Abstract
N6-2-hydroxyethyl-adenosine (HEA) is one of the main bioactive components found in Cordyceps cicadae and it has been reported to display antioxidant and anti-inflammatory activities. Cisplatin (CP) is one of the most commonly used chemotherapeutic drug for treating various cancers and tumors, but the use is widely curtailed due to its toxicity of various organs including the kidney. This study was aimed at investigating the protective effect of HEA on cisplatin-induced kidney injury. Mice were pretreated with HEA for 7 days and administered with cisplatin. Kidney function index including blood urea nitrogen (BUN), creatinine, renal oxidative stress and pro-inflammatory indices such as malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), tumor necrosis factor (TNF-α), interleukin 1 beta (IL-1β) and interleukin 6 (IL-6) were measured. Histopathological assessment of the kidney was also performed. The results indicated that HEA noticeably modulated the levels of BUN and creatinine as well as decreased the expression of MDA, TNF-α, IL-1β and IL-6 in the kidney. In addition, HEA up regulated the activities of antioxidant enzymes SOD, CAT and GSH-Px. Therefore, we concluded that HEA could effectively alleviate cisplatin-induced nephrotoxicity by counteracting oxidative stress and inflammation.
Introduction
Cisplatin (CP) is an anticancer drug that is extensively used as the first line of treatment for various tumors and cancers including breast, bladder, lung, testicular, ovarian, head and neck cancers (Arany and Safirstein Citation2003; Dasari and Tchounwou Citation2014). The mechanism of action of cisplatin is thought to mediated through its interaction with purine bases in the DNA to form DNA–protein crosslink leading to apoptosis (Chválová et al. Citation2007; Dasari and Tchounwou Citation2014). Despite the effectiveness of cisplatin as a chemotherapeutic agent, the side effects and toxicities associated with the use has greatly limited its clinical application. Side effects such as nephrotoxicity, myelotoxicity, neurotoxicity, electrolyte disturbance, hemolytic anemia and ototoxicity have been widely reported (Fuertes et al. Citation2003; Florea and Busselberg Citation2011; Schanz et al. Citation2017; Zhang et al. Citation2020). Nephrotoxicity is one of the main side effects induced by cisplatin due to its accumulation in the renal proximal tubules and it has been estimated that 25–30% of patients using cisplatin experience renal dysfunction (Ciarimboli et al. Citation2010; Crona et al. Citation2017; Sanchez-Gonzalez et al. Citation2017; Ning et al. Citation2018).
The pathogenesis involved in cisplatin-induced nephrotoxicity is complex, involving an interplay of inflammation, apoptosis, oxidative stress, production of high levels of reactive oxygen species (ROS) and deterioration of antioxidant enzyme levels (Manohar and Leung Citation2018; Sun et al. Citation2019; Yang et al. Citation2019). Furthermore, ROS and oxidative stress induced by cisplatin can activate several inflammatory mediators and pathways such as TNF-α and IL-1β and nuclear factor-κB (Sahu et al. Citation2014). As such, therapeutic agents that can effectively combat inflammatory response, oxidative stress and increase anti-oxidation could be beneficial in the treatment of cisplatin-induced nephrotoxicity. At present, there are no effective treatment for alleviating cisplatin-induced kidney injury.
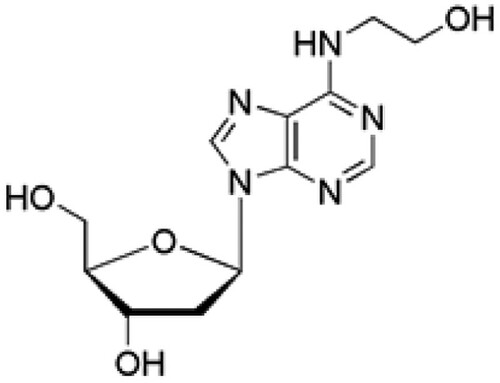
Natural products have been used in the treatment of several diseases due to their unique advantages attributed of their multiple effects, multi-active constituents and limited side effects. N6-2-hydroxyethyl-adenosine (HEA; Figure ) is a bioactive nucleoside isolated from the medicinal mushroom Cordyceps cicadae. HEA has been demonstrated to manifest a host of pharmacological properties including antitumor, antioxidant, anti-inflammatory, antidiabetic, renal protective, neuroprotective, Ca2+ antagonist, radiation resistance and analgesic activities. For instance, HEA protected against H2O2 and LPS induced oxidative toxicity as well as renal interstitial fibrosis through its anti-oxidation and anti-inflammatory effect (Lu et al. Citation2015; Zheng et al. Citation2018; Wang et al. Citation2019; Zhang et al. Citation2019). However, the potential protective effect of HEA on cisplatin-induced nephrotoxicity have not been explored. In this study, we examined the protective effects of HEA on cisplatin-induced nephrotoxicity.
