still questions: Does TIPE2 overexpression induce a better and more durable immune tolerance? What effect and mechanism is the cytokines secreted by hAD-MSCs on microenvironment to induce an immune tolerance? Is the TIPE2 expression in hAD-MSCs positively correlated with the immune tolerance persistence in vitro or in vivo? Based on the previous researches and these questions, we hypothesized that TIPE2 inactivation increases cardiac allograft rejection/ischemic reperfusion injury. If the hypothesis is established, it will bring new ideas to transplant immunization and regenerative medicine. This research is to discover the positive role of TIPE2 in immune tolerance of heart transplantation.
Materials and methods
Extraction of human umbilical cord blood serum
The placenta specimens were obtained from the amniotic membrane of pregnant women who had been delivered by cesarean section. The whole process is informed and consented. The experiment was approved by the West China Hospital Ethics Committee. The collected cord blood was stored in a 50 mL centrifuge tube at 4°C, treated within 6 h, centrifuged at 3000 r/min for 10 min, and the serum was aspirated, filtered and dispensed, and stored at −20°C.
Animals
A total of 40 SPF male C57BL/6 (H-2d) and BALB/c (B6; H-2b) mice (8–12 wk) that weighed 25–30 g (Ensiweier biotechnology co. LTD, Chongqing, China) was used for transplantation. The hearts of BALB/c mice (20) were transplanted into the neck of C57BL/6 mice (20). The inbreeding algebra of BALB/c mice were the 108th generation and C57BL/6 mice were 32th generation. All mice were housed under specific pathogen-free facility. We performed heterotopic heart transplantation, in which the descending aorta or infrarenal aorta from the donor mouse was anastomosed to the infrarenal aorta using a cuff technique as describe by P. Zhu et al. (Citation2016). All animals received humane care in compliance with the National Institutes of Health Principles of Laboratory Animal Care and approved by the West China Hospital Ethics Committee.
Isolation, culture and enrichment of human amniotic mesenchymal stem cells
Under aseptic condition, the amniotic membrane was peeled off from the placenta tissue by a mechanical method and washed with D-Hank’s liquid several times to remove residual blood, amnion was cut into pieces and digested 4 times with 0.05% trypsin (Biyuntian, China) containing 0.02% EDTA. Used 0.75 mg/mL type 2 collagen solution (Biyuntian, China) with 0.075 mg/mL DnaseI for shaking amniotic membrane at 37°C, rotated at 2000 r/min for about 2 h until the tissue was digested. Subsequently, filtered through 300 mesh steel mesh to collect the cell filtrate, centrifuged at 1500 r/min for 10 min. The cell pellet was resuspended in LG-DMEM medium (Gibco, USA) containing 5–10% human cord blood serum, inoculated in a 25 cm2 culture flask (Costar, USA) at a cell density of 5 × 105/mL under 37°C, 5% CO2 incubation condition, saturated humidity the CO2 incubator (Sanyo, Japan), and the new medium was replaced on the 3rd day.
Identification of human amniotic mesenchymal stem cells
Flow cytometry was used to detect the surface markers of human amniotic mesenchymal stem cells. The third-generation cells after 80% fusion were taken, digested with 0.125% trypsin, washed twice with PBS (Beijing Zhongshan, China), and made to a concentration of 2 × 10 with PBS. The 9L-1 suspension was filtered through a 300 mesh nylon mesh. The number of CD105, CD44, CD73, CD90 positive cells and CD34, CD45, CD19, CD11B negative cells were detected by flow cytometry (BD FACSCalibur, Becton Dickinson, Mountain View, CA, USA). The following antibodies were used: anti-CD105 (Abcam, ab11414, 1:100), anti-CD44 (Abcam, ab157107, 1:100), anti-CD73 (Abcam, ab175396, 1:100), anti-CD90 (Abcam, ab133350, 1:100), anti-CD34 (Abcam, ab81289, 1:100), anti-CD45 (Abcam, ab10558, 1:100), anti-CD19 (Abcam, ab134114, 1:100), anti-CD11B (Abcam, ab25533, 1:100).
Isolation, culture and identification of cardiomyocytes
The isolation and culture of mouse cardiomyocytes were described by D. X. Li et al. (Citation2014). The cultured cardiomyocytes were digested with 0.25% trypsin (Biyuntian, China). The supernatant was removed after centrifugation for 5 min. The cells were resuspended by adding 1 mL of pre-cooled PBS. Centrifuge again to remove the supernatant. Add 50 µL a-actin antibody (Abcam, USA) to each well (antibody diluted with Staining Buffer). Then, 50 µL cell suspension into each well and mix gently, incubation in 4°C refrigerator for 20 min. After centrifugation and washing, 100 µL resuspended cells was detected by flow cytometry.
