Abstract
Severe acute pancreatitis (SAP) usually causes multiple-organ dysfunction syndrome and exhibits a high mortality rate. It has been previously reported that berberine, as a dominant alkaloid, can be effectively used against inflammation. However, there is limited data on the effects of berberine hydrochloride (BBRH) on inflammation in SAP rat models. Male Sprague–Dawley rats were given preliminary intraperitoneal injections of BBRH 30 min prior to retrograde infusion of 5% sodium taurocholate in their biliopancreatic ducts. The rats were sacrificed 12 h after taurocholate administration, and tissues and blood samples were then acquired for histological, chemical, and molecular analyses. The SAP models exhibited enhanced edema, ascitic fluid volume, neutrophil infiltration, serum amylase and lipase levels, and interleukin (IL)-6, tumor necrosis factor-α, and IL-1β levels, and reduced IL-10 concentration, while BBRH administration reversed these changes. Further study showed that in the SAP model, protein and mRNA levels of nuclear factor kappa-light-chain-enhancer of activated B (NFκB) p65 and toll-like receptor 4 (TLR4) were increased and inhibitor of nuclear factor kappa B (IκBα) expression was decreased, while pretreatment with BBRH significantly decreased the activation of the TLR4/IκBα/NFκB pathway. The data obtained displayed that BBRH mitigated acute pancreatitis by suppressing the TLR4/IκB/NFκB pathway.
Introduction
Severe acute pancreatitis (SAP), a threatening acute abdominal disease, exhibits a high rate of mortality (Felderbauer et al. Citation2005; Yuan et al. Citation2009). Excellent advances have been achieved in the diagnosis and treatment of SAP, but the rate of mortality remains high (Zhang et al. Citation2010). Consequently, SAP management is complicated. It is crucial to explore the molecular etiology of SAP development, especially the mechanisms that stimulate innate immune reactions (Sharif et al. Citation2009). It was previously indicated that the extent and prognosis of pancreatitis might rely on events that occurred subsequent to acinar cell damage (Ding et al. Citation2010).
Despite considerable research in this area, the exact SAP etiology has not been completely understood yet. Previous studies demonstrated enhanced generation of inflammatory cytokines and stimulation of inflammatory pathways in patients and animals with SAP (Satoh et al. Citation1999; Makhija and Kingsnorth Citation2002). It has been suggested that stimulation of nuclear factor kappa-light-chain-enhancer of activated B (NFκB) serves as an initial and essential event during the development of inflammation in SAP (Jakkampudi et al. Citation2016). As a fully-characterized transcriptional factor, NFκB contributes to the regulation of multiple inflammatory and immune reactions (Blackwell and Christman Citation1997), unites the early acinar damage with systemic inflammatory reactions, and preserves the latter (Long et al. Citation2005). The results of the SAP animal studies suggested that the NFκB pathway was stimulated by various agents, including sodium taurocholate, cholecystokinin, and inflammatory cytokines (Rakonczay et al. Citation2008). Toll-like receptor 4 (TLR4) is a rare mammalian pattern-recognition receptor, which participates in innate immune reactions. It is stimulated by damage-associated molecular pattern (DAMP) and prototypical pathogen-associated molecular pattern (PAMP) proteins (Li et al. Citation2016). It has been recently proven that TLR4, instead of TLR2, participates in the regulation of inflammation and pancreatic tissue injury in SAP that is triggered by retrograde taurocholate infusion (Awla et al. Citation2011). Consequently, regulation of the stimulation of the TLR4/NFκB pathway, followed by attenuation of nonspecific inflammation, could serve as a promising strategy to treat SAP.
As a traditional Chinese medicine, berberine is used as a therapeutic agent for various inflammatory disorders (Jia et al. Citation2012). Previous research revealed that berberine significantly reduced interleukin (IL)-1 and tumor necrosis factor (TNF)-α levels in the lipopolysaccharide (LPS)-triggered murine hepatic damage model (Vento et al. Citation2009). Berberine hydrochloride (BBRH) (C20H19ClNO4), a type of quaternary ammonium compound, exists in plants that contain isoquinoline alkaloids. Such plants include those from the Ranunculaceae and Rutaceae families (Kim et al. Citation2014). Besides, relevant studies confirmed that BBRH showed anti-inflammatory, lipid-reducing, and anticancer activities (Vento et al. Citation2009; Li et al. Citation2014). Further, numerous studies reported its anti-inflammatory effects. Zhang et al. found that BBRH showed a notably protective effect in mice with LPS-triggered hepatic impairment and decreased the release of TNF-α and IL-1 (Zhang et al. Citation2014). Moreover, Nemoto et al. demonstrated that BBRH could prevent intestine adhesion and inflammation after surgery, dramatically decrease the adherent density and severity, and likewise visibly diminish the expression of relevant inflammatory mediators (Nemoto et al. Citation2002). Choi et al. affirmed that BBRH mitigated the severity of pancreatitis by suppressing the activation of NFκB, c-Jun N-terminal kinases (JNK), and p38 and modulating acute pancreatitis (AP) (Choi et al. Citation2017). The pharmacological effects of BBRH in rats with SAP have been extensively reported, while the underlying molecular mechanisms remain uninvestigated.
