ABSTRACT
In this study, endophytic fungi were isolated from Euphorbia larica Boiss. and their antagonistic potential against phytopathogenic fungi viz. Fusarium sp., Rosellinia sanctae-cruciana and Cladosporium sp. was evaluated using an in vitro confrontation assay. A total of four endophytic fungi were isolated from the roots of E. larica. Among them, two fungal isolates (SQUCC-F1-1 and SQUCC-F6-2) showed inhibitory activity against Fusarium sp. None of the fungal endophytes restricted the growth of Rosellinia sanctae-cruciana and Cladosporium sp. On the basis of multi-gene phylogenetic analysis, these two endophytic fungi were identified as Neocosmospora sp. (SQUCC-F1-1) and Alternaria alternata (SQUCC-F6-2). Scanning electron microscopic observation of hyphae of the phytopathogenic fungi from the dual culture plate revealed morphological abnormalities such as loss of turgidity, shrinkage and disintegration of the hyphae. Analysis of the metabolites produced by these endophytic fungi by GC-MS revealed the existence of various fatty acids, fatty acid methyl esters, hydrocarbons and alkanes, which have been previously demonstrated to have antimicrobial activity. The antagonistic activities of fungal endophytes from E. larica are reported for the first time.
Introduction
The existence of endophytic fungi in a wide range of plant species has been documented (Alvin et al. Citation2014). These endophytes are non-pathogenic microorganisms that colonize internal plant tissues for at least part of their life cycles (Rosenblueth and Martinez-Romero Citation2006; Alvin et al. Citation2014; Bacon and White Citation2016; Khare et al. Citation2018) and establish a mutualistic relationship with their hosts (Carroll Citation1988; Freeman and Rodriguez Citation1993; Rodriguez et al. Citation2009). The endophytic fungi spread through seeds or enter through roots during seed germination and colonize the host plants (Partida-Martinez and Heil Citation2011). The distribution and species diversity of fungal endophytes are influenced by location, developmental stage of host plants and prevailing climatic conditions (Petrini Citation1991; Larran et al. Citation2001). Roots, in general, contain more endophytes compared with the above-ground parts of the plants (Rosenblueth and Martinez-Romero Citation2006). Several reports indicate that the endophytes act as a biological defense for the host plant and offer protection against invading plant pathogens either directly by releasing antimicrobial metabolites or indirectly by triggering host defense mechanisms (Waller et al. Citation2005; Zabalgogeazcoa Citation2008; Johnston-Monje and Raizada Citation2011; Mousa and Raizada Citation2013). Mousa et al. (Citation2015) demonstrated that extracts from fungal endophytes isolated from Eleusine coracana inhibited the growth of Fusarium graminearum, the causal agent of Gibberella ear rot in maize and Fusarium head blight in wheat. Plants containing endophytes have been reported to be healthier than those without endophytes (Martinez-Klimova et al. Citation2017).
Endophytic fungi have been isolated and characterized from various medicinal plants (Jia et al. Citation2016). The endophytes greatly influence the quality and quantity of the drugs derived from those medicinal plants (Chen et al. Citation2016). Many bioactive compounds are co-produced by the host plant and endophytes that reside in the medicinal plants (Aly et al. Citation2010; Heinig et al. Citation2013). For instance, compounds such as Azadirachtin (Kusari et al. Citation2012), Camptothecin (Puri et al. Citation2005) and Podophyllotoxin (Puri et al. Citation2006) are produced by both endophytes and their respective host plants. Some endophytic fungi are known to induce the production of secondary metabolites in plants. For instance, the fungal endophyte Colletotrichum gloeosporioides induces the production of Artemisinin in Artemisia annua (Wang et al. Citation2006).
Euphorbia larica Boiss. is a deciduous, dense, erect shrub with yellow/green branching stems. It is widely distributed in Oman (Al-Mahmooli et al. Citation2013). The latex of E. larica is used to treat the parasites in camels (Pickering and Patzelt Citation2008). In addition, it is used for the treatment of bites, boils, burns (Divakar et al. Citation2016), skin diseases, intestinal parasites, gonorrhea, migraines and warts (Genovese et al. Citation2009). It shows pharmacological properties like anticancer and antimicrobial activities (Saleem et al. Citation2019). However, no information is available in the literature about endophytic fungi inhabiting E. larica. The objectives of this study were (1) to isolate and characterize endophytic fungi from E. larica; (2) to evaluate antagonistic potential of the endophytes against three fungal pathogens of medicinal plants viz., Fusarium sp., Rosellinia sanctae-cruciana and Cladosporium sp.; and (3) to identify volatile secondary metabolites produced by the selected endophytes.
