 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Many traditional plants have been used to fight life-threatening diseases such as diabetes, atherosclerosis and cancer. These plants have been shown to possess antioxidant activities, decrease the diabetes nuisance. The effect of 250 mg/kg b.wt of polyherbal drug (PHD) was investigated on high-fat diet-induced diabetic rats and impact of PHD administration was observed. The blood biochemical parameters such as glucose, insulin, hemoglobin, HbA1c, lipid profile and plasma antioxidants were evaluated. The increased levels of blood glucose, cholesterol, triglycerides and low-density lipoprotein cholesterol were noticed. In contrast, the levels of insulin and HDL-cholesterol were diminished. The oral administration of PHD showed a decrease in glucose level, cholesterol, triglycerides and LDL-cholesterol, whereas an increase in insulin level and HDL-cholesterol were recorded. What’s more, the antioxidant enzymes in diabetic control rats showed significantly decreased activities of SOD, catalase, glutathione peroxide activities and reduced glutathione (GSH) compared to treated diabetic rats and the extent of LPO. These results showed that our formulated PHDs have possessed antioxidant potential. The effect produced by the PHD on various parameters was comparable to that of Metformin – a standard anti-diabetic drug.
Introduction
The metabolic disorders such as hyperglycemia, altered lipid profile, carbohydrates and protein metabolisms and increased risk of cardiovascular disease complications are associated with diabetes mellitus (Sharafeldin and Rizvi Citation2015). In recent years, there has been an increased inclination towards the herbal formulations due to the trend towards the natural sources that are with minimal side effects. The mixture of several crude extracts, when used in formulation, enhances the beneficial effects through synergistic amplification and diminishes any possible adverse effects and offers an advantage over a single isolated ingredient.
Gymnema sylvestre is a highly effective anti-diabetic medicinal herb. Leaves contain lupeol, β-amyrin, stigmasterol, pentriacontane, hentricontane, α and β chlorophyll, resin, tartaric acid, gymnemic acid (anti-sweet compounds), the mixture of triterpene saponins, anthraquinone derivatives, alkaloids, betaine choline and trimethylamine (Karadeniz et al. Citation2005). Traditionally, the leaves of G. sylvestre were used for the treatment of diabetes and other disorders, while the flowers and bark are given in diseases related to phlegm (Kirtika and Basu Citation1975). A recent review described the antimicrobial, hepatoprotective, antihypercholesterolemic and anti-inflammatory activities of leaves of this plant (Agarwal et al. Citation2000; Kanetkar et al. Citation2007). They are also used for making anti-diabetic formulations in folk, ayurvedic and homoeopathic medicines. Cassia auriculata Linn.is found throughout central and southern parts of India and it has been widely used in Ayurvedic medicine, especially as an anti-diabetic, in the treatments of rheumatism, dysentery, asthma, cough and renal disorders (Kirtika and Basu Citation1975; Kumar et al. Citation2002; Kumar Rajagopal et al. Citation2003). All the parts of this plant are an excellent source of antioxidants which has proved their immense potency against degenerative diseases. The effect of aqueous extract of the flowers was examined on antioxidants and lipid peroxidation in the brain of streptozotocin-induced diabetic rats which showed the significant decrease in thiobarbituric reactive substances and hydroperoxide in the brain (Latha and Pari Citation2003).
Trigonella foenum graecum (Fenugreek) is belonging to the family of Fabaceae which is an annual herbaceous legume and consumed as a spice as well as a vegetable in different parts of the world. The recent researches have proved its potential against atherosclerosis, constipation, diabetes, high cholesterol and hyper-triglyceridemia (Ansari Citation2005; Taylor and Zaman Citation1997; Raju et al. Citation2001; Kaviarasan et al. Citation2007). The seeds of fenugreek contain alkaloids, flavonoids, saponins, amino acids, tannins and some steroidal glycosides, proteins and act as antioxidants or inhibitors of oxidation which retard or prevent the oxidation in general and prolong the life of oxidizable matter (Ministry of Health and Family Welfare Citation1996; Sharma et al. Citation1990). It has also been used as an appetite stimulant (Petit et al. Citation1993) and as a laxative (Riad and El-Baradie Citation1959).