Material and methods
Animal treatment, grouping, and model construction
Sixty healthy clean male Sprague Dawley rats, aged 3–4 months and weighing 180–250 g, were bought from the experimental animal center of our facility. The animal experiments were approved by the local ethical committee and conducted following the Guideline for the Care and Use of Laboratory Animals of The Affiliated Hospital of Qingdao University. The rats were acclimatized to the laboratory conditions 1 week prior to the experiment. Water was freely available, but food consumption was restrained 12 h before the experiment.
The rats were randomized into four categories: normal group, SAP group, preliminary BBRH-treated group, and preliminary vehicle-treated group. Each group was further divided into three categories (each category comprised five rats) based on the duration of the experiment (6, 12, and 24 h).
SAP modeling
To anaesthetize the rats, 4% chloral hydrate (200 mg/kg body) was administered as an intraperitoneal injection. The rats went into a deep sleep, and this was the major parameter to ensure that the rats were completely anesthetized. After shaving and draping the rats, their abdomens were cut along the lower abdominal midline under aseptic conditions. The tip of a syringe needle (1 mL) was set into the pancreatic duct through the opening of the duodenal papilla. Two small artery clippings were used to occlude the pancreatic puncture and bile duct, and 5% sodium taurocholate was injected at a rate of 0.1 mL/min for 4 min. After the injection, a 5-min suspension was conducted to examine pancreatic tissue edema in the rats. The vessel clamp was then removed, and the abdomen was closed in layers.
The same procedure, excluding the sodium taurocholate injection in the pancreatic duct, was performed in the rats from the normal group. The BBRH- and vehicle-treated groups received intraperitoneal BBRH injection (100 mg/kg) and normal saline, respectively, 30 min prior to model construction. All rats received a subcutaneous saline injection (0.2 mL/kg) after the surgery to compensate for the volume of liquid lost. The rats were allowed to drink water freely, but food consumption was restrained. Degrees of edema larger than 8 represented humane endpoints. After the experiments, the rats (150–180 mg) were humanely sacrificed by spinal dislocation after anesthesia, according to the guidelines of animal ethics.
Histological assessment
Paraffin slices of pancreatic specimens were stained with hematoxylin and eosin (HE) and observed under a light microscope. Histopathological assessment was by two pathologists blinded to the study design. Levels of necrosis and inflammatory cell infiltration were scored on a scale from 0 to 3 (0 being normal and 3 being severe) (Jo et al. Citation2014).
Ascitic fluid volume
Dry cotton balls were used to determine the ascitic fluid weight. After sacrificing the rats at the indicated time-points, suction of the ascitic fluid was performed using dry cotton balls. The weight of the dry and wet cotton balls was measured before and after draining the ascitic fluid. Ascitic fluid volume was then determined by calculating the variation in the weight of the cotton balls (ascitic density was estimated to be 1 mg/mL, and the maximum level of ascitic fluid was ∼5% per animal body weight).
Serum amylase and lipase analysis
The rats were anesthetized using 4% isoflurane. Image retro-orbital bleeding was performed to obtain blood samples (1.5–2 mL). Serum lipase and amylase levels were assessed using enzymatic approaches. Amylase and lipase activities were evaluated using specific kits from Zhongsheng Beikong Bio-Technology, Beijing, China and Nanjing Jiancheng Corp., Nanjing, China, respectively, according to the manufacturers’ instructions.
Immunohistochemistry (IHC)
Paraffin slices of pancreatic specimens were deparaffinized and dehydrated with graded ethanol. The specimens were washed with phosphate-buffered saline (PBS) thrice after the antigen was retrieved using citric acid. This was followed by blocking of the specimens using blocking liquid [tri-buffered saline with tween (TBST)/5% bovine serum albumin (BSA)] at room temperature for 20 min. The slices were incubated with rabbit anti-myeloperoxidase (MPO) antibodies (Abcam, ab9535, 1:500) at 4°C overnight and subsequently with secondary goat anti-rabbit antibodies at 37°C for 1 h. They were then counterstained with hematoxylin and observed under a light microscope.