Materials and methods
Animals
Healthy specific pathogen free male ICR mice (20–22 g; 7 weeks old) were used for the experimental study. The experimental animal ethics committee of China–Japan Union Hospital of Jilin University approved the study protocol (ethics approval number 20190043) and the handling and care of the animals were in accordance with the requirement of the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). All the mice were housed in a standard room with temperature and relative humidity of 22 ± 2°C and 55 ± 5%, respectively and a 12-h occulting cycle. The animals were allowed free access to standard diet and water ad libitum unless stated otherwise. After seven days of adaptation, the mice were randomly divided into five groups (eight mice in each group). Group 1: healthy control group (HCG), group 2: cisplatin control group (CPG), group 3: CP + HEA (10 mg/kg), group 4: CP + HEA (20 mg/kg), group 5: CP + HEA (40 mg/kg). The mice in groups 3–5 were orally gavaged with HEA for seven consecutive days. On the 7th day, mice in groups 2–5 received a single injection of 20 mg/kg body weight of CP intraperitoneally. The mice designated as healthy control were given equal volume of normal saline. The mice were sacrificed three days after CP administration, blood and kidney tissues were collected for further experiments. Doses of HEA and CP used in this study were selected based on previous studies (Guo et al. Citation2018; Wang et al. Citation2019; Jin et al. Citation2020).
Histological analysis and determination of biochemical indicators
The kidneys were quickly excised and fixed in 10% neutral buffered formalin. The fixed tissues were further dehydrated, embedded in paraffin, cut into sections of 5μm thickness and stained with hematoxylin and eosin (H&E). The stained slides were further visualized under light microscopy. The serum was obtained from the blood collected and used for determining the levels of creatinine and blood urea nitrogen (BUN).
Assessment of renal inflammatory cytokine levels
The levels of inflammatory cytokines including TNF-α, IL-1β and IL-6 in kidney tissue homogenates were detected using ELISA kits according to the manufacturer’s protocols (Shanghai Enzyme-linked Biotechnology Co., Ltd., China).
Assessment of renal oxidative stress levels
The levels of renal antioxidant enzymes namely SOD, CAT, GSH-Px as well as MDA levels were estimated by using commercially available assay kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions.
Statistical analysis
GraphPad Prism (version 7.0) was used for statistical analysis. Data were expressed as mean ± SD. Differences among groups were analyzed by one-way analysis of variance (ANOVA) and Tukey post hoc test. P < .05 was considered statistically significant.
Results
HEA attenuates cisplatin-induced renal dysfunction
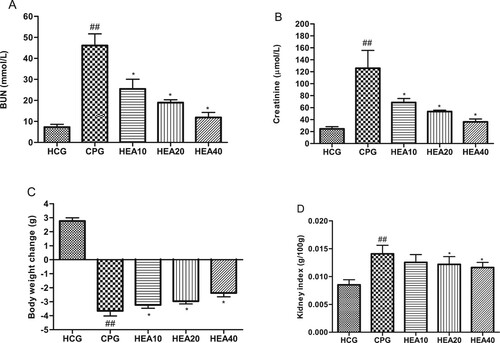
We analyzed the effect of HEA on levels of the biomarkers of kidney function, including creatinine and BUN. As shown in Figure (A, B), cisplatin caused a dramatic increase in creatinine and BUN levels when compared to the levels observed in the healthy control mice (P < .05). In contrast, pretreatment with HEA led to a marked decrease in creatinine and BUN levels in a concentration dependent manner. Cisplatin also led to a significant weight loss when compared to the weight of mice in the healthy control group. Whereas, HEA considerably reduced this phenomenon (Figure (C)). Similarly, HEA improved kidney index in the treated mice when compared to the untreated cisplatin mice group (Figure (D)).