Materials and methods
Collection of medicinal plant
The medicinal plant, Euphorbia larica Boiss. (Euphorbiaceae) was collected from the greenhouse of Oman Botanic Garden (OBG), Muscat, Oman. Five disease-free potted plants were randomly sampled for isolation of endophytic fungi.
Fungal pathogens
Three fungal pathogens were used in this study. The cultures of Cladosporium sp. isolate SQUCC F2-1 (GenBank accession number MK583570) and Rosellinia sanctae-cruciana SQUCC-F7-2 (GenBank accession number MK583575) isolated from Euphorbia larica plants showing symptoms of stem blight and Fusarium sp. isolate SQUCC F1-2 (GenBank accession number MK583572) isolated from Aloe dhufarensis plants with symptoms of leaf spot were obtained from the Department of Crop Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University and maintained in Potato Dextrose Agar (PDA) (Oxoid Ltd., Basingstoke, UK) at room temperature (25 ± 2°C). Pathogenicity of these isolates was confirmed by artificially inoculating the respective host plants (Al-Rashdi et al. unpublished).
Isolation of endophytic fungi
The plants were washed thoroughly in tap water and then in distilled water. The roots were separated and cut into 1–2 cm segments and surface disinfected by rinsing in 70% ethanol for 1 min, followed by washing with 1% NaOCl for 3 min. The plant tissues were finally washed three times with sterilized distilled water. The surface-sterilized tissue pieces were cut into smaller fragments (0.2–0.5 cm in length) using a sterile scalpel and placed carefully on PDA medium and incubated at 27°C for 5–7 days. Four root tissue segments were placed in each Petri dish. The endophytic fungal isolates were subsequently purified by hyphal tip culture method. The purified cultures were stored on PDA slants at 4 °C.
In vitro antagonistic activity
Each endophytic fungal isolate and phytopathogenic fungus were co-cultured at the two opposite sides of the PDA plates by placing mycelial plugs (5 mm diameter) taken from actively growing cultures. Control plates were inoculated with the phytopathogenic fungal discs alone. Three replicate plates were used for each isolate and incubation was at 27°C. The % growth inhibition was recorded when the control plates were completely colonized by the phytopathogenic fungi tested as described by Toghueo et al. (Citation2016).
Scanning electron microscopy
To observe the changes in the hyphal morphology of the susceptible fungus (Fusarium sp.) due to inhibitory activity of endophytes, pieces of agar medium (0.5 cm) containing mycelium of Fusarium sp. were taken from the edge of inhibition zone. The samples were processed as described by Goldstein et al. (Citation2003) and examined with a scanning electron microscope (JEOL, model JSM-7600F).
Molecular characterization of endophytic fungi
Total genomic DNA was extracted from the fungal mycelium as per the procedure described by Lee and Taylor (Citation1990). Briefly, freeze-dried mycelium was ground into fine powder. Seven hundred µl of lysis buffer (50 mM Tris-HCl, 50 mM EDTA, 3% SDS, 1% 2-mercaptoethanol) was mixed with 80 mg of ground mycelia and incubated for 1 h at 65 °C. After incubation, the lysate was mixed with an equal volume of phenol: chloroform: isoamyl alcohol (25:24:1) and then centrifuged at 10,000 ×g for 15 min at 4 °C. This step was repeated again followed by precipitation of DNA using isopropanol and sodium acetate. The DNA pellet was washed using 70% ethanol. The pellet was dried and then dissolved in 100 µl of sterile distilled water. The quality and quantity of the DNA were determined by using a NanoDrop 1000 Spectrophotometer.
The fungal DNA was used as a template to amplify the internal transcribed spacer (ITS) region of nuclear ribosomal DNA by PCR using ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) primers (White et al. Citation1990) as described by Al-Sadi et al. (Citation2012). Subsequently, additional loci were amplified and sequenced, namely: major allergen alt a1 (Alt a 1) (Hong et al. Citation2005) for Alternaria, and translation elongation factor (TEF) (O'Donnell et al. Citation1998) and RNA polymerase II subunit (RPB2) (Lofgren et al. Citation2018) for Neocosmospora. The PCR reaction mixture contained 100 ng of DNA, 20 pmol of each primers and a puReTaq Ready-to-Go PCR bead (GE Healthcare, UK) in a total volume of 25 μl. Amplifications were performed using a thermal cycler as per the conditions and primers described by Al-Nadabi et al. (Citation2018). Aliquots of 5 μl of PCR amplified products were analyzed in 1.2% agarose gels in TBE buffer. The amplified PCR products were sequenced at the Macrogen Inc., Seoul, Korea.