TIPE2 gene modification hAD-MSCs
TIPE2 was identified by the BLAST search of the National Center for Biotechnology Information (GenBank accession number NM_001014039.1). The full-length TIPE2 was then sequenced and cloned into the expression vector pDC316 with a GFP tag. TIPE2 gene overexpressing adenovirus were produced by Chongqing Biomedicine biotechnology co. LTD (Chongqing, China) according to the two plasmid system instructions as described by Bett et al. (Citation1994). The recombinant plasmid was transfected into 293A cells with the transfection reagent (Lipofectamine 2000, Invitrogen), transfection methods followed the manufacturer’s instructions. The fusion rate of 293A cells was 60–80% at transfection, and on the third day, the medium was changed to fresh medium. Then, the 293A cells were cultured for 48 h for fluorescence observation (CKX53, OLYMPUS, Japan) and flow cytometry to determine transfection efficiency, the positive rate of GFP protein was more than 80%. The virus titer of TIPE2 adenovirus was 1.5 × 1010 TU/mL, control was 2.0 × 1010 TU/mL. The hAD-MSC cell were grown in 6-well plates and transfected with TIPE2 adenovirus, cells were harvested 72 h after transfection for protein. Western blot was used to detect transfection efficiency.
In vitro co-culture experiment
The mechanism of proliferation and differentiation of null-hAD-MSCs (or TIPE2 gene-modified hAD-MSCs) and their interaction were studied in vitro. After the heart transplantation mouse model was established, the damaged cardiomyocytes and hAD-MSCs were co-cultured in two ways: non-contact Transwell (Corning, CA, USA) co-culture and direct contact co-culture to observe the proliferation and differentiation of hAD-MSCs after co-culture.
Cell cycle analysis by propidium iodide (PI) staining
Cells in the growing period were added with 3 mL PBS, removing the liquid, and digested 1–5 min with 1 mL trypsin and resuspended with 5 mL PBS. Then, transferred it to a 15 mL centrifuge tube for centrifuging at 1500 rpm/min to remove the supernatant. Adding 2 mL cold ethanol (95%) to fix 30 min, after centrifugation, adding 500 µL PBS to incubate for 30 min. Finally, 800 µL of PI staining solution (Becton Dickinson, USA) was added at 37°C for staining and analyzed using the flow cytometry.
Determination of apoptotic cells by Annexin V assay
The apoptosis of co-culture cells was assessed using the Annexin-APC-A staining kit (Becton Dickinson, USA), according to the manufacturer’s instructions. Briefly, the cells were collected directly into a 10 mL centrifuge tube, and the number of cells in each sample was 1–5 × 106/mL, centrifuged at 1000 r/min for 5 min to discarded the culture solution, and resuspended with phosphate buffer solution. Annexin V-FITC labeling and PI solution were added and mixed uniformly. The apoptotic rate was detected using a flow cytometry.
Examination of cell viability by MTT assay
The co-cultured cells were inoculated into a medium containing 10% fetal bovine serum to form a single cell suspension, and 1000–10,000 cells per well with 200 µL were inoculated into 96 well-plate. The cells were cultured for 3–5 days under normal conditions (37°C, 5% CO2 incubator). Then, 20 µL MTT solution (5 mg/mL, Biyuntian,China) was added to each well and the cells incubated for 4 h. Carefully discarded the culture supernatant in each well and added 150 µL DMSO (Biyuntian, China) for oscillating 10 min to fully melt the crystals. The absorbance of each well was read 570 nm with a multiskan spectrum (Thermo Fisher, USA), and the results were recorded at 12, 24, 48, 72, 96 h.
Immunochemistry
The cell patches were washed three times with PBS. Adding the vWF antibody (Abcam, USA) incubated overnight at 4°C. After rewarming for 30 min, the slides were washed three times with PBS. Then, they were incubated for 1 h with secondary antibodies (Abcam, USA). Immunostaining was examined using an inverted microscope (Leica, Heidelberg, Germany).
Real-time PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) from cells according to the manufacturer’s instructions. First-strand cDNA using the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Japan). And then qRT-PCR was performed using a Real-time PCR System (Bio-rad, USA) with the SYBR Green PCR Kit (Takara, Otsu, Japan), reaction procedure of qRT-PCR followed the manufacturer’s protocol. Using 2-ΔΔCT method to calculate the relative expression levels. The expressions in cardiomyocytes were quantified. The relative expression levels were normalized to β-actin.