Effect of HEA on histological examination
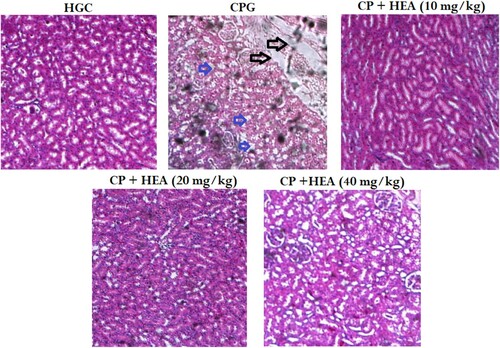
As shown in Figure (A), the kidney tissues of the healthy control group showed normal kidney architecture, with well and closely arranged renal tubule and epithelial cells. Whereas, the CP group showed gross renal tubules damage, congestion of renal capillaries, neutrophils infiltration, necrosis of renal cells, nucleus contraction and cellular vacuolation (Figure (B)). In the HEA pretreated groups, there were obvious signs of alleviated pathological changes at varying degrees (Figure (C)–(E)).
Figure 3. Effect of HEA on cisplatin-induced histopathological changes in kidney tissues (A) Normal control group, (B) Cisplatin group, (C) Cisplatin group + HEA (10 mg kg−1 body mass) group, (D) Cisplatin group + HEA (20 mg kg−1 body mass) group (D) Cisplatin group + HEA (40 mg kg-1 body mass) group.
HEA alleviated cisplatin-induced renal oxidative stress
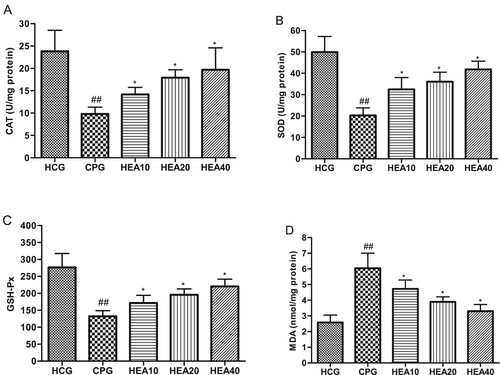
Oxidative stress is considered as a critical parameter in the pathogenesis of cisplatin-induced renal injury. As such, we investigated the effect of HEA on renal oxidative stress parameters. The results indicated that MDA levels in the kidneys of CP model group were significantly elevated compared to the healthy control group (P < .05). In addition, cisplatin caused a marked and evident reduction in antioxidant enzymes activities (CAT, SOD and GSH-Px) in the CP model group compared to the healthy control group. Whereas, compared to the CP model group, the levels of CAT, SOD and GSH-Px were significantly increased in the groups that received HEA pretreatment, while MDA levels were noticeably reduced (Figure (A)–(D)).
HEA alleviated cisplatin-induced renal inflammation
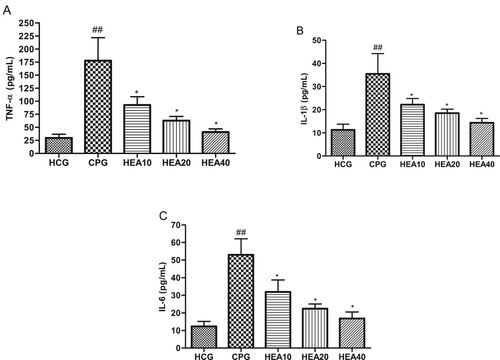
As showed in Figure (A)–(C), TNF-α, IL-6 and IL-1β were obviously elevated in the kidney after the administration cisplatin in the CP model group compared with the healthy control group (P < .05). However, in groups that received pretreatment with HEA these inflammatory cytokines were significantly reduced by varying degrees, suggesting that HEA could alleviate kidney inflammation induced by cisplatin.
Discussion
Cisplatin is one of the most frequently used drugs in the treatment of a number of malignancies, cancers and solid tumors. However, its clinical application has been limited due to multiple toxicities and side effects accrued from its usage (Xing et al. Citation2019; Yucetas et al. Citation2019; Zhang et al. Citation2019). A lot of convincing evidences have implicated ROS generation, oxidative stress and inflammation as the main culprit responsible for cisplatin-evoked toxicity including nephrotoxicity (Arafa and Atteia Citation2019; Li et al. Citation2018). At present, amifostine, erythropoietin and dexamethasone are the most commonly used chemoprotective agent used for alleviating or preventing cisplatin-induced nephrotoxicity. However, unpleasant side effects have impeded their use (Tessoulin et al. Citation2017; Volarevic et al. Citation2019). Agents with antioxidant as well as anti-inflammatory activities have been considered as an alternative protective agent against cisplatin-induced nephrotoxicity (Ali and Al Moundhri Citation2006). As such, this study was designed to evaluate the therapeutic effect of HEA on cisplatin-induced acute kidney injury by exploring it antioxidant and inflammation effects. The results obtained in this study showed that the nephro-protective effect of HEA against cisplatin-induced toxicity is through the regulation of inflammation, oxidative stress and kidney function parameters.