Western blotting (WB)
Tissues were homogenized and lysed using the RIPA lysis buffer. After incubation of the tissues in the buffer at 4°C for 1 h, cell debris was eliminated by centrifugation at a high speed for 20 min. SDS-PAGE was conducted to resolve proteins, which were then transferred to PVDF membranes (Millipore). The membranes were blocked by incubating them with 5% BSA in PBS-Tween (PBST) at 4°C for 1 h. The membranes were then incubated with specific primary antibodies (anti-IL-1β antibody, Abcam, ab9722, 1:2000; anti-IL-6 antibody, Abcam, ab6672, 1:2000; anti-IL-10 antibody, Abcam, ab9969, 1:1000; anti-TNF-α antibody, Abcam, ab6671, 1:2000; anti-TLR4 antibody, Abcam, ab13867, 1:2000; anti-NFκB antibody, Abcam, ab231481, 1:1000; and anti-IκBα antibody, ab7217, 1:2000) and then with anti-mouse or anti-rabbit secondary antibodies that were conjugated with horseradish peroxidase.
Quantitative real-time polymerase chain reaction (Q-PCR)
TRIzol (1 mL) was utilized to extract total RNA from breast cancer cells. Reverse transcription PCR of RNA (10 µg) of every group was performed using random hexamers. A control reaction, without reverse transcriptase, was also performed. ABI 7300 was adopted to assess the related specific expression of RNA using the PCR primers. The sequences for the Q-PCR primers are listed in Table . SYBR green was utilized to perform Q-PCR, according to the following procedure: initial denaturation at 95°C for 0.5 h and 40 two-step cycles (95°C for 1–15 s and 62°C for 5–30 s). Dissociation curve assessment was performed after every test to ensure the examination of specific targets.
Table 1. Primer sequences used in this study.
Statistical analysis
SPSS 19.0 was used for statistical analysis. ANOVA with Tukey’s post hoc test was used for comparison of multiple groups, while student’s t-test was utilized for comparison of two groups. The differences were regarded significant when P <0.05.
Results
Effects of BBRH on pancreatic histopathology
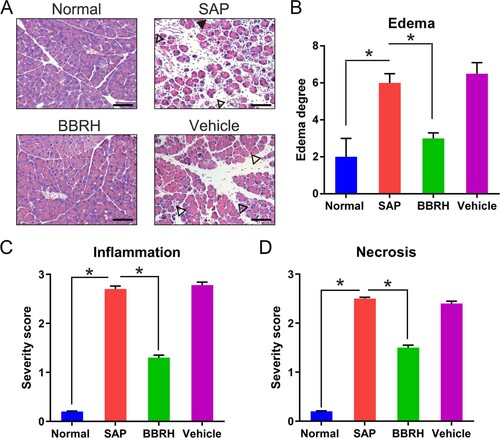
Pancreatic specimens of the normal rats displayed normal structure without any histopathological alteration, while those of rats with SAP displayed pathological alterations, such as enhanced edema, vacuolization, inflammatory cell infiltration, and necrosis (Figure (A,B)). Nevertheless, these alterations were significantly reversed after BBRH administration. Furthermore, to examine the effects of BBRH on the development and subsequent severity of SAP, we investigated the histological score of the pancreas against SAP. In normal rats, no histological abnormalities were observed in the pancreas. In rats with SAP, histological examination revealed tissue damage, characterized by inflammatory cell infiltration, interstitial edema, and necrosis, suggesting successful SAP modeling in the rats. However, BBRH treatment significantly reduced pancreatic edema and inflammation (Figure (C,D)).
Figure 1. Effects of BBRH on the histology of the pancreas in SAP. The pancreas was cut 24 h after SAP modeling. (A) Representative HE staining and (B) histological extent of edema are displayed. Scale bar, 50 µm. Result is presented in the form of mean ± SD (n = 15 per group). Differences among various groups are assessed using ANOVA. *P < 0.05.
Effects of BBRH on the volume of ascitic fluid and serum amylase and lipase levels
Volume of the ascitic fluid in rats of the SAP group, compared with that in rats of the normal and BBRH-treated groups, was significantly increased. The values were considerably elevated 24 h post model construction. Furthermore, volume of the ascitic fluid in rats of the SAP group was elevated, while that in rats of the BBRH-treated group rapidly plateaued during model construction (Figure (A)).