Phylogenetic analysis
To identify the fungal isolates, the nucleotide sequences were subjected to BLAST search with the NCBI database (http://www.ncbi.nlm.nih.gov). Further, phylogenetic analyses were carried out to estimate the correct placement of our isolate within the respective genera. Multiple sequence alignments were made with the MEGA v.6 (Tamura et al. Citation2013) and were visually improved where necessary. Phylogenetic trees were constructed with raxmlGUI v.1.3 software (Silvestro and Michalak Citation2012) using the Maximum Likelihood (ML) method. Bootstrap analysis based on 1000 bootstrap replication was performed with the GTRGAMMA substitution model. The resulting phylogenetic trees were printed using MEGA v.6 (Tamura et al. Citation2013) and the layout was made with Adobe Illustrator CC 2018.
Analysis of volatile metabolites production by endophytes
Preparation of extracts of endophytic fungi
The fungal endophytes were grown at 27°C in 200 ml of Czapek Dox broth under stationary conditions. After 2 weeks, ethyl acetate (1:1, v/v) was added to the fungal culture and mixed with a homogenizer for 2 min. The mixtures were filtered through a muslin cloth and the organic layer and aqueous phase were separated using a separating funnel. The aqueous phase was re-extracted with ethyl acetate (1:1, v/v). The organic fractions were pooled and evaporated in vacuo at 50°C to dryness using a rotary evaporator. The residues were dissolved in 1 ml of methanol and subjected to Gas chromatography-mass spectrometry (GC-MS) analysis.
GC-MS analysis
GS-MS analysis was carried out on a Shimadzu GC-2010 Plus gas chromatograph (Shimadzu, Kyoto, Japan). Compounds were separated using a Supelco Omegawax 250 capillary column having dimensions of 30 m length, internal diameter of 0.25 mm and 0.25 μm film thickness. The injection volume of the sample was 1 μl and the split mode used was 10:1. The identification of the compounds after gas-chromatographic separation was carried out using Shimadzu QP-2010 ultra-mass spectrometer as the detector. Helium (99.9999% purity) was used as the carrier gas with a flow rate of 1.0 ml/min. The ionization voltage was 70 eV and the mass spectral scan range was 35–500 amu. The oven temperature settings were programmed to begin at 50°C, followed by an increase at a rate of 4°C/min to 250°C and a hold for 5 min. By using Wiley and NIST mass spectrum libraries the compounds were identified.
Results
Antagonistic activity of endophytes
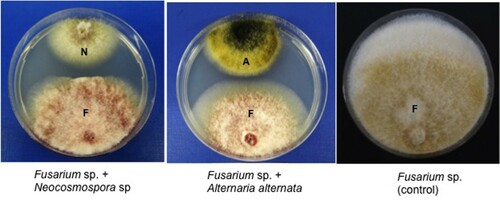
A total of four endophytic fungi were obtained from the roots of E. larica. Among them, two fungal isolates viz., SQUCC-F1-1 and SQUCC-F6-2 exhibited in vitro antagonistic activities against Fusarium sp. and recorded % inhibition of 26.7 and 20.0, respectively. None of the endophytic fungi inhibited the growth of Cladosporium sp. and Rosellinia sanctae-cruciana. The inhibition of mycelial growth of Fusarium sp. by the endophytic fungal isolates SQUCC-F1-1 and SQUCC-F6-2 is shown in Figure .
Scanning electron microscopy
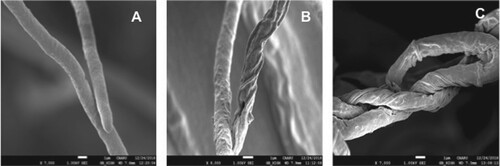
The scanning electron microscopic observations of Fusarium sp. from the dual culture plate revealed that the endophytes SQUCC-F1-1 and SQUCC-F6-2 induced morphological abnormalities on the hyphae of Fusarium sp. including loss of turgidity, shrinkage, twisting and disintegration (Figure ).
Figure 2. Scanning electron micrographs showing morphological changes in the hyphae of Fusarium sp. strain SQUCC-F1-2 due to antagonistic activity of Alternaria alternata strain SQUCC-F6-2 and Neocosmospora sp. strain SQUCC-F1-1. A, Fusarium sp. control; B, Fusarium sp. + Alternaria alternata; C, Fusarium sp.+ Neocosmospora sp.