Western blot analysis
Total proteins were extracted from cells using ice-cold lysis buffer, the extracted protein was quantified by BCA kit (Takara, Japan). Briefly, 500 µg protein was taken from the total protein of each sample and mixed with 5 × SDS loading buffer at a ratio of 4:1. The concentration of the mixed protein was about 6 µg/µL, then boiled in boiling water for 5 min to denature the protein. 60 µg/lane proteins were separated by SDS-PAGE gels (Jinruisi, China), then transferred into polyvinylidene difuoride (PVDF) membranes (Millipore, USA). The PVDF membranes were blocked with 5% non-fat powdered milk for 2 h at room temperature and incubated with primary antibodies overnight at 4°C. The following primary antibodies were used: anti-p38 (Biyuntian, AF7668, 1:1000), anti-phospho-p38 (Biyuntian, AF5884, 1:1000), anti-ERK (Biyuntian, AF1315, 1:1000), anti-phospho-ERK (Biyuntian, AF1891, 1:1000), anti-TGF-β (Biyuntian, AF0297, 1:1000), anti-phospho-TGF-β (Biyuntian, AF5863, 1:1000), anti-IFN-gamma (Abcam, ab77246, 1:1000) anti-IL-10 (Abcam, ab34843, 1:1000), anti-β-actin (Abcam, ab8227, 1:1000). After washing the PVDF membranes with TBST for three times (5 min per wash), the membranes were incubated with the secondary antibodies (Biyuntian, China) for 1.5–2 h at room temperature. The membranes were then washed three times again with TBST. Protein expression levels were visualized using enhanced chemiluminescence (ECL) detection system (Thermo Fisher Scientific, USA). The proteins were quantified using ImageJ software (National Institutes of Health, USA). The expression levels were normalized to β-actin.
Statistical analyses
Data are expressed as mean with standard deviation (SD). The significant analysis of data between the two groups was analyzed using one-way ANOVA followed by post hoc Tukey’s test in SPSS 23.0 (SPSS, Inc., Chicago, IL, USA), and p < 0.05 was considered to be statistically significant.
Result
The immunophenotype of hAD-MSCs and cardiomyocytes was analyzed by flow cytometry
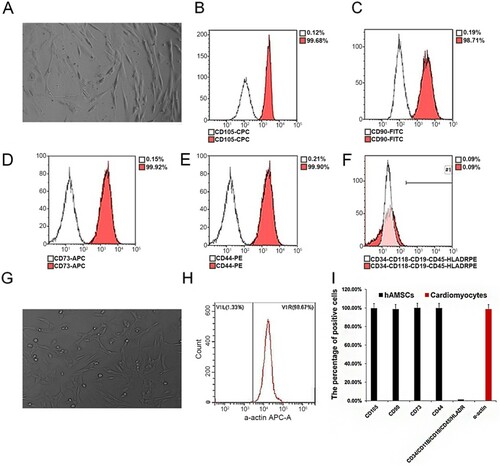
The results showed that the hAD-MSCs expressed CD44, CD73, CD105 and CD90, the percentage of positive expression was >90%, while CD34, CD45, CD11B, CD19, HLA-DR were negatively expressed, indicating that the hAD-MSCs isolated in this study were in higher purity (Figure (A–F, I)). The positive rate of isolated mouse cardiomyocytes was 98.67%, indicating that the purity of cardiomyocytes isolated in this study was very considerable (Figure (G–I)).
Figure 1. hAD-MSCs and cardiomyocyte purity identification. (A) hAD-MSCs cells; (B–F) Flow cytometry detection of CD44, CD73, CD105, CD90, CD34, CD45, CD11B, CD19 and HLA-DR expression in isolated cultured hAD-MSCs; (G) Cardiomyocytes; (H) The expression of a-actin in isolated and cultured cardiomyocytes was detected by flow cytometry; (I) Bar chart of statistical results.