Figure 2. Effects of BBRH on the volume of ascitic fluid and serum amylase and lipase levels in all groups. (A) Ascitic fluid volume in pancreatic tissue. (B, C) Blood samples were collected at 6, 12, and 24 h for amylase and lipase assay after SAP modeling. Result is presented in the form of mean ± SD (n = 15 per group). Differences among various groups are assessed using ANOVA. *P < 0.05.
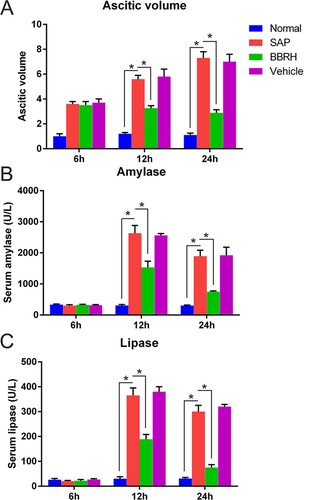
Serum lipase and amylase levels are commonly recognized as SAP markers, which represent the severity of the disease. BBRH administration significantly reduced lipase and amylase levels, as shown in Figure (B,C).
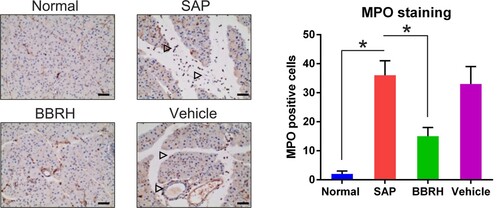
BBRH reduced MPO concentration in the pancreas of the animals with SAP
Rats with SAP showed a higher number of MPO-positive cells and more pancreatic neutrophil infiltration (Figure ) than did BBRH-treated rats.
Figure 3. Effects of BBRH on pancreatic neutrophil infiltration observed after MPO staining. Representative IHC images of the MPO stained pancreas. The frequencies of pancreatic MPO-positive cells are calculated. Scale bar, 50 µm. Result is presented in the form of mean ± SD (n = 15 per group). Differences among various groups are assessed using ANOVA. *P < 0.05.
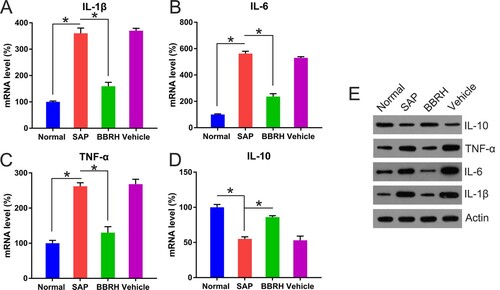
Effects of BBRH on serum levels of inflammatory and anti-inflammatory cytokines
Serum levels of inflammatory cytokines (IL-1β, TNF-α, and IL-6) were elevated, whereas those of anti-inflammatory cytokines (IL-10) were repressed in rats with SAP, compared to normal rats, at not only the transcriptional level but also the translational level. Furthermore, these alterations were reversed after BBRH administration (Figure (A–E)).
Figure 4. Transcription and translation of inflammatory (IL-1β, IL-6, and TNF-α) and anti-inflammatory (IL-10) cytokines in pancreatic tissue. (A-D) Q-PCR is conducted to examine the expression of IL-1β, IL-6, TNF-α, and IL-10 in pancreatic specimens after 12 h. GAPDH serves as an internal control. (E) WB is employed to determine protein concentration of these cytokines. Result is presented in the form of mean ± SD (n = 15 per group). Differences among various groups are assessed using ANOVA. *P < 0.05.
Effects of BBRH on the expression of TLR4 and NFκB p65 in the pancreas
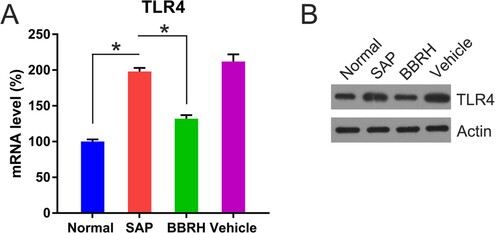
TLR4 transcription in the pancreas of rats with SAP, compared with that in the pancreas of normal rats, was significantly enhanced, while TLR4 expression in the pancreas of BBRH-treated rats was significantly reduced (Figure (A)). The protein level of TLR4 followed a similar trend, as detected by WB (Figure (B)).