Cinnamon (Cinnamomum verum, synonym C. zeylanicum) is a small evergreen tree, 10–15 m tall, belonging to the family Lauraceae, native to Sri Lanka and South India. The available in vitro and animal in vivo studies suggest that cinnamon has anti-inflammatory, antimicrobial, antibacterial, antioxidant, antitumor, cardiovascular, cholesterol-lowering and immunomodulatory effects (Denys Citation2012). Aqueous extracts from cinnamon have been shown to increase in vitro glucose uptake and glycogen synthesis, increase phosphorylation of the insulin receptor and likely help trigger the insulin cascade system (Imparl-Radosevich et al. Citation1998; Jarvill-Taylor et al. Citation2001).
Syzygium cumini Linn (Family: Myrtaceae), commonly known as Jam, is a popular seasonal fruit in India, Bangladesh and many other South East Asian countries. It is not only a delicious fruit but also an important traditional and modern medicine. Different parts of the plant, especially fruits, seeds and stem bark, possess promising activity against diabetes mellitus and it has been confirmed by several experimental and clinical studies and several earlier investigations have been reported from the different parts of the plant with antioxidant (Pandey and Khan Citation2002; Banerjee et al. Citation2005; Sultana et al. Citation2007; Mandal et al. Citation2008; Bopp et al. Citation2009; Bhuyan et al. Citation2010). The plant possesses a large number of chemical compounds such as vitamin C, gallic acid, tannins, anthocyanins, acetyl oleanolic acid, triterpenoids, ellagic acid, isoquercitin, quercetin, kaempferol and myricetin in different concentrations. Most of these compounds have been reported to possess nutritive and therapeutic potentials (Gupta and Sharma Citation1974; Bhatia and Bajaj Citation1975; Noomrio and Dahot Citation1996). Cassia auriculata, Gymnema sylvestre, Trigonella foenum graecum, Syzygium cumini (L.) and Cinnamomum zeylanicum are some of the herbal drugs which have been used individually for the treatment of various ailments in Traditional medicine and are known to have hypoglycemic, hypolipidemic and antioxidant properties. Hence, we have combined all these above-mentioned plants mixed in the defined ratio. The present study was designed to elucidate the oxidative stress behind the anti-diabetic effect of the polyherbal preparation using in vivo studies.
Material and methods
Sources of chemicals
High-fat diet (HFD) components such as cholesterol, cholic acid and sucrose were obtained from Sisco Research Laboratories Pvt. Ltd, Mumbai, India. Standard flavonoids were obtained from Sigma Chemical Company (St Louis, MO, USA) and coconut oil was obtained from the local market in Chennai. All other chemicals used were of analytical grade.
Preparation of polyherbal
The flower of Cassia auriculata and leaves of Gymnema sylvestre were collected from a local garden in the southern part of India (Kanchipuram and Villupuram District, Tamil Nadu). Seeds of Trigonella foenum graecum (Fenugreek), bark of Cinnamomum Zeylanicum and fruit of Syzygium cumini (L.) at the commercially matured stage were purchased from the market and the plant materials were air-dried under the shade to fine power using cutting mill and mixed with the defined ratio.
Determination of flavonoid constituents using HPLC
Chromatographic analysis was carried out by HPLC Shimadzu CLASS- VPV6.14 SP2. The operating parameters were as follows: Column: C18; Mobile Phase: Solvent A – Water (Millipore)–Acetic acid (25:1 v/v), Solvent B – Methanol; Pumps (Binary Gradient); T.Flow: 1.000 ml/min; P.Max: 400.0 kgf/cm2; P.Min: 0.0 kgf/cm2; CTO-10ASvp, Temperature: 40°C; SPD-10 Avp (Det.A): Lamp: D2; Polarity: +ve. The extraction was carried out using 2 g of powdered plant material with 50 ml of 95% ethanol under 80 kHz, 45°C in ultrasonic extraction device for 30 min, repeated twice. The extract was collected and filtered; the filtrate was dried at 50°C under reduced pressure in a rotary evaporator. The dried crude extract was dissolved in the 100 ml of the mobile phase. After filtering through a filter paper and a 0.45 mm membrane filter (Millipore), the extract was injected into HPLC by the autosampler. The gradient elution of solvent and solvent B had a significant effect on the resolution of compounds. As a result, solvent gradients were formed, using the dual-pumping system, by varying the proportion of solvent A to solvent B. Solvent B was increased to 50% in 4 min and subsequently increased to 80% in 10 min at a flow rate of 1.0 ml/min. The detection wavelength was 280 nm. Flavonoid constituents were identified by comparing their retention times to those of authentic standards under identical analysis conditions and UV spectra, using in house PDA library.