Overexpressing TIPE2 gene in hAD-MSCs
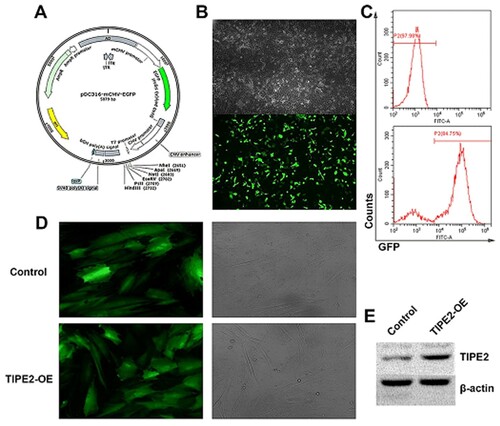
After inserting the TIPE2 gene into the shuttle plasmid (Figure (A)), 293A cells were co-transfected with the adenoviral backbone plasmid, and the mature adenovirus was packaged in 293 T cells (Figure (B)), the transfection efficiency was 84.75% by flow cytometry (Figure (C)). After concentration and purification, the adenovirus was added to the hAD-MSCs cultured in vitro. After 48 h of infection, green fluorescent expression was observed in the hAD-MSCs by inverted fluorescence microscopy, fluorescence intensity of the TIPE2-overexpressed was obviously higher than that of the control (Figure (D)). Subsequent Western blotting also confirmed that TIPE2 protein expression was higher in the overexpressed (Figure (E)). These results indicated that the adenovirus successfully infected into the cells.
Figure 2. TIPE2 overexpressing adenoviral vector construction and infection. (A) Viral expression vector backbone; (B) Adenovirus packaging; (C) Transfection efficiency of adenovirus. (D) Empty virus and TIPE2 overexpressing virus respectively infect hAD-MSCs. (E) Detection of the TIPE2 expression by Western blot.
Cell viability assay
The cells were divided into four groups: (1) Contact co-culture: TIPE2 gene-modified hAD-MSCs. (2) Contact co-culture of null-hAD-MSCs. (3) Non-contact co-culture of TIPE2 gene-modified hAD-MSCs. (4) Non-contact co-culture of null-hAD-MSCs. The cell cycle was detected by flow cytometry. The proportion of S phase cells in the four groups was 21.76%, 21.51%, 21.71%, and 20.33%, respectively (Figure (A–D, I)). The proportion of apoptosis in the four groups was 13.83%, 16.26%, 8.57%, and 16.51%, respectively (Figure (E–H, J)).
Figure 3. Cell viability assay. (A–D), Cell cycle assay; (E–H), Groups of cells. Apoptosis detection; (I) Statistical results of cell cycle; (J) Statistical results of apoptosis; (K) MTT results. The four groups of cells were (1) Contact co-culture: TIPE2 gene-modified hAD-MSCs. (2) Contact co-culture of null-hAD-MSCs. (3) Non-contact co-culture of TIPE2 gene-modified hAD-MSCs. (4) Non-contact co-culture of null-hAD-MSCs.
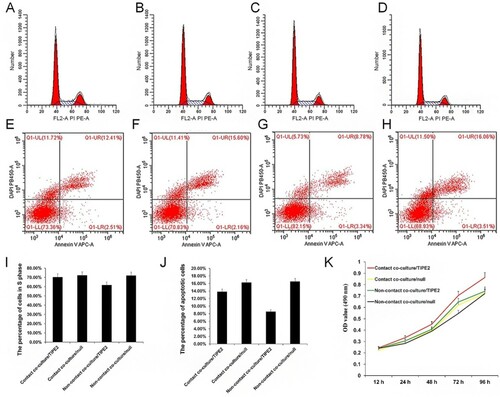
The results of MTT assay showed that the OD values of the cells increased gradually at 12, 24, 48, 72, and 96 h. After TIPE2 overexpression, the OD values in the contact and the non-contact were lower than those in the empty virus control (Figure (K)). These results showed that TIPE2 accelerated cell viability and reduced apoptosis rates during the cell co-culture.
The detection to the expression of related gene and protein
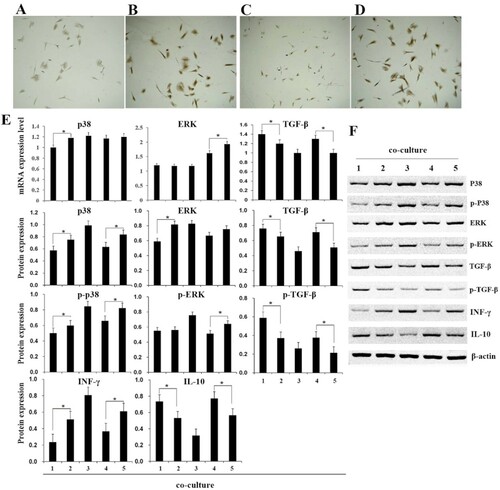
Immunohistochemistry was used to detect the expression of vWF, a protein marker of immune rejection (Brouland et al. Citation1999; Lagoo et al. Citation2000; Ozdemir et al. Citation2006), and it was found that overexpression of TIPE2 could reduce the level of vWF in both contact co-culture and non-contact co-culture groups (Figure (A–D)). To further clarify the regulation of TIPE2 on cellular immune response, the expressions of p38, ERK, TGF-β, IL-10, and INF-γ in different groups were detected by qPCR and western blot. p38, ERK, and TGF-β are important regulators involved in the TIPE2-mediated signaling pathway. INF-γ and IL-10 are cellular cytokines secreted respectively by Th1 and Th2 cells, maintaining the immune balance in organism. The results implied that after overexpression of TIPE2, the mRNA expression level of p38, ERK, and INF-γ were reduced when compared with those of the empty virus. The protein expression and the phosphorylation levels of these proteins were also relatively reduced in both contact co-culture and non-contact co-culture groups (Figure (E,F)). In contrast, the expressions of TGF-β and IL-10 increased after TIPE2 overexpression. Demonstrating that TIPE2 was enabled to regulate the expression of cellular immune response factors.