Figure 5. Transcription and translation of TLR4 in pancreatic tissue. (A) Q-PCR is conducted to examine the expression of TLR4 mRNA in pancreatic specimens after 12 h. GAPDH serves as an internal control. (B) WB is used to examine the protein concentration of TLR4. Result is presented in the form of mean ± SD (n = 15 per group). Differences among various groups are assessed using ANOVA. *P < 0.05.
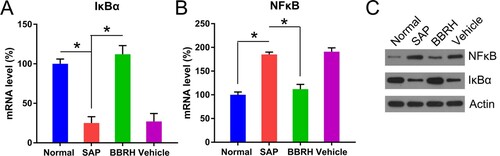
For the downstream IκBα and NFκB expression analysis, both Q-PCR and WB were performed. Q-PCR data showed that both transcription and translation of IκBα were reduced in rats with SAP, compared with normal rats. However, an increase in the expression of IκBα was observed in BBRH-treated rats (Figure (A,C)). NFκB expression followed a reverse trend. SAP modeling significantly increased p65 expression; however, BBRH administration restored p65 levels to normal (Figure (B,C)).
Figure 6. Transcription and translation of IκBα and NFκB p65 in pancreatic tissue. (A, B) Q-PCR is conducted to examine the expression of IκBα and NFκB p65 mRNA in pancreatic specimens after 12 h. GAPDH serves as an internal control. (C) WB is used to examine the protein concentration of IκBα and NFκB p65. Result is presented in the form of mean ± SD (n = 15 per group). Differences among various groups are assessed using ANOVA. *P < 0.05.
Discussion
Our study aimed to verify that BBRH noticeably attenuated the extent of SAP by repressing the TLR4/NFκB pathway. Manifestations of SAP, triggered via sodium taurocholate injection, in rats resembled those of SAP in humans (Mareninova et al. Citation2006). Previous studies showed that berberine chloride demonstrated a prophylactic effect on propyl thiouracil induced-thyroidal dysfunction in female rats (Maurya et al. Citation2016). It was also suggested that BBRH was a suitable prophylactic drug for colorectal adenoma recurrence (Chen et al. Citation2019), colon cancer (Ghareeb et al. Citation2018), and diabetic complications (Zhang and Chen Citation2012). We examined the effects of BBRH administration on SAP in rats. BBRH significantly deterred pancreatic damage in SAP, as evidenced by the histological features, serum lipase and amylase levels, and MPO staining. Besides, BBRH could modulate the expression of inflammatory cytokines. Although BBRH was given before the taurocholate injection, no data that supports that BBRH treatment improves existing SAP has been reported. These findings indicated that BBRH was a potential prophylactic drug for SAP.
BBRH has been widely used as a therapeutic agent for infections and heart failure (Fu et al. Citation2015) due to its antiviral, antibacterial, and blood pressure-reducing activities. It was previously demonstrated that BBRH could be effectively used against LPS-triggered endometritis in mice (Wang et al. Citation2018). Neutrophil infiltration and MPO function was repressed by BBRH via NFκB stimulation (Shao et al. Citation2015). Several studies reported that treatment of mice with BBRH decreased pancreatic and pancreatitis-relevant pulmonary impairment and activities of amylase and lipase and suppressed the expression of inflammatory cytokines and inducible nitrous oxide synthase. Additionally, BBRH administration dramatically suppressed JNK activation in cerulein-triggered AP. Deactivating JNK improved pancreatitis and suppressed inflammatory mediators (Choi et al. Citation2016). In our study, anti-inflammatory effects of BBRH on SAP in rats, in terms of multiple parameters, were examined. It was discovered that BBRH could ameliorate SAP by regulating the TLR4/NFκB pathway.
The molecular etiology of SAP development remains unclear [5]. However, it has been generally acknowledged that SAP is included in systemic inflammatory response syndrome (SIRS) and can cause multiple-organ dysfunction syndrome (MODS) (Khanna et al. Citation2013; Gasparović et al. Citation2014) by stimulation of trypsin, release-triggered pancreatic self-digestion, necrosis, and hemorrhage (Armstrong et al. Citation2013). It has been proved that inflammatory cytokines, including TNF-α, IL-1β, and IL-6, trigger, augment, and preserve the inflammatory reactions in SAP, contributing to SAP development. They also significantly affect SAP prognosis because SAP can stimulate SIRS and MODS, which is a severe complication, resulting in a high rate of mortality (De Beaux et al. Citation1996; Kusske et al. Citation1996). IL-10 counteracts inflammation, prohibits generation of inflammatory cytokines, and represses SAP severity (Zou et al. Citation2002). IL-10 expression has been proven to decrease lung damage and the rate of mortality in SAP models (Osman et al. Citation1998). The study indicated that levels of IL-1β, IL-6, and TNF-α were dramatically elevated, while that of IL-10 was reduced in rats with SAP after 12 and 24 h. BBRH administration suppressed the expression of TNF-α, IL-1β, and IL-6, but enhanced that of IL-10. These findings proved that the defensive action of BBRH could be attributed to the suppression of inflammatory mediators and generation of anti-inflammatory cytokines.