The samples are calculated using the following formula.
Animals
Adult male rats of Wistar strain weighing 195–205 g were procured from Central Animal House facility, Sri Muthukumaran Medical College Hospital & Research Institute, Chennai, Tamil Nadu, India. The animals were maintained under standard conditions of humidity, temperature (25 ± 2°C) and light (12 h light/dark). They were fed with standard rat pellet diet and water ad libitum. The study has got the approval from the Institutional Animal Ethical Committee (IAEC), regulated by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment & Forests (Animal Welfare Division), Government of India (IAEC No. 06/2008).
Induction of type 2 diabetes
Rats were fed an HFD containing cholesterol 1.5%, cholic acid 0.5%, coconut oil 30%, standard rat feed 68% and 30% sucrose through drinking water for the period of 60 days. On the 59th day of feeding, after overnight fasting, blood glucose was checked and the rats which have blood glucose above 200 mg/dl were chosen as type 2 diabetic rats. Treatment was started on the next day after confirmation of type 2 diabetes and this was considered as 1st day of treatment and it was continued for 45 days. The rats were fed with HFD during this period. Body weights of the various groups were recorded prior to induction of type 2 diabetes and end of the 45 days of treatment with the polyherbal drug (PHD) (Nampurath et al. Citation2008).
Experimental design
The animals were divided into five groups of six animals in each. PHD dissolved in distilled water and administered orally using an intragastric tube for the period of 45 days. Metformin is an anti-diabetic drug, dissolved in distilled water and used as a reference drug.
At the end of the experimental period (45th day), the animals were subjected to anesthesia and sacrificed by cervical decapitation. Blood samples from experimental animals were also collected in collection tubes coated with or without EDTA for the separation of plasma and serum to determine blood parameters.
Biochemical analysis
Plasma glucose was estimated by the method of Trinder using a reagent kit (Trinder Citation1969) and plasma insulin was measured by the method of Burgi et al. (Citation1988). HOMA-IR was calculated by the following formula (Kirubananthan et al. Citation2019)
Hemoglobin (Hb) and glycated hemoglobin (HbA1c) were estimated by the method of Drabkin and Austin (Citation1932) and Sudhakar and Pattabiraman (Citation1981), respectively. The activities of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were estimated (by using commercially available kits) by the method of Reitman and Frankel (Citation1957). Serum urea and creatinine were measured by the diacetyl monoxime method by Wybenga et al. (Citation1971) and Slot (Citation1965). Serum total cholesterol, triglyceride and high-density lipoprotein were estimated according to the methods of Zlatkis et al. (Citation1953), Foster and Dunn (Citation1973) and Burstein et al. (Citation1970). The serum levels of very low-density lipoprotein and low-density lipoprotein were calculated using the Friedewald formula (Friedewald et al. Citation1972).
LPO in serum was estimated by measuring TBARS and hydroperoxides using the methods of Fraga et al. (Citation1988) and Jiang et al. (Citation1992), respectively. The activity of CAT was estimated by the method of Sinha (Citation1952). The activity of SOD was assayed by the method of Kakkar et al. (Citation1998). The activity of glutathione peroxidase (GPx) was measured by the method described by Rotruck et al. (Citation1973). Glutathione S-transferase (GST) activity was determined spectrophotometrically by the method of Habig et al. (Citation1974). Glutathione reductase (GR) was assayed by the method of Horn and Burns (Citation1978). Reduced glutathione was measured according to the method of Beutler and Kelly (Citation1963). Vitamin C was estimated by the method of Omaye et al. (Citation1979). Vitamin E was determined by the method of Baker et al. (Citation1951).
Statistical analysis
The results are expressed as mean ± standard deviation (SD). Differences between groups were assessed by ANOVA using the SPSS software package for Windows. Post hoc testing was performed for inter-group comparisons using the least significance difference (LSD) test; P-values of <.05 were considered as significantly changed.