Molecular identification of endophytes
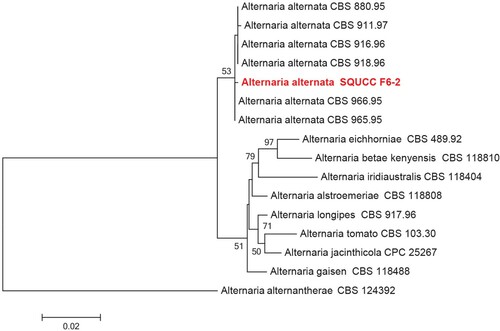
BLAST search of the ITS sequences of the endophytic fungal isolates viz., SQUCC F6-2 and SQUCC F1-1 revealed that these isolates belong to Alternaria section Alternaria, and the genus Neocosmospora, respectively. The combined ITS and Alt a 1 sequence alignment was used subsequently to identify the species in the Alternaria section Alternaria (Figure ). Based on the phylogenetic analysis, the Alternaria isolate (SQUCC F6-2) was identified as Alternaria alternata.
Figure 3. Phylogenetic tree obtained from the combined ITS and Alt a 1 sequence alignment of the species of Alternaria. The tree was rooted using Alternaria alternantherae (CBS 124392). The phylogenetic tree was constructed by Maximum likelihood methods and GTRGAMMA model. The bootstrap values are expressed as percentage of 1000 replications.
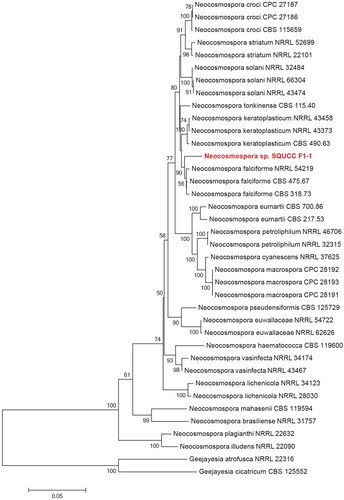
Similarly, Neocosmospora phylogenetic tree was constructed using the combined ITS, TEF and RPB2 sequence data. The Neocosmospora isolate (SQUCC F1-1) in the present study formed a distinct clade, which is sister to species including Neocosmospora falciforme and N. keratoplastica (Figure ). Phylogenetic analysis revealed that Neocosmospora isolate (SQUCC F1-1) is distinct and therefore, we identified it as Neocosmospora sp. The ITS sequences of the endophytic fungi were deposited in the GenBank under the accession numbers: MK583569 (Neocosmospora sp. strain SQUCC-F1-1) and MK583574 (Alternaria alternata strain SQUCC-F6-2).
Figure 4. Phylogenetic tree obtained from combined ITS, TEF and RPB2 sequence alignment of the species of Neocosmospora. The tree was rooted using Geejayesia atrofusca (NRRL 22316) and Geejayesia cicatricum (CBS 125552). The phylogenetic tree was constructed by Maximum likelihood methods and GTRGAMMA model. The bootstrap values are expressed as percentage of 1000 replications.
Analysis of volatile metabolites of fungal endophytes
The production of volatile metabolites by the fungal endophytes viz., Neocosmospora sp. and Alternaria alternata was analyzed by GC-MS and the results are summarized in Tables and . The most commonly produced metabolites by these endophytes were Dodecanoic acid, Tetradecanoic acid and Hexadecanoic acid methyl ester. In addition, the production of 2-methyloctacosane was observed in Neocosmospora sp.
Table 1. Compounds detected in the extract of Neocosmospora sp. strain SQUCC-F1-1.
Table 2. Compounds detected in the extract of Alternaria alternata strain SQUCC-F6-2.
Discussion
Euphorbia larica is an important medicinal plant in Oman. However, there is a dearth of reports on the endophytes associated with this plant. In this study, two endophytic fungi viz., Neocosmospora sp. strain SQUCC-F1-1 and Alternaria alternata strain SQUCC-F6-2 showing antagonistic potential were isolated from the roots of Euphorbia larica. These endophytes were identified based on multigene phylogenies. Alternaria sp. has been previously described as an endophyte in Zingiber officinale (Uzma et al. Citation2016). To our knowledge, this is the first study of endophytes from Euphorbia larica. The less colonization of endophytes in this medicinal plant may be due to their habitat since the samples were collected from greenhouse-grown plants. Hardoim et al. (Citation2012) reported that the diversity of endophytes from plants grown under natural field conditions was more when compared to the endophytes from plants grown under controlled environmental conditions, suggesting that endophytic microorganisms are acquired largely from the local environment.