TLR4 is a type of inflammatory transmembrane receptor (Vaure and Liu Citation2014). TLR4, generated from the epithelia of pancreatic ducts and acini, stimulates multiple signaling agents via a combination of certain ligands through the MyD88 pathway (Zhai et al. Citation2004). NFκB is sequestered in the cytoplasm of resting cells by a group of inhibitory proteins of NFκB (IκBs) under normal circumstances. Signaling sensors, including TAK1, TRAF-6, and IRAK, stimulate NFκB and trigger transcription of its target and secretion of inflammatory mediators, such as TNF-α and IL-10, thereby regulating the inflammatory reactions in SAP (Doyle et al. Citation2005). In the present study, the expression of IκBα, TLR4, and NFκB was examined to assess the effects of BBRH on the stimulation of the NFκB pathway during SAP. Sodium taurocholate activation significantly up-regulated TLR4, and this, consequently, led to IκBα degeneration and NFκB stabilization. Nevertheless, TLR4 up-regulation, NFκB stimulation, and IκBα degeneration were significantly reversed on preliminary BBRH administration, conforming to down-regulation of TNF-α, IL-1β, and IL-6, and this finding was consistent with that of a previous report (Choi et al. Citation2016). These findings indicated that down-regulation of inflammatory cytokines could be attributed to the impaired stimulation of the TLR4/NFκB pathway by BBRH.
Collectively, the study revealed that BBRH mitigated SAP and BBRH administration could possibly be a potential prophylactic strategy for SAP treatment.
Supplemental Material
Download PDF (56.8 KB)Data availability statement
The raw data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Armstrong J, Cash N, Soares P, Souza M, Sutton R, Criddle D. 2013. Oxidative stress in acute pancreatitis: lost in translation? Free Radical Res. 47(11):917–933. doi: https://doi.org/10.3109/10715762.2013.835046
- Awla D, Abdulla A, Regnér S, Thorlacius H. 2011. TLR4 but not TLR2 regulates inflammation and tissue damage in acute pancreatitis induced by retrograde infusion of taurocholate. Inflammation Res. 60(12):1093–1098. doi: https://doi.org/10.1007/s00011-011-0370-1
- Blackwell TS, Christman JW. 1997. The role of nuclear factor-κ B in cytokine gene regulation. Am J Respir Cell Mol Biol. 17(1):3–9. doi: https://doi.org/10.1165/ajrcmb.17.1.f132
- Chen Y-X, Gao Q-Y, Zou T-H, Wang B-M, Liu S-D, Sheng J-Q, Ren J-L, Zou X-P, Liu Z-J, Song Y-Y. 2019. Berberine Hydrochloride for the Prevention of Colorectal Adenomas: A Double-Blind, Randomised Controlled Multicenter Clinical Trial.
- Choi S-B, Bae G-S, Jo I-J, Song H-J, Park S-J. 2017. Effects of berberine on acute necrotizing pancreatitis and associated lung injury. Pancreas. 46(8):1046–1055. doi: https://doi.org/10.1097/MPA.0000000000000877
- Choi S-B, Bae G-S, Jo I-J, Wang S, Song H-J, Park S-J. 2016. Berberine inhibits inflammatory mediators and attenuates acute pancreatitis through deactivation of JNK signaling pathways. Mol Immunol. 74:27–38. doi: https://doi.org/10.1016/j.molimm.2016.04.011
- De Beaux A, Goldie A, Ross J, Carter D, Fearon K. 1996. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg. 83(3):349–353. doi: https://doi.org/10.1002/bjs.1800830317
- Ding S-Q, Li Y, Zhou Z-G, Wang C, Zhan L, Zhou B. 2010. Toll-like receptor 4-mediated apoptosis of pancreatic cells in cerulein-induced acute pancreatitis in mice. Hepatobiliary Pancreat Dis Int. 9(6):645–650.