Figure 4. Detection of related gene and protein expression of immune responses. (A–D), vWF detection by immunohistochemistry, the four groups of cells were contact co-culture/TIPE2 (A), contact co-culture/null (B), non-contact co-culture/TIPE2 (C), and non-contact co-culture of/null (D). E, qRT-PCR and western blot detect the expression of p38, ERK, TGF-β, INF-γ, and IL-10. F, Western blot detect the protein expression. (1) contact co-culture/TIPE2; (2) contact co-culture/null; (3) cardiomyocytes; (4) non-contact co-culture/TIPE2; (5) non-contact co-culture of/null. ‘*’ indicate p < 0.05.
Discussion
Anti-immunological rejection and ischemia-reperfusion injury are the core issues for heart transplantation research. From the perspective of applied technology, it is a key technology that to control stem cells to induction of tolerance after organ transplantation (Sordi and Piemonti Citation2011). However, limited self-tolerance, limited differentiation rate, and microenvironment in hAD-MSCs are not conducive to the survival of homing stem cells. This is a major obstacle for the effective use of stem cell-induced immune tolerance therapy and the exploration to the enhancement of stem cell-induced immune tolerance (Richter et al. Citation2017).
In this study, we found that overexpression of TIPE2 in hAD-MSCs decreased the expression of p38, ERK, and INF-γ, but increased the expression TGF-β and IL-10, thereby we suggested that TIPE2 suppresses the immune response of the co-culture system of hAD-MSCs and the injured cardiomyocyte in vitro. The previous study has found that TIPE2 acts as a negative regulator of the immune response, and it can regulate the adaptive and the innate immune response by simultaneously conducting T-Cell Receptor and Toll-like Receptor signal transduction pathway, thus preventing from a hyperresponsiveness and maintaining the immune homeostasis (Sun et al. Citation2008). In the rat’s allogeneic heart allograft models, the survival time of the donor’s heart was significantly longer and the percentage of CD4+CD25+ regulatory T cells also was significantly higher after overexpressing TIPE2 (Youbin et al. Citation2015). Both ERK and p38 are mitogen-activated protein kinases, and they have participated in one of the central pathways activated by innate immune signals and inflammation (G. Huang et al. Citation2009; Dong et al. Citation2019). A strong activation of ERK and p38 was observed in a mouse model of heart transplantation that published in 2009 (Sucher et al. Citation2009). Of note, these report described that TIPE2 is involved in multiple signaling pathways and the TIPE2 overexpression inhibits the expression and the phosphorylation of ERK and P38 (Y. Zhu et al. Citation2016; R. Liu et al. Citation2017), this is consistent with our results. In immunosuppressive therapy for heart transplant rejection, the survival time of the mice was prolonged along with the increased IL-10/IL-6 expression and suppressed INF-γ/IL-2 expression (Lv et al. Citation2018). When TIPE2 silenced by siRNA, IL-10 and TGF-β expressions were remarkably down-regulated (Luan et al. Citation2011). Besides, it was reported that the down-regulation of TIPE2 in T cells could efficiently promote the secretion of IL-2 and IFN-γ. Basing on our results, we hypothesized that the TIPE2 might mediate the immune balance of heart transplantation via inducing expression of immunosuppressive factors, reducing the expression of immunoactivator.