Several reports indicate that endophytic fungi from plants exhibit antagonistic effect towards phytopathogenic fungi (Mejia et al. Citation2008; Lahlali and Hijri Citation2010; Kusari et al. Citation2013; Katoch and Pull Citation2017). The results revealed that the endophytic fungi Neocosmospora sp. and Alternaria alternata recovered from Euphorbia larica showed antagonistic potential against Fusarium sp., the pathogen of Aloe dhufarensis; but not against Cladosporium sp. and Rosellinia sanctae-cruciana, the pathogens of E. larica. Partida-Martinez and Heil (Citation2011) reported that endophytes are former pathogens of plants, which have reduced their virulence and establish asymptomatic interactions with host plants. Freeman and Rodriguez (Citation1993) reported that a single mutation was adequate to transform a pathogenic strain of Colletotrichum magna into a non-pathogenic endophyte. Romao et al. (Citation2011) demonstrated that Guignardia citricarpa, the citrus pathogen and the endophyte Guignardia mangiferae vary only in a limited number of enzymes and the endophyte was phylogenetically derived from the pathogen. It can be speculated that Cladosporium sp., Rosellinia sanctae-cruciana, Neocosmospora sp. and Alternaria alternata might have co-existed in E. larica as pathogens. At later stages, Neocosmospora sp. and Alternaria alternata might have lost their virulence and changed into endophytes due to alterations in the genetic makeup (Freeman and Rodriguez Citation1993) and failed to show antagonism towards the pathogens of their own host plant.
Scanning electron microscopic observations clearly showed morphological abnormalities in the hyphae of Fusarium sp. and confirmed the antagonistic effect of the endophytes. In general, shrinkage, deformation and loss of turgidity were observed in hyphae of the pathogenic fungi. The shrinkage of the hyphae of phytopathogenic fungi indicates a possible loss of cytoplasmic volume. Changes in the intracellular osmotic pressure generally results in distortion of fungal hyphae. Ezziyyani et al. (Citation2007), while studying the interaction between the pathogenic fungus, Phytophthora capsici and the antagonist, Trichoderma harzianum also observed deformation, disintegration and vacuolization of P. capsici hyphae. The disintegration of hyphae of the pathogenic fungi in the inhibition zone in this study suggests alterations in the permeability of the cell membrane (Halo et al. Citation2018).
The production of antifungal compounds is one of the major mechanisms involved in the inhibition of plant pathogens by endophytes (Lugtenberg et al. Citation2016). Hence the metabolites of the endophytic fungi were analyzed by GC-MS. Dodecanoic acid, Hexadecanoic acid, methyl ester and Tetradecanoic acid were commonly detected in the ethyl acetate extracts of Alternaria alternata strain SQUCC-F6-2 and Neocosmospora sp. strain SQUCC-F1-1. These compounds have been shown previously to have antifungal activity (Abubacker and Deepalakshmi Citation2013; Hameed et al. Citation2015; Abubakar and Majinda Citation2016). Lou et al. (Citation2013) reported the production of several compounds by Alternaria sp. including Tenuazonic acid, Z-Hydroxyzinnimidine, Octadecane-1,3,4-triol, Cyclo-(L-Ala-trans-4-hydroxy-L-Pro-) and Fumitremorgin B. The production of bioactive metabolites such as altechromone A, alternariol 9-methyl ether and 3beta-hydroxy-(22E, 24R)-ergosta-5,8,22-trien-7-one by Alternaria brassicicola has been documented (Gu Citation2009). The results of this study suggest that the volatile organic compounds produced by the endophytes viz., Alternaria alternata strain SQUCC-F6-2 and Neocosmospora sp. strain SQUCC-F1-1 from Euphorbia larica might have contributed for their antimicrobial activity against the phytopathogenic fungus Fusarium sp. Further research is required to confirm the biological activity of these metabolites.
Authors’ contributions
RV, AMA, BZA designed the study, FKHA, HKA, SSNM conducted lab experiments, RV, AMA, BZA supervised the research project, FKHA, RV, AMA, SSNM, BZA wrote the manuscript.
Compliance with ethical standards
Ethical approval
This article is original and not published elsewhere. All authors discussed the results, read and approved the final manuscript. The authors confirm that there are no ethical issues in the publication of the manuscript.
Acknowledgements
We thank Dr. Khalid Al-Farsi and Dr. Annette Patzelt, Oman Botanic Garden for providing Euphorbia larica and Dr. Jamal Nasser Al-Sabahi, CAMS, SQU for helping in GC-MS analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability
All data generated or analyzed during this study are included in this article.