- Doyle SL, Jefferies CA, O'Neill LA. 2005. Bruton's tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFκB activation by lipopolysaccharide. J Biol Chem. 280(25):23496–23501. doi: https://doi.org/10.1074/jbc.C500053200
- Felderbauer P, Müller C, Bulut K, Belyaev O, Schmitz F, Uhl W, Schmidt WE. 2005. Pathophysiology and treatment of acute pancreatitis: new therapeutic targets–a ray of hope? Basic Clin Pharmacol Toxicol. 97(6):342–350. doi: https://doi.org/10.1111/j.1742-7843.2005.pto_274.x
- Fu K, Lv X, Li W, Wang Y, Li H, Tian W, Cao R. 2015. Berberine hydrochloride attenuates lipopolysaccharide-induced endometritis in mice by suppressing activation of NF-κB signal pathway. Int Immunopharmacol. 24(1):128–132. doi: https://doi.org/10.1016/j.intimp.2014.11.002
- Gasparović V, Daković K, Gornik I, Radonić R. 2014. Severe acute pancreatitis as a part of multiple dysfunction syndrome. Coll Antropol. 38(1):125–128.
- Ghareeb AE, Moawed FS, Ghareeb DA, Kandil EI. 2018. Potential prophylactic effect of berberine against Rat colon Carcinoma Induce by 1, 2-Dimethyl Hydrazine. Asian Pac J Cancer Prev. 19(6):1685.
- Jakkampudi A, Jangala R, Reddy BR, Mitnala S, Reddy DN, Talukdar R. 2016. NF-κB in acute pancreatitis: mechanisms and therapeutic potential. Pancreatology. 16(4):477–488. doi: https://doi.org/10.1016/j.pan.2016.05.001
- Jia L, Liu J, Song Z, Pan X, Chen L, Cui X, Wang M. 2012. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J Pharm Pharmacol. 64(10):1510–1521. doi: https://doi.org/10.1111/j.2042-7158.2012.01529.x
- Jo I-J, Bae G-S, Choi SB, Kim D-G, Shin J-Y, Seo S-H, Choi M-O, Kim T-H, Song H-J, Park S-J. 2014. Fisetin attenuates cerulein-induced acute pancreatitis through down regulation of JNK and NF-κB signaling pathways. Eur J Pharmacol. 737:149–158. doi: https://doi.org/10.1016/j.ejphar.2014.05.018
- Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, Dixit V. 2013. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI scores, IL-6, CRP, and procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surg. 2013:1–10. doi: https://doi.org/10.1155/2013/367581
- Kim M, Shin MS, Lee JM, Cho HS, Kim CJ, Kim YJ, Choi HR, Jeon JW. 2014. Inhibitory effects of isoquinoline alkaloid berberine on ischemia-induced apoptosis via activation of phosphoinositide 3-kinase/protein kinase B signaling pathway. Int Neurourol J. 18(3):115. doi: https://doi.org/10.5213/inj.2014.18.3.115
- Kusske A, Rongione A, Reber H. 1996. Cytokines and acute pancreatitis. Gastroenterology. 110(2):639–642. doi: https://doi.org/10.1053/gast.1996.v110.agast960639
- Li G, Wu X, Yang L, He Y, Liu Y, Jin X, Yuan H. 2016. TLR4-mediated NF-κB signaling pathway mediates HMGB1-induced pancreatic injury in mice with severe acute pancreatitis. Int J Mol Med. 37(1):99–107. doi: https://doi.org/10.3892/ijmm.2015.2410
- Li X, Zhao S-J, Shi H-L, Qiu S-P, Xie J-Q, Wu H, Zhang B-B, Wang Z-T, Yuan J-Y, Wu X-J. 2014. Berberine hydrochloride IL-8 dependently inhibits invasion and IL-8-independently promotes cell apoptosis in MDA-MB-231 cells. Oncol Rep. 32(6):2777–2788. doi: https://doi.org/10.3892/or.2014.3520
- Long J, Song N, Liu X-P, Guo K-J, Guo R-X. 2005. Nuclear factor-kappaB activation on the reactive oxygen species in acute necrotizing pancreatitic rats. World J Gastroenterol. 11(27):4277. doi: https://doi.org/10.3748/wjg.v11.i27.4277
- Makhija R, Kingsnorth AN. 2002. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 9(4):401–410. doi: https://doi.org/10.1007/s005340200049
- Mareninova OA, Sung K-F, Hong P, Lugea A, Pandol SJ, Gukovsky I, Gukovskaya AS. 2006. Cell death in pancreatitis caspases protect from necrotizing pancreatitis. J Biol Chem. 281(6):3370–3381. doi: https://doi.org/10.1074/jbc.M511276200