MSCs not only exhibit cardiovascular differentiation potential, but also display strong paracrine capacity and low immunity properties, the MSC – immunoinduction therapy seems very promising for heart transplantation (Zhang et al. Citation2013). MSC transplantation was able to prevent left ventricular dilation and dysfunction after the operation, attenuate the cytotoxic activity of spleen lymphocytes and increase the expression of IL-10 (Du et al. Citation2008). A new study reports the co-transplantation of MSCs enhances the graft survival of allogeneic transplanted induced pluripotent stem cell-derived cardiomyocyte through increasing Tregs numbers and IL-10 and TGF-β expression (Yoshida et al. Citation2020). Similar findings were reported in allogeneic heart grafts of mice, MSC-induced myeloid-derived immunosuppressive cells mediate operational transplant tolerance and significantly prolong the graft survival (Obermajer et al. Citation2014). Additionally, intravenous injection of the MSCs alters Th1/Th2 cell balance and upregulates Tregs expression to extend cardiac graft survival (H. Huang et al. Citation2013). hAD-MSC is also considered as a suitable material for transplantation. The researchers discovered that hAD-MSCs could promote the formation of stable neovasculature and angiogenesis thus it could be much meaningful for multiple sclerosis therapy with their unique properties, including immunomodulation and inflammation suppression, angiogenesis promotion, oxidative stress inhibition (Abbasi-Kangevari et al. Citation2019). Tsuji et al. (Citation2010) found that the hAD-MSCs xenografted to cure myocardial ischemia-reperfusion injury will differentiate into myocardial cells and restore partial myocardial function without rejection (Tsuji et al. Citation2010). The hAD-MSCs transplantation can ameliorate ischemia/reperfusion injury of the lung by downregulating proinflammatory factors and upregulating anti-inflammatory in a dog model of cardiopulmonary bypass (Qiang et al. Citation2016). Moreover, hAD-MSCs can inhibit the immune responses by reducing the proliferation of lymphocytes and the secretion of IFN-γ in vitro, and the inhibitory effect is increased as well as the number of hAD-MSCs (Song et al. Citation2015). Our study indicated that TIPE2-modified hAD-MSCs exhibit the properties of inhibiting the pro-immune factors and up-regulated the immunosuppressive factors in vitro. The TIPE2-modified hAD-MSCs accelerate cell viability and reduce apoptosis rates. Basing on our results, we speculated that TIPE2-modified hAD-MSCs may play a protective role in myocardial ischemia-reperfusion/immune rejection injury.
Taken together, this study demonstrates that the co-culture of TIPE2-modified hAD-MSCs and injured cardiomyocytes accelerates cell viability and reduces the cell apoptosis rates. Furthermore, TIPE2-modified hAD-MSCs inhibit the expressions of pro-immune factors (p38, ERK, and INF-γ), and then heighten the expressions of immunosuppressive factors (TGF-β and IL-10). Thus, we speculated that the TIPE2 may enhance a cellular immune tolerance in allogeneic heart transplantation. This study provides a theoretical basis for the study of allogeneic heart transplantation.
Ethical approval
The study complies with the Declaration of Helsinki and was approved by West China Hospital Ethics Committee.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (# 81660074). FW and SP collected the data and wrote the manuscript. GY, YY, XM, GL and LY contributed to collecting data and reviewing the manuscript. YG conceived the study and contributed to reviewing/editing the manuscript. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in https://share.weiyun.com/590ocgl
Additional information
Funding
References
- Abbasi-Kangevari M, Ghamari SH, Safaeinejad F, Bahrami S, Niknejad H. 2019. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPS regulation, and remyelination stimulation. Front Immunol. 10:238. doi: https://doi.org/10.3389/fimmu.2019.00238
- Alikarami F, Yari F, Amirizadeh N, Nikougoftar M, Jalili MA. 2015. The immunosuppressive activity of amniotic membrane mesenchymal stem cells on T lymphocytes. Avicenna J Med Biotechnol. 7:90–96.