Blood urea nitrogen (BUN) and creatinine are two key markers of kidney function that are obviously elevated in cisplatin-induced renal injury (Lee et al. Citation2013). BUN is an end product of protein metabolism and it is considered as a vital index of glomerular filtration function, while creatinine is a waste product that is excreted by the kidney as a result of muscle metabolism. High levels of serum creatinine and BUN is indicative of renal malfunctioning. Numerous studies have linked cisplatin-induced toxicity to a decrease in glomerular filtration rate and subsequently increased serum BUN and creatinine levels (Malik et al. Citation2016; Boroja et al. Citation2018). Our findings are consistent with previous studies showing CP-induced significant elevation of serum creatinine and BUN levels (Qin et al. Citation2019; Sioud et al. Citation2020). Our study also revealed an increase in serum BUN and creatinine levels in the CP model group suggesting kidney damage and HEA effectively attenuated cisplatin-induced kidney damages by reducing serum BUN and creatinine levels.
The impact of ROS and oxidative stress in cisplatin-induced nephrotoxicity has been extensively documented. Cisplatin can evoke the generation of ROS and mitochondrial dysfunction, which inevitably leads to increase in the levels of lipid peroxidation by product (MDA) (Xiao et al. Citation2003; Berndtsson et al. Citation2007). Oxidative stress is one of the main mechanisms associated with cisplatin-induced organ damages and it is a common pathway involved in cognitive dysfunction, kidney, liver and testicular injuries caused by cisplatin (Afsar et al. Citation2017; Lomeli et al. Citation2017; Adeoye et al. Citation2019; Jing et al. Citation2019). In addition, numerous reports have clearly portrayed that cellular antioxidant enzyme activities are impeded during ROS and oxidative stress induced damages. SOD, CAT and GSH-Px antioxidant enzymes offers protection against oxidative insults in the cells and the reduction in their activities makes the target cells and organs vulnerable to oxidative damages (Hassan et al. Citation2017; Meng et al. Citation2017). Our results indicated that cisplatin impaired the activities of SOD, CAT and GSH-Px and increased the content of MDA in the kidney which was consistent with results from other studies (Adeoye et al. Citation2019; Kandemir et al. Citation2019; Qin et al. Citation2019). Treatment with HEA improved the activities of CAT, SOD and GSH-Px in the kidney. This suggests that HEA can reduce cisplatin-induced oxidative stress in the kidney by upregulating the activities of antioxidant enzymes.
Aside the direct effect on oxidative damage, ROS and oxidative stress can also significantly trigger several other avalanches of inflammatory related reactions. The interplay between ROS, oxidative stress and inflammation has been well illustrated in cisplatin-induced acute kidney injury (Kang et al. Citation2009; Jing et al. Citation2019). Cisplatin-induced kidney damage can activate macrophages triggered inflammation resulting in the production of an array of inflammatory related cytokines, including TNF-α, IL-1β and IL-6 (Hagar et al. Citation2015). The results from this study are in accordance and consistent with the previous studies indicating the toxicity of CP in renal tissues by increasing the levels of pro-inflammatory cytokines (Hagar et al. Citation2015; Al Fayi et al. Citation2020). In agreement with previous studies, our results indicated that CP administration caused significant increase in pro-inflammatory cytokines suggesting the initiation of inflammation. HEA attenuated cisplatin-evoked release of pro-inflammatory cytokines, suggesting the anti-inflammatory ability of HEA.