Result and discussion
Many herbal products have been reported earlier in Siddha and Ayurvedha for the treatment of diabetes mellitus. The polyherbal formulations might involve an extra-pancreatic action and produce a hypoglycemic effect, for example, possibly by stimulating glucose utilization in peripheral tissues. Sustained reduction in hyperglycemia may decrease the risk of developing microvascular complications and most likely reduce the risk of macrovascular complications (Rahul et al. Citation2019).
Active principles of PHD
The flavonoids such as gallic acid, caffeic acid, rutin, quercetin and Ferulic acid were identified in ethanolic extract of PHD by comparing their retention time with those of standard solutions (Figures and ). Under the selected operating conditions, the retention times (min) for the studied compounds were as follows: 5.500 (gallic acid), 9.117 (caffeic acid), 10.950 (rutin), 12.008 (quercetin) and 23.633 (ferulic acid). HPLC analysis revealed that gallic acid is the major active principles of the SM extract. The major flavonoids in the extract are gallic acid (230.2 µg/g), quercetin (201.1 µg/g), caffeic acid (700.0 µg/g), followed by rutin (100.2 µg/g) and ferulic acid (210.1 µg/g) (Table ).
Figure 1. HPLC-DAD chromatogram of the flavonoid standards used in the study. The flavonoid chromatogram of gradient HPLC was detected using C18 column monitored at 280 nm: 1 gallic acid, 2 caffeic acid, 3 rutin, 4 quercetin and 5 ferulic acid.
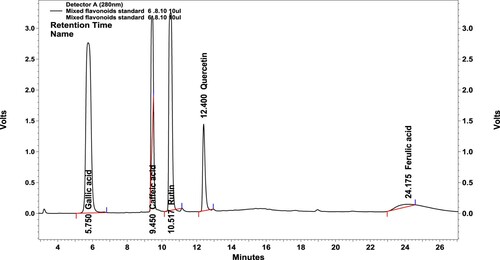
Figure 2. Chromatogram of representative sample (extract of herbal constituents of SM) tested in the study. The flavonoids were detected using C18 column monitored at 280 nm: 1 gallic acid, 2 caffeic acid, 3 rutin, 4 quercetin and 5 ferulic acid
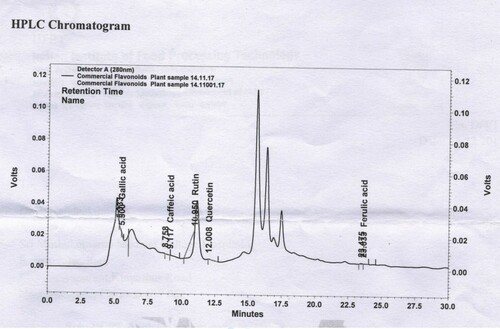
Table 1. Retention time, area, and concentrations of five different flavonoid constituents present in the ethanol extract of herbal constituents of PHD.
Effect of PHD in body weight and food intake
Table shows food intake, initial and final body weight in control and treated groups. The body weight was significantly decreased, whereas food intake was significantly increased in diabetic rats when compared with normal control rats. The decrease in body weight of diabetic rats is due to catabolism of fats and proteins. Due to insulin deficiency, the protein content is decreased in muscular tissue by proteolysis (Sundara et al. Citation2013). On oral administration of PHD (250 mg/kg b.w) and metformin (50 mg/kg b.w) for 45 days significantly increased the body weight and significantly decreased food intake when compared with untreated diabetic control rats. Our results are in agreement with previous reports (Castaneda Citation2002; Wei et al. Citation2003; Gillespie Citation2006) which observed the following characteristics in diabetic animals such as polyuria, increased water intake, dehydration, weight loss and muscle wasting, excessive hair loss and scaling, diarrhea, cataracts and increased food intake. Moreover, the decrease in body weight caused in diabetic rats may be due to the conversion of glucose into glycogen, catabolism of fats and inhibition of lipolysis, which are depending on insulin action.
Table 2. Effect of PHD on body weight and food intake in control and high-fat-induced type 2 diabetic rats.