Additional information
Funding
References
- Abubacker MN, Deepalakshmi T. 2013. In vitro antifungal potential of bioactive compound methyl ester of hexadecanoic acid isolated from Annona muricata Linn (Annonaceae) leaves. Biosci Biotech Res Asia. 10:879–884. doi: https://doi.org/10.13005/bbra/1211
- Abubakar M, Majinda R. 2016. GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines. 3:3. doi: https://doi.org/10.3390/medicines3010003
- Al-Mahmooli IH, Al-Bahri YS, Al-Sadi AM, Deadman ML. 2013. First report of Euphorbia larica dieback caused by Fusarium brachygibbosum in Oman. Plant Dis. 97:687–687. doi: https://doi.org/10.1094/PDIS-09-12-0828-PDN
- Al-Nadabi HH, Maharachchikumbura SSN, Agrama H, Al-Azri M, Nasehi A, Al-Sadi AM. 2018. Molecular characterization and pathogenicity of Alternaria species on wheat and date palms in Oman. Eur J Plant Pathol. 152:577–588. doi: https://doi.org/10.1007/s10658-018-1550-4
- Al-Sadi AM, Al-Ghaithi AG, Al-Balushi ZM, Al-Jabri AH. 2012. Analysis of diversity in Pythium aphanidermatum populations from a single greenhouse reveals phenotypic and genotypic changes over 2006 to 2011. Plant Dis. 96:852–858. doi: https://doi.org/10.1094/PDIS-07-11-0624
- Alvin A, Miller KI, Neilan BA. 2014. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol Res. 169:483–495. doi: https://doi.org/10.1016/j.micres.2013.12.009
- Aly AH, Debbab A, Kjer J, Proksch P. 2010. Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 41:1–16. doi: https://doi.org/10.1007/s13225-010-0034-4
- Bacon CW, White JF. 2016. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis. 68:87–98. doi: https://doi.org/10.1007/s13199-015-0350-2
- Carroll G. 1988. Fungal endophytes in stems and leaves: from latent pathogen to mutualistic symbiont. Ecology. 69:2–9. doi: https://doi.org/10.2307/1943154
- Chandrasekaran M, Senthilkumar A, Venkatesalu V. 2011. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmcol. Sci. 15:775–780.
- Chen L, Zhang QY, Jia M, Ming QL, Yue W, Rahman K, Qin LP, Han T. 2016. Endophytic fungi with antitumor activities: their occurrence and anticancer compounds. Crit Rev Microbiol. 42:454–473.
- Divakar MC, Al-Siyabi A, Varghese SS, Al Rubaie M. 2016. The practice of ethnomedicine in the Northern and Southern provinces of Oman. Oman Med J. 31:245. doi: https://doi.org/10.5001/omj.2016.49
- Ezziyyani M, Requena ME, Egea-Gilabert C, Candela ME. 2007. Biological control of Phytophthora root rot of pepper using Trichoderma harzianum and Streptomyces rochei in combination. J Phytopathol. 155:342–349. doi: https://doi.org/10.1111/j.1439-0434.2007.01237.x
- Freeman S, Rodriguez RJ. 1993. Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science. 260:75–78. doi: https://doi.org/10.1126/science.260.5104.75
- Genovese S, Curini M, Epifano F. 2009. Chemistry and biological activity of azoprenylated secondary metabolites. Phytochemistry. 70:1082–1091. doi: https://doi.org/10.1016/j.phytochem.2009.06.016
- Goldstein J, Newbury DE, Joy DC, Lyman CE, Echlin P, Lifshin E, Sawyer L, Michael JR. 2003. Scanning electron microscopy and X-Ray microanalysis, 3rd ed. New York: Springer.
- Gu W. 2009. Bioactive metabolites from Alternaria brassicicola ML-P08, an endophytic fungus residing in Malus halliana. World J Microbiol Biotechnol. 25:1677–1683. doi: https://doi.org/10.1007/s11274-009-0062-y
- Halo BA, Al-Yahyai RA, Al-Sadi AM. 2018. Aspergillus terreus inhibits growth and induces morphological abnormalities in Pythium aphanidermatum and suppresses Pythium-induced damping-off of cucumber. Front Microbiol. 9:95. doi: https://doi.org/10.3389/fmicb.2018.00095
- Hameed I, Fadhil L, Kamal S. 2015. Analysis of bioactive chemical compounds of Aspergillus Niger by using gas chromatography-mass spectrometry and Fourier-transform infrared spectroscopy. J Pharmacognosy Phytother. 7:132–163. doi: https://doi.org/10.5897/JPP2015.0354
- Hardoim PR, Hardoim CC, Van Overbeek LS, Van Elsas JD. 2012. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS One. 7:e30438. doi: https://doi.org/10.1371/journal.pone.0030438
- Heinig U, Scholz S, Jennewein S. 2013. Getting to the bottom of Taxol biosynthesis by fungi. Fungal Divers. 60:161–170. doi: https://doi.org/10.1007/s13225-013-0228-7