Conclusion
In conclusion, this study illustrated the protective effect of HEA against cisplatin-induced kidney injury. HEA pretreatment protected against oxidative stress and inflammation by elevating the renal antioxidant enzymes as well as inhibiting the release of inflammatory related cytokines in the kidney. These results may provide useful hints in the utilization of HEA for the treatment of cisplatin-induced nephrotoxicity.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
- Adeoye BO, Oyagbemi AA, Asenuga ER, Omobowale TO, Adedapo AA. 2019. The ethanol leaf extract of Andrographis paniculata blunts acute renal failure in cisplatin-induced injury in rats through inhibition of Kim-1 and upregulation of Nrf2 pathway. J Basic Clin Physiol Pharmacol. 30:205–217. doi: https://doi.org/10.1515/jbcpp-2017-0120
- Afsar T, Razak S, Khan MR, Almajwal A. 2017. Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC Cancer. 17:883. doi: https://doi.org/10.1186/s12885-017-3898-9
- Al Fayi M, Otifi H, Alshyarba M, Dera AA, Rajagopalan P. 2020. Thymoquinone and curcumin combination protects cisplatin-induced kidney injury, nephrotoxicity by attenuating NFκB, KIM-1 and ameliorating Nrf2/HO-1 signalling. J Drug Target. 5:1–10. doi: https://doi.org/10.1080/1061186X.2020.1722136
- Ali BH, Al Moundhri MS. 2006. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol. 44:1173–1183. doi: https://doi.org/10.1016/j.fct.2006.01.013
- Arafa MH, Atteia HH. 2019. Protective role of epigallocatechin gallate in a rat model of cisplatin-induced cerebral inflammation and oxidative damage: impact of modulating NF-κB and Nrf2. Neurotox Res. 36:551–562. doi: https://doi.org/10.1007/s12640-019-00044-8
- Arany I, Safirstein RL. 2003. Cisplatin nephrotoxicity. Semin Nephrol. 23:460–464. doi: https://doi.org/10.1016/S0270-9295(03)00089-5
- Berndtsson M, Hagg M, Panaretakis T, Havelka AM, Shoshan MC, Linder S. 2007. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int J Cancer. 120:175–180. doi: https://doi.org/10.1002/ijc.22132
- Boroja T, Katanić J, Rosić G, Selaković D, Joksimović J, Mišić D, Stanković V, Jovičić N, Mihailović V. 2018. Summer savory (Satureja hortensis L.) extract: Phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem Toxicol. 118:252–263. doi: https://doi.org/10.1016/j.fct.2018.05.001
- Chválová K, Brabec V, Kaspárková J. 2007. Mechanism of the formation of DNA–protein cross-links by antitumor cisplatin. Nucl Acids Res. 35:1812–1821. doi: https://doi.org/10.1093/nar/gkm032
- Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstädt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH, et al. 2010. Organic cation transporter 2 mediates cisplatin induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 176:1169–1180. doi: https://doi.org/10.2353/ajpath.2010.090610
- Crona DJ, Faso A, Nishijima TF, McGraw KA, Galsky MD, Milowsky MI. 2017. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist. 22:609–619. doi: https://doi.org/10.1634/theoncologist.2016-0319
- Dasari S, Tchounwou PB. 2014. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 740:364–378. doi: https://doi.org/10.1016/j.ejphar.2014.07.025
- Florea AM, Busselberg D. 2011. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel). 3:1351–1371. doi: https://doi.org/10.3390/cancers3011351
- Fuertes MA, Castilla J, Alonso C, Perez JM. 2003. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 10:257–266. doi: https://doi.org/10.2174/0929867033368484
- Guo Y, Wang M, Mou J, Zhao Z, Yang J, Zhu F, Pei G, Zhu H, Wang Y, Xu G, et al. 2018. Pretreatment of Huaiqihuang extractum protects against cisplatin induced nephrotoxicity. Sci Rep. 8:7333. doi: https://doi.org/10.1038/s41598-018-25610-6
- Hagar H, Medany AE, Salam R, Medany GE, Nayal OA. 2015. Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp Toxicol Pathol. 67:133–141. doi: https://doi.org/10.1016/j.etp.2014.11.001