Effect of PHD on blood glucose, insulin, HOMA-IR, Hb and HbA1c
Table depicts the alterations in the levels of blood glucose, insulin, HOMO-IR, Hb and HbA1c in control and experimental animals. The glucose and insulin levels were observed in experimental groups on the end of the experimental day after a 12 h fasting period. The levels of glucose, HOMA-IR and HbA1c were significantly increased, whereas the levels of insulin and Hb were significantly decreased in the diabetic rats compared with control rats. Treatment with PHD or metformin reversed these values to near normal in diabetic rats. Oral administration of PHD to normoglycemic rats showed no significant changes in the above-said parameters.
Table 3. Effect of PHD on blood glucose, insulin, HOMA-IR, Hb and HbA1c in control and high-fat-induced type 2 diabetic rats.
Generally, insulin stimulates protein synthesis and retards protein degradation which may be responsible for increased level of Hb (Murray et al. Citation2000). HbA1c was found to increase in patients with diabetes mellitus due to glycosylation of hemoglobin and the amount of increase was directly proportional to the fasting blood glucose levels (Babu et al. Citation2007). During diabetes, the hemoglobin level is decreased in diabetic rats due to an excess amount of blood glucose reacts with hemoglobin (Sheela and Augusti Citation1992). However, upon administration of PHD to diabetic rats, the above-said parameters were significantly restored to near normal. Some PHDs produce hypoglycemic action by potentiating the insulin effect, either by increasing the pancreatic secretion of insulin from the β-cells or its release from bound insulin, whereas they act through an extra-pancreatic mechanism by inhibition of hepatic glucose production or correction of insulin resistance (Eddouks et al. Citation2003; Pari and Satheesh Citation2004). Our formulated PHD may have acted through one of the above mechanisms which is involved in improving insulin levels, stabilization of blood glucose in diabetic rats and thereby inhibited the bodyweight loss.
Effect of PHD on serum amino transferases activities and urea and creatinine levels
The activities of serum amino transferases (AST and ALT) and the levels of renal markers (urea and creatinine) in control and experimental animals are shown in Table . The activities of AST, ALT and the levels of serum urea and creatinine were increased significantly in diabetic rats when compared with control animals. Upon PHD and metformin administrations, the levels were significantly reverted back to near-normal levels when compared with untreated diabetic rats. This result was in agreement with Shanmugasundaram et al. (Citation1983) and El Shafey et al. (Citation2013) who reported that administration of dried leaf powder of G. sylvestre decreased glucose levels as it controlled gluconeogenic enzymes (ALT and AST). The transaminases AST and ALT are used as normal indicators of liver function and as biomarkers for predicting toxicity. Damage to parenchymal liver cells results in elevated levels of both of these transaminases in the blood (Nandhakumar et al. Citation2013). Therefore, the elevated levels of AST and ALT in serum might be mainly due to the leakage of these enzymes from the liver cytosol into the bloodstream (Navarro et al. Citation1993). Increased gluconeogenesis and ketogenesis may also be due to elevated levels of AST and ALT activity in diabetic rats (Ghosh and Suryawanshi Citation2001). From this point of view, PHD may act as a hepatoprotective agent. Kidney function was assessed by determining the levels of urea and creatinine in the blood. Increased blood urea and non-protein levels have been observed with impaired renal function and in acute renal failure (Nandhakumar et al. Citation2013). In the present study, the elevated levels of urea and creatinine in diabetic rats were brought back to near normal by the treatment with PHD and metformin. Therefore, the administration of PHD prevents the further progression of renal damage in diabetic rats.
Table 4. Effect of polyherbal preparation on liver function test in control and high-fat-induced type 2 diabetic rats.
Effect of PHD on serum lipid profile
The levels of lipid profile such as total cholesterol, triglycerides, HDL, VLDL and LDL in plasma of control and experimental animals are illustrated in Table . The levels of total cholesterol, triglycerides, VLDL and LDL in plasma were increased significantly, whereas the level of HDL was decreased significantly in high-fat-induced diabetic rats when compared with control rats. However, on treatment with PHD, the levels of the above-said parameters were brought back to near-normal levels compared to untreated diabetic control rats. Decreasing levels of triglyceride, cholesterol and LDL-cholesterol and increasing level of HDL-cholesterol might be due to an increase in insulin which caused an increased activity of lipoprotein lipase (Facilitated chylomicron transport through cell membranes) and decreased activity of hormone-sensitive lipase (converted neutral fats into free fatty acids). The elevated levels of serum lipids were noticed in diabetic rats which represent the risk of coronary heart disease. These results were in agreement with Daisy et al. (Citation2009), Aralelimath and Bhise (Citation2012) and El Shafey et al. (Citation2013) who reported that increasing insulin secretion after administration of PHD led to a decrease in cholesterogenesis and fatty acid synthesis.