- Maurya H, Dhiman S, Dua K, Gupta G. 2016. Pharmacological effect of berberine chloride in propyl thiouracil induced thyroidal dysfunction - A time Bound study in female rats. Recent Pat Drug Delivery Formulation. 10(2):165–173. eng. doi: https://doi.org/10.2174/1872211310666160321123610
- Nemoto S, Vallejo JG, Knuefermann P, Misra A, Defreitas G, Carabello BA, Mann DL. 2002. Escherichia coli LPS-induced LV dysfunction: role of toll-like receptor-4 in the adult heart. Am J Physiol-Heart Circ Physiol. 282(6):H2316–H2323. doi: https://doi.org/10.1152/ajpheart.00763.2001
- Osman MO, Jacobsen NO, Kristensen JU, Deleuran B, Gesser B, Larsen CG, Jensen SL. 1998. IT 9302, a synthetic interleukin-10 agonist, diminishes acute lung injury in rabbits with acute necrotizing pancreatitis. Surgery. 124(3):584–592. doi: https://doi.org/10.1016/S0039-6060(98)70106-0
- Rakonczay Z, Hegyi P, Takacs T, McCarroll J, Saluja AK. 2008. The role of NF-κB activation in the pathogenesis of acute pancreatitis. Gut. 57(2):259–267. doi: https://doi.org/10.1136/gut.2007.124115
- Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. 1999. Inhibition of nuclear factor-κB activation improves the survival of rats with taurocholate pancreatitis. Gut. 44(2):253–258. doi: https://doi.org/10.1136/gut.44.2.253
- Shao G, Tian Y, Wang H, Liu F, Xie G. 2015. Protective effects of melatonin on lipopolysaccharide-induced mastitis in mice. Int Immunopharmacol. 29(2):263–268. doi: https://doi.org/10.1016/j.intimp.2015.11.011
- Sharif R, Dawra R, Wasiluk K, Phillips P, Dudeja V, Kurt-Jones E, Finberg R, Saluja A. 2009. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 58(6):813–819. doi: https://doi.org/10.1136/gut.2008.170423
- Vaure C, Liu Y. 2014. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 5:316. doi: https://doi.org/10.3389/fimmu.2014.00316
- Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, Roberts LJ, Arduini A, Escobar JJ, Sastre J, et al. 2009. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 124(3):e439–e449. doi: https://doi.org/10.1542/peds.2009-0434
- Wang X, Feng S, Ding N, He Y, Li C, Li M, Ding X, Ding H, Li J, Wu J. 2018. Anti-Inflammatory effects of berberine hydrochloride in an LPS-induced murine model of Mastitis. Evid-Based Complement Altern Med. 2018:1–9.
- Yuan H, Jin X, Sun J, Li F, Feng Q, Zhang C, Cao Y, Wang Y. 2009. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas. 38(2):143–148. doi: https://doi.org/10.1097/MPA.0b013e31818166b4
- Zhai Y, O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. 2004. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 173(12):7115–7119. doi: https://doi.org/10.4049/jimmunol.173.12.7115
- Zhang M, Chen L. 2012. Berberine in type 2 diabetes therapy: a new perspective for an old antidiarrheal drug? Acta Pharm Sin B. 2(4):379–386. doi: https://doi.org/10.1016/j.apsb.2012.06.004
- Zhang Y, Li X, Zhang Q, Li J, Ju J, Du N, Liu X, Chen X, Cheng F, Yang L. 2014. Berberine hydrochloride prevents postsurgery intestinal adhesion and inflammation in rats. J Pharmacol Exp Ther. 349(3):417–426. doi: https://doi.org/10.1124/jpet.114.212795
- Zhang ZW, Zhang QY, Zhou MT, Liu NX, Chen TK, Zhu YF, Wu L. 2010. Antioxidant inhibits HMGB1 expression and reduces pancreas injury in rats with severe acute pancreatitis. Dig Dis Sci. 55(9):2529–2536. doi: https://doi.org/10.1007/s10620-009-1073-0
- Zou W-G, Wang D-S, Lang M-F, Jin D-Y, Xu D-H, Zheng Z-C, Wu Z-H, Liu X-Y. 2002. Human interleukin 10 gene therapy decreases the severity and mortality of lethal pancreatitis in rats. J Surg Res. 103(1):121–126. doi: https://doi.org/10.1006/jsre.2001.6327