- Hong SG, Cramer RA, Lawrence CB, Pryor BM. 2005. Alt a 1 allergen homologs from Alternaria and related taxa: analysis of phylogenetic content and secondary structure. Fungal Genet Biol. 42:119. doi: https://doi.org/10.1016/j.fgb.2004.10.009
- Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin LP. 2016. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol. 7:906. doi: https://doi.org/10.3389/fmicb.2016.00906
- Johnston-Monje D, Raizada MN. 2011. Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS One. 6:e20396. doi: https://doi.org/10.1371/journal.pone.0020396
- Katoch M, Pull S. 2017. Endophytic fungi associated with Monarda citriodora, an aromatic and medicinal plant and their biocontrol potential. Pharm Biol. 55:1528–1535. doi: https://doi.org/10.1080/13880209.2017.1309054
- Khare E, Mishra J, Arora NK. 2018. Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol. 9:2732. doi: https://doi.org/10.3389/fmicb.2018.02732
- Kusari P, Kusari S, Spiteller M, Kayser O. 2013. Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant specific phytopathogens. Fungal Divers. 60:137–151. doi: https://doi.org/10.1007/s13225-012-0216-3
- Kusari S, Verma VC, Lamshoeft M, Spiteller M. 2012. An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J Microbiol Biotechnol. 28:1287–1294. doi: https://doi.org/10.1007/s11274-011-0876-2
- Lahlali R, Hijri M. 2010. Screening, identification and evaluation of potential biocontrol fungal endophytes against Rhizoctonia solani AG3 on potato plants. FEMS Microbiol Lett. 311:152–159. doi: https://doi.org/10.1111/j.1574-6968.2010.02084.x
- Larran S, Monaco C, Alippi HE. 2001. Endophytic fungi in leaves of Lycopersicon esculentum Mill. World J Microbiol Biotechnol. 17:181–184. doi: https://doi.org/10.1023/A:1016670000288
- Lee SB, Taylor JW. 1990. Isolation of DNA from fungal mycelia and single spores. In: Innis M, Gelfand D, Sninsky J, Thomas JW, editors. PCR Protocols: A Guide to methods and Applications. Orlando: Academic Press; p. 282–287.
- Lofgren LA, LeBlanc NR, Certano AK, Nachtigall J, LaBine KM, Riddle J, Broz K, Dong Y, Bethan B, Kafer CW, Kistler HC. 2018. Fusarium graminearum: pathogen or endophyte of North American grasses? New Phytol. 217:1203–1212. doi: https://doi.org/10.1111/nph.14894
- Lou J, Fu L, Peng Y, Zhou L. 2013. Metabolites from Alternaria fungi and their bioactivities. Molecules. 18:5891–5935. doi: https://doi.org/10.3390/molecules18055891
- Lugtenberg BJ, Caradus JR, Johnson LJ. 2016. Fungal endophytes for sustainable crop production. FEMS Microbiol Ecol. 92:12. doi: https://doi.org/10.1093/femsec/fiw194
- Martinez-Klimova E, Rodriguez-Pena K, Sanchez S. 2017. Endophytes as sources of antibiotics. Biochem Pharmacol. 134:1–17. doi: https://doi.org/10.1016/j.bcp.2016.10.010
- Mejia LC, Rojas EI, Maynard Z, Van Bael S, Arnold AE, Hebbar P, Samuels GJ, Robbins N, Herre EA. 2008. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol Control. 46:4–14. doi: https://doi.org/10.1016/j.biocontrol.2008.01.012
- Mousa WK, Raizada MN. 2013. The diversity of anti-microbial secondary metabolites produced by fungal endophytes: an interdisciplinary perspective. Front Microbiol. 4:65. doi: https://doi.org/10.3389/fmicb.2013.00065
- Mousa WK, Schwan A, Davidson J, Strange P, Liu H, Zhou T, Auzanneau FI, Raizada MN. 2015. An endophytic fungus isolated from finger millet (Eleusine coracana) produces anti-fungal natural products. Front Microbiol. 6:1157. doi: https://doi.org/10.3389/fmicb.2015.01157
- O'Donnell K, Kistler HC, Cigelnik E, Ploetz RC. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. U.S.A. 95:2044–2049. doi: https://doi.org/10.1073/pnas.95.5.2044
- Parang K, Knaus EE, Wiebe LI, Sardari S, Daneshtalab M, Csizmadia F. 1996. Synthesis and antifungal activities of myristic acid analogs. Arch Pharm. 329:475–482. doi: https://doi.org/10.1002/ardp.19963291102
- Partida-Martinez LP, Heil M. 2011. The microbe-free plant: fact or artifact? Front. Plant Sci. 2:100. doi: https://doi.org/10.3389/fpls.2011.00100
- Petrini O. 1991. Fungal endophytes of tree leaves. In: Andrew IA, Hirano SS, editors. Microbial ecology of leaves. New York: Springer-Verlag; p. 179–197.