Table 5. Effect of PHD on lipid profile in control and high-fat-induced type 2 diabetic rats.
Effect of PHD on enzymic and non-enzymic antioxidants levels in serum and liver
Levels of LPO, enzymic antioxidants (SOD, CAT, GPx and GR) and non-enzymic antioxidants activities with response to PHD in the control and experimental groups of rats are shown in Figure , Tables and , respectively. Treatment groups of rats showed increased activities of enzymic and non-enzymic antioxidants which were decreased HFD-induced rats when compared with their control rats. These data show the protective and cytoprotective effect of PHD through its antioxidant effect by modulating enzymic and non-enzymic antioxidants activities in the diabetic condition. This could be correlated with previous studies reporting that Gymnema sylvestre (Patel et al. Citation2009), Syzygium cumini (Gupta and Saxena Citation2011), Trigonella foenum (Xue et al. Citation2007) and Cinnamomum zeylanicum (Hassan et al. Citation2012) have antiperoxidative and antihyperlipidemic effects in diabetic animals.
Figure 3. Effect of PHD on lipid peroxides in control and high-fat-induced type 2 diabetic rats Values are given as mean ± SD for six animals in each group. Values are considered significantly different at *P < .05 with post hoc LSD test. a, Control vs diabetic rats. b, Diabetic rats vs Diabetic + PHD-treated rats. c, Diabetic vs Diabetic + Metformin-treated rats. d, Control vs Diabetic + PHD-treated rats
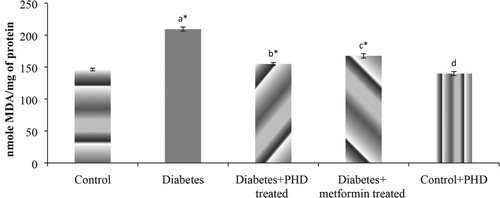
Table 6. Effect of PHD on the activities of serum and liver enzymic antioxidants in control and high-fat-induced type 2 diabetic rats.
Table 7. Effect of PHD on the activities of serum and liver non-enzymic antioxidants in control and high-fat-induced type 2 diabetic rats.
Enzymic antioxidants like SOD and CAT are inactivated in the diabetic condition; it may be caused by proteins glycation, thus promoting the free radical generation, which results in lipid peroxidation (Kennedy and Lyons Citation1997). The key role of GSH is protecting the cells from oxidative damage. Most prominent antioxidant GSH present in liver cells. Hence, GSH level in the liver reflects the detoxification potential of the liver. Our results showed that diabetes caused a significant increase in lipid peroxides, as indicated by a significant increase in TBARS concentration accompanied by a concomitant significant decrease in the activity of GSH, SOD and CAT in the liver tissue of diabetic rats when compared with normal control rats (Zhang et al. Citation2010; Wang et al. Citation2011). Administration with PHD in diabetic rats inverted the activities of this antioxidant in the liver and plasma which might be due to decreased oxidative stress as evidenced by decreased lipid peroxidation. These findings suggested that PHD treatment enhances the antioxidant defense mechanism in diabetes and, in this way, may improve blood glucose and lipid profile.
Conclusion
In the present study, the PHD have strong anti-diabetic potential in HFD-induced type 2 diabetic rats as evidenced by increasing the body weight and decreasing hyperglycemia, insulin resistance, hyperlipidemia and oxidative stress in diabetic rats. These findings suggested the antihyperglycemic potential of PHD in type 2 diabetes. Further studies are needed to elucidate the exact mechanism of anti-diabetic and antihyperlipidemic actions of PHD so as to develop it as a potent anti-diabetic drug.