- Bao W, Qin X, Guan N, Wang S, Zhu J, Sun X, Zhou H, Zhu Z, Zhu C. 2015. MyD88-silenced dendritic cells induce T-cell hyporesponsiveness and promote Th2 polarization in vivo. Cytotherapy. 17:1240–1250. doi: https://doi.org/10.1016/j.jcyt.2015.05.008
- Bett AJ, Haddara W, Prevec L, Graham FL. 1994. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 91:8802–8806. doi: https://doi.org/10.1073/pnas.91.19.8802
- Brouland JP, Egan T, Roussi J, Bonneau M, Pignaud G, Bal C, et al. 1999. In vivo regulation of von willebrand factor synthesis: von Willebrand factor production in endothelial cells after lung transplantation between normal pigs and von Willebrand factor-deficient pigs. Arterioscler Thromb Vasc Biol. 19:3055–3062. doi: https://doi.org/10.1161/01.ATV.19.12.3055
- Chamberlain G, Fox J, Ashton B, Middleton J. 2007. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 25:2739–2749. doi: https://doi.org/10.1634/stemcells.2007-0197
- Dong N, Xu X, Xue C, Wang C, Li X, Bi C, Shan A. 2019. Ethyl pyruvate inhibits LPS induced IPEC-J2 inflammation and apoptosis through p38 and ERK1/2 pathways. Cell Cycle. 18:2614–2628. doi: https://doi.org/10.1080/15384101.2019.1653106
- Du YY, Zhou SH, Zhou T, Su H, Pan HW, Du WH, Liu B, Liu QM. 2008. Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction. Cytotherapy. 10:469–478. doi: https://doi.org/10.1080/14653240802129893
- Huang H, He J, Teng X, Yu Y, Ye W, Hu Y, Shen Z. 2013. Combined intrathymic and intravenous injection of mesenchymal stem cells can prolong the survival of rat cardiac allograft associated with decrease in miR-155 expression. J Surg Res. 185:896–903. doi: https://doi.org/10.1016/j.jss.2013.06.015
- Huang G, Shi LZ, Chi H. 2009. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine. 48:161–169. doi: https://doi.org/10.1016/j.cyto.2009.08.002
- Jia L, Gui B, Tian P, Yao G, Fu R, Wang L, Ge H, Ou Y. 2013. TIPE2, a novel biomarker for clinical chronic kidney allograft rejection. Artif Organs. 37:221–225. doi: https://doi.org/10.1111/j.1525-1594.2012.01527.x
- Kariminekoo S, Movassaghpour A, Rahimzadeh A, Talebi M, Shamsasenjan K, Akbarzadeh A. 2016. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 44:749–757. doi: https://doi.org/10.3109/21691401.2015.1129620
- Klaeske K, Lehmann S, Büttner P, Palitzsch R, Fischer J, Jawad K, et al. 2020. Identification of the immunological profile in rejection-free heart transplantation. Transpl Immunol. 59:101259. doi: https://doi.org/10.1016/j.trim.2019.101259
- Lagoo AS, Buckley PJ, Burchell LJ, Peters D, Fechner JH, Tsuchida M, et al. 2000. Increased glomerular deposits of von Willebrand factor in chronic, but not acute, rejection of primate renal allografts. Transplantation. 70:877–886. doi: https://doi.org/10.1097/00007890-200009270-00005
- Li DX, Wu J, Bai Y, Zhao XC, Liu LJ. 2014. Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. Jove-Journal of Visualized Experiments. 8:1–8.
- Liu R, Fan T, Geng W, Chen YH, Ruan Q, Zhang C. 2017. Negative immune regulator TIPE2 promotes M2 macrophage differentiation through the activation of PI3K-AKT signaling pathway. PLoS One. 12:e0170666. doi: https://doi.org/10.1371/journal.pone.0170666
- Luan YY, Yao YM, Zhang L, Dong N, Zhang QH, Yu Y, Sheng ZY. 2011. Expression of tumor necrosis factor-alpha induced protein 8 like-2 contributes to the immunosuppressive property of CD4(+)CD25(+) regulatory T cells in mice. Mol Immunol. 49:219–226. doi: https://doi.org/10.1016/j.molimm.2011.08.016
- Lv Y, Pang X, Jia PY, Jia DL. 2018. Combined therapy of infusion of DC from rats with higher expression of IDO and CD40L on rejection post heart transplantation. Eur Rev Med Pharmacol Sci. 22:7977–7984.
- Madsen JC. 2017. Advances in the immunology of heart transplantation. J Heart Lung Transplant. 2017(36):1299–1305. doi: https://doi.org/10.1016/j.healun.2017.10.003
- Obermajer N, Popp FC, Soeder Y, Haarer J, Geissler EK, Schlitt HJ, Dahlke MH. 2014. Conversion of Th17 into IL-17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. J Immunol. 193:4988–4999. doi: https://doi.org/10.4049/jimmunol.1401776
- Oho M, Nakano R, Nakayama R, Sakurai W, Miyamoto A, Masuhiro Y, Hanazawa S. 2016. TIPE2 (tumor necrosis factor alpha-induced protein 8-like 2) is a novel negative regulator of TAK1 signal. J Biol Chem. 291:22650–22660. doi: https://doi.org/10.1074/jbc.M116.733451
- Ozdemir BH, Sar A, Haberal M. 2006. The importance of glomerular deposits of von Willebrand factor in human renal allografts. Ren Fail. 28:315–321. doi: https://doi.org/10.1080/08860220600577759
- Podesta MA, Remuzzi G, Casiraghi F. 2019. Mesenchymal stromal cells for transplant tolerance. Front Immunol. 10:1287. doi: https://doi.org/10.3389/fimmu.2019.01287
- Qiang Y, Liang G, Yu L. 2016. Human amniotic mesenchymal stem cells alleviate lung injury induced by ischemia and reperfusion after cardiopulmonary bypass in dogs. Lab Invest. 96:537–546. doi: https://doi.org/10.1038/labinvest.2016.37
- Richter M, Stone D, Miao C, Humbert O, Kiem HP, Papayannopoulou T, et al. 2017. In vivo hematopoietic stem cell transduction. Hematol Oncol Clin North Am. 31:771–785. doi: https://doi.org/10.1016/j.hoc.2017.06.001
- Shah KS, Kittleson MM, Kobashigawa JA. 2019. Updates on heart transplantation. Current Heart Failure Reports.