- Hassan SM, Khalaf MM, Sadek SA, Abo-Youssef AM. 2017. Protective effect of apigenin and myricetin against cisplatin-induced nephrotoxicity in mice. Pharm Biol. 55:766–774. doi: https://doi.org/10.1080/13880209.2016.1275704
- Jin F, Chen X, Yan H, Xu Z, Yang B, Luo P, He Q. 2020. Bisdemethoxycurcumin attenuates cisplatin-induced renal injury through anti-apoptosis, anti-oxidant and anti-inflammatory. Eur J Pharmacol. 874:173026. doi: https://doi.org/10.1016/j.ejphar.2020.173026
- Jing T, Liao J, Shen K, Chen TZ, Wang Y, Jin B, Pan H. 2019. Protective effect of urolithin a on cisplatin-induced nephrotoxicity in mice via modulation of inflammation and oxidative stress. Food Chem Toxicol. 129:108–114. doi: https://doi.org/10.1016/j.fct.2019.04.031
- Kandemir FM, Yildirim S, Caglayan C, Kucukler S, Eser G. 2019. Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environ Sci Pollut Res Int. 26:22562–22574. doi: https://doi.org/10.1007/s11356-019-05505-3
- Kang KP, Kim DH, Jung YJ, Lee AS, Lee S, Lee SY, Jang KY, Sung MJ, Park SK, Kim W. 2009. Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol Dial Transplant. 24:3012–3020. doi: https://doi.org/10.1093/ndt/gfp242
- Lee HS, Kim BK, Nam Y, Sohn UD, Park ES, Hong SA, Lee JH, Chung YH, Jeong JH. 2013. Protective role of phosphatidylcholine against cisplatin-induced renal toxicity and oxidative stress in rats. Food Chem Toxicol. 58:388–393. doi: https://doi.org/10.1016/j.fct.2013.05.005
- Li YZ, Ren S, Yan XT, Li HP, Li W, Zheng B, Wang Z, Liu YY. 2018. Improvement of cisplatin-induced renal dysfunction by Schisandra chinensis stems via anti-inflammation and anti-apoptosis effects. J Ethnopharmacol. 217:228–237. doi: https://doi.org/10.1016/j.jep.2018.01.033
- Lomeli N, Di K, Czerniawski J, Guzowski JF, Bota DA. 2017. Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic Biol Med. 102:274–286. doi: https://doi.org/10.1016/j.freeradbiomed.2016.11.046
- Lu MY, Chen CC, Lee LY, Lin TW, Kuo CF. 2015. N(6)-(2-hydroxyethyl)adenosine in the medicinal mushroom Cordyceps cicadae attenuates lipopolysaccharide-stimulated pro-inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathways. J Nat Prod. 78:2452–2460. doi: https://doi.org/10.1021/acs.jnatprod.5b00573
- Malik S, Suchal K, Bhatia J, Khan S, Vasisth S, Tomar A, Goyal S, Kumar R, Arya DS, Ojha SK. 2016. Therapeutic potential and molecular mechanisms of Emblica officinalis gaertn in countering nephrotoxicity in rats induced by the chemotherapeutic agent cisplatin. Front Pharmacol. 7:350. doi: https://doi.org/10.3389/fphar.2016.00350
- Manohar S, Leung N. 2018. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 31:15–25. doi: https://doi.org/10.1007/s40620-017-0392-z
- Meng H, Fu G, Shen J, Shen K, Xu Z, Wang Y, Jin B, Pan H. 2017. Ameliorative effect of daidzein on cisplatin-induced nephrotoxicity in mice via modulation of inflammation, oxidative stress, and cell death. Oxid Med Cell Longev. 2017:1. doi: https://doi.org/10.1155/2017/3140680
- Ning Y, Shi Y, Chen J, Song N, Cai J, Fang Y, Yu X, Ji J, Ding X. 2018. Necrostatin-1 attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis and oxidative stress and retains klotho expression. Front Pharmacol. 9:384. doi: https://doi.org/10.3389/fphar.2018.00384
- Qin X, Meghana K, Sowjanya NL, Sushma KR, Krishna CG, Manasa J, Sita GJA, Gowthami M, Honeyshmitha D, Srikanth G, SreeHarsha N. 2019. Embelin attenuates cisplatin-induced nephrotoxicity: involving inhibition of oxidative stress and inflammation in addition with activation of Nrf-2/Ho-1 pathway. Biofactors. 45:471–478. doi: https://doi.org/10.1002/biof.1502
- Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M, Rachamalla SS, Sistla R. 2014. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-kappa B activation and antioxidant defense. PLoS One. 9:e105070. doi: https://doi.org/10.1371/journal.pone.0105070
- Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Duenas M, Prieto M, Sánchez-López E, Thomale J, Ruiz-Ortega M, López-Novoa JM, Morales AI. 2017. Differential effect of quercetin on cisplatin-induced toxicity in kidney and tumor tissues. Food Chem Toxicol. 107:226–236. doi: https://doi.org/10.1016/j.fct.2017.06.047
- Schanz M, Schricker S, Pfister F, Alscher MD, Kimmel M. 2017. Renal complications of cancer therapies. Drugs Today (Barc). 54:561–575. doi: https://doi.org/10.1358/dot.2018.54.9.2874064
- Sioud F, Ben Toumia I, Lahmer A, Khlifi R, Dhaouefi Z, Maatouk M, Ghedira K, Chekir-Ghedira L. 2020. Methanolic extract of Ephedra alata ameliorates cisplatin-induced nephrotoxicity and hepatotoxicity through reducing oxidative stress and genotoxicity. Environ Sci Pollut Res Int. 27:12792–12801. doi: https://doi.org/10.1007/s11356-020-07904-3
- Sun CY, Nie J, Zheng ZL, Zhao J, Wu LM, Zhu Y, Su ZQ, Zheng GJ, Feng B. 2019. Renoprotective effect of scutellarin on cisplatin-induced renal injury in mice: impact on inflammation, apoptosis, and autophagy. Biomed Pharmacother. 112:108647. doi: https://doi.org/10.1016/j.biopha.2019.108647
- Tessoulin B, Thomare P, Delande E, Moynard J, Gastinne T, Moreau A, Bossard C, Mahé B, Blin N, Dubruille V, et al. 2017. Carboplatin instead of cisplatin in combination with dexamethasone, high-dose cytarabine with or without rituximab (DHAC+/-R) is an effective treatment with low toxicity in Hodgkin’s and non-Hodgkin’s lymphomas. Ann Hematol. 96:943–950. doi: https://doi.org/10.1007/s00277-017-2981-2
- Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, Arsenijevic N. 2019. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 26:25. doi: https://doi.org/10.1186/s12929-019-0518-9
- Wang X, Qin A, Xiao F, Olatunji OJ, Zhang S, Pan D, Han W, Wang D, Ni Y. 2019. N6-(2-hydroxyethyl)-adenosine from Cordyceps cicadae protects against diabetic kidney disease via alleviation of oxidative stress and inflammation. J Food Biochem. 43:e12727. doi: https://doi.org/10.1111/jfbc.12727
- Xiao T, Choudhary S, Zhang W, Ansari NH, Salahudeen A. 2003. Possible involvement of oxidative stress in cisplatin-induced apoptosis in LLC-PK1 cells. J Toxicol Environ Health A. 66:469–479. doi: https://doi.org/10.1080/15287390306449
- Xing JJ, Hou JG, Liu Y, Zhang RB, Jiang S, Ren S, Wang YP, Shen Q, Li W, Li XD, Wang Z. 2019. Supplementation of saponins from leaves of Panax quinquefolius mitigates cisplatin-evoked cardiotoxicity via inhibiting oxidative stress-associated inflammation and apoptosis in mice. Antioxidants. 8:9. doi: https://doi.org/10.3390/antiox8090347
- Yang L, Yang Q, Li J, Hou G, Chen T, Ye M. 2019. Nephroprotective effects of Lachnum melanin against acute kidney injury induced by cisplatin in mice. Process Biochem. 83:198–205. doi: https://doi.org/10.1016/j.procbio.2019.05.001
- Yucetas S, Ucler N, Cakir T. 2019. The effects of agomelatine on the biochemical and pathological features of cisplatin-induced peripheral neuropathy: the first experimental study in rats. Turk Neurosurg. 29:901–908.
- Zhang K, Weng H, Yang J, Wu C. 2020. Protective effect of Liuwei Dihuang Pill on cisplatin-induced reproductive toxicity and genotoxicity in male mice. J Ethnopharmacol. 247:112269. doi: https://doi.org/10.1016/j.jep.2019.112269
- Zhang L, Wu T, Olatunji OJ, Tang J, Wei Y, Ouyang Z. 2019. N6-(2-hydroxyethyl)-adenosine from Cordyceps cicadae attenuates hydrogen peroxide induced oxidative toxicity in PC12 cells. Metab Brain Dis. 34:325–334.
- Zheng R, Zhu R, Li X, Li X, Shen L, Chen Y, Zhong Y, Deng Y. 2018. N6-(2-hydroxyethyl) adenosine from Cordyceps cicadae ameliorates renal interstitial fibrosis and prevents inflammation via TGF-β1/Smad and NF-κB signaling pathway. Front Physiol. 9:1229. doi: https://doi.org/10.3389/fphys.2018.01229