Acknowledgements
The authors gratefully acknowledged the DST-SERB for providing financial support. Award-File No. ECR/2016/001579, New Delhi, India
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agarwal SK, Singh SS, Verma S, Lakshmi V, Sharma A. 2000. Chemistry and medicinal uses of Gymnema sylvestre (Gur-mar) leaves: a review. Indian Drugs. 37:354–360.
- Ansari SH. 2005. Essentials of pharmacognosy, 1st ed. New Delhi: Birla Publication Pvt. Ltd; p. 357–384.
- Aralelimath VR, Bhise SB. 2012. Anti-diabetic effects of Gymnema sylvestre extract on streptozotocin induced diabetic rats and possible b-cell protective and regenerative evaluations. Dig J Nanomater Biostruct. 7(1):135–142.
- Babu H, Cheung G, Kettenmann H, Palmer TD, Kempermann G. 2007. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS ONE. 2:e388. doi:https://doi.org/10.1371/journal.pone.0000388.
- Baker H, Frank O, Angelis B, Feingold S. 1951. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr Report Intern. 21:531–536.
- Banerjee A, Dasgupta N, De B. 2005. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem. 90:727–733. doi: https://doi.org/10.1016/j.foodchem.2004.04.033
- Beutler E, Kelly BM. 1963. The effect of sodium nitrate on RBC glutathione. Experientia. 19:96–97. doi: https://doi.org/10.1007/BF02148042
- Bhatia IS, Bajaj KL. 1975. Chemical constituents of the seeds and bark of Syzygium cumini. Planta Med. 28:346–352. doi: https://doi.org/10.1055/s-0028-1097868
- Bhuyan ZA, Rokeya B, Masum N, Hossain S, Mahmud I. 2010. Antidiabetic effect of Syzygium cumini (L) seed on type 2 diabetic rats. Dhaka Univer J Biol Sci. 19:157–164. doi: https://doi.org/10.3329/dujbs.v19i2.8959
- Bopp A, De Bona KS, Bellé LP, Moresco RN, Moretto MB. 2009. Syzygium cumini inhibits adenosine deaminase activity and reduces glucose levels in hyperglycemic patients. Fundam Clin Pharmacol. 23:501–507. doi: https://doi.org/10.1111/j.1472-8206.2009.00700.x
- Burgi M, Briner N, Franken ACH, Kessler K. 1988. One step sandwich enzyme immunoassay for insulin using monoclonal antibodies. Clin Biochem. 21:311–314. doi: https://doi.org/10.1016/S0009-9120(88)80087-0
- Burstein MSHR, Scholnick HR, Morfin R. 1970. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 11:583–595.
- Castaneda C. 2002. Muscle wasting and protein metabolism. J Anim Sci. 80:E98–E105. doi: https://doi.org/10.2527/animalsci2002.80E-Suppl_2E98x
- Daisy P, Eliza J, Farook KAM. 2009. A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J Ethnopharmacol. 126:339–344. doi: https://doi.org/10.1016/j.jep.2009.08.018
- Denys JC. 2012. Sources of natural antioxidants and their activities. Antioxidant properties of spices, herbs and other sources. New York: Springer; p. 65–138.
- Drabkin DL, Austin JM. 1932. Spectrophotometric studies, spectrophotometric constants for common haemoglobin derivatives in human, dog and rabbit blood. J Biol Chem. 9:719–733.
- Eddouks M, Jouad H, Maghrani M, Lemhadri A, Burcelin A. 2003. Inhibition of endogenous glucose production accounts for hypoglycemic effect of Spergularia purpurea in streptozotocin mice. Phytomedicine. 10:594–599. doi: https://doi.org/10.1078/094471103322331890
- El Shafey AA, El-Ezabi MM, Seliem MM, Ouda HH, Ibrahim DS. 2013. Ibrahim effect of gymnema sylvestre R. Br. leaves extract on certain physiological parameters of diabetic rats. J King Saud Univer Sci. 25:135–141. doi: https://doi.org/10.1016/j.jksus.2012.11.001
- Foster LB, Dunn RT. 1973. Stable reagents for determination of serum triglycerides by colorimetric Hantzsch condensation method. Clin Chem. 19(3):338–340. doi: https://doi.org/10.1093/clinchem/19.3.338
- Fraga CG, Leibouitz BE, Toppel