- Soleymaninejadian E, Pramanik K, Samadian E. 2012. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 67:1–8. doi: https://doi.org/10.1111/j.1600-0897.2011.01069.x
- Song J, Cong S, Li Y, Bai L, Cao G. 2015. Human amniotic mesenchymal stem cells inhibit allogeneic lymphocyte proliferation and reduce the secretion of interferon gamma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 31:333–337.
- Sordi V, Piemonti L. 2011. Therapeutic plasticity of stem cells and allograft tolerance. Cytotherapy. 13:647–660. doi: https://doi.org/10.3109/14653249.2011.583476
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, et al. 2002. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 99:16899–16903. doi: https://doi.org/10.1073/pnas.242603899
- Sucher R, Gehwolf P, Kaier T, Hermann M, Maglione M, Oberhuber R, et al. 2009. Intracellular signaling pathways control mitochondrial events associated with the development of ischemia/ reperfusion-associated damage. Transpl Int. 22:922–930. doi: https://doi.org/10.1111/j.1432-2277.2009.00883.x
- Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, et al. 2008. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 133:415–426. doi: https://doi.org/10.1016/j.cell.2008.03.026
- Tsuji H, Miyoshi S, Ikegami Y, Hida N, Asada H, Togashi I, et al. 2010. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res. 106:1613–1623. doi: https://doi.org/10.1161/CIRCRESAHA.109.205260
- Valarmathi MT, Fuseler JW, Potts JD, Davis JM, Price RL. 2018. Functional tissue Engineering: A Prevascularized cardiac Muscle Construct for Validating human mesenchymal stem cells Engraftment potential In vitro. Tissue Eng Part A. 24:157–185. doi: https://doi.org/10.1089/ten.tea.2016.0539
- Yoshida S, Miyagawa S, Toyofuku T, Fukushima S, Kawamura T, Kawamura A, et al. 2020. Syngeneic mesenchymal stem cells reduce immune rejection after induced pluripotent stem cell-derived allogeneic cardiomyocyte transplantation. Sci Rep. 10:4593. doi: https://doi.org/10.1038/s41598-020-58126-z
- Youbin Z, Yunsheng Y, Zhenya S, Xiaoming Z, Xiaomei T. 2015. Tumor necrosis factor-α-induced protein 8-like 2 gene overexpression prolongs the survival of rat allogeneic heart allografts. Transplant Proc. 47:2517–2522. doi: https://doi.org/10.1016/j.transproceed.2015.08.002
- Yusen RD, Edwards LB, Dipchand AI, Goldfarb SB, Kucheryavaya AY, Levvey BJ, et al. 2016. The registry of the international society for heart and lung transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 35:1170–1184. doi: https://doi.org/10.1016/j.healun.2016.09.001
- Zhang Y, Liang X, Lian Q, Tse HF. 2013. Perspective and challenges of mesenchymal stem cells for cardiovascular regeneration. Expert Rev Cardiovasc Ther. 11:505–517. doi: https://doi.org/10.1586/erc.13.5
- Zhu P, Esckilsen S, Atkinson C, Chen XP, Nadig SN. 2016. A simplified cuff technique for abdominal aortic transplantation in mice. J Surg Res. 200:707–713. doi: https://doi.org/10.1016/j.jss.2015.08.039
- Zhu Y, Tao M, Wu J, Meng Y, Xu C, Tian Y, et al. 2016. Adenovirus-directed expression of TIPE2 suppresses gastric cancer growth via induction of apoptosis and inhibition of AKT and ERK1/2 signaling. Cancer Gene Ther. 23:98–106. doi: https://doi.org/10.1038/cgt.2016.6