- Pickering H, Patzelt A. 2008. Field guide to the wild plants of Oman. Kew Richmond: Royal Botanic Gardens.
- Puri SC, Nazir A, Chawla R, Arora R, Riyaz-ul-Hasan S, Amna T, Sharma A. 2006. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. J Biotechnol. 122:494–510. doi: https://doi.org/10.1016/j.jbiotec.2005.10.015
- Puri SC, Verma V, Amna T, Qazi GN, Spiteller M. 2005. An endophytic fungus from Nothapodytes foetida that produces Camptothecin. J Nat Prod. 68:1717–1719. doi: https://doi.org/10.1021/np0502802
- Rodriguez RJ, White JF, Arnold AE, Redman RS. 2009. Fungal endophytes: diversity and functional roles. New Phytol. 182:314–330. doi: https://doi.org/10.1111/j.1469-8137.2009.02773.x
- Romao AS, Sposito MB, Andreote FD, Azevedo JL, Araujo WL. 2011. Enzymatic differences between the endophyte Guignardia mangiferae (Botryosphaeriaceae) and the citrus pathogen G. citricarpa. Genet Mol Res. 10:243–252. doi: https://doi.org/10.4238/vol10-1gmr952
- Rosenblueth M, Martinez-Romero E. 2006. Bacterial endophytes and their interactions with hosts. Mol Plant-Microbe Interact. 19:827–837. doi: https://doi.org/10.1094/MPMI-19-0827
- Saleem H, Zengin G, Locatelli M, Mollica A, Ahmad I, Mahomoodally FM, Ahemad N. 2019. In vitro biological propensities and chemical profiling of Euphorbia milii Des Moul (Euphorbiaceae): A novel source for bioactive agents. Ind Crops Prod. 130:9–15. doi: https://doi.org/10.1016/j.indcrop.2018.12.062
- Shobier AH, Ghani SAA, Barakat KM. 2016. GC/MS spectroscopic approach and antifungal potential of bioactive extracts produced by marine macroalgae. Egypt J Aquat Res. 42:289–299. doi: https://doi.org/10.1016/j.ejar.2016.07.003
- Silvestro D, Michalak I. 2012. RaxmlGUI: a graphical frontend for RAxML. Org Divers Evol. 12:335–337. doi: https://doi.org/10.1007/s13127-011-0056-0
- Spikes AE, Paschen MA, Millar JG, Moreira JA, Hamel PB, Schiff NM, Ginzel MD. 2010. First contact pheromone identified for a longhorned beetle (Coleoptera: Cerambycidae) in the subfamily Prioninae. J Chem Ecol. 36:943–954. doi: https://doi.org/10.1007/s10886-010-9837-8
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics analysis version 6.0. Mol Biol Evol. 30:2725–2729. doi: https://doi.org/10.1093/molbev/mst197
- Toghueo RMK, Eke P, Zabalgogeazcoa I, de Aldana BRV, Nana LW, Boyom FF. 2016. Biocontrol and growth enhancement potential of two endophytic Trichoderma spp. from Terminalia catappa against the causative agent of common bean root rot (Fusarium solani). Biol Control. 96:8–20. doi: https://doi.org/10.1016/j.biocontrol.2016.01.008
- Uzma F, Konappa NM, Chowdappa S. 2016. Diversity and extracellular enzyme activities of fungal endophytes isolated from medicinal plants of Western Ghats. Karnataka. Egypt J Basic Appl Sci. 3:335–342. doi: https://doi.org/10.1016/j.ejbas.2016.08.007
- Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Huckelhoven R, Neumann C, Von Wettstein D, Franken P. 2005. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci U.S.A. 102:13386–13391. doi: https://doi.org/10.1073/pnas.0504423102
- Walters DR, Walker RL, Walker KC. 2003. Lauric acid exhibits antifungal activity against plant pathogenic fungi. J Phytopathol. 151:228–230. doi: https://doi.org/10.1046/j.1439-0434.2003.00713.x
- Wang JW, Zheng LP, Tan RX. 2006. The preparation of an elicitor from a fungal endophyte to enhance artemisinin production in hairy root cultures of Artemisia annua L. Chin J Biotechnol. 22:829–834.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. San Diego: Academic Press; p. 315–322.
- Zabalgogeazcoa I. 2008. Fungal endophytes and their interaction with plant pathogens: a review. Span J Agric Res. 6:138–146. doi: https://doi.org/10.5424/sjar/200806S1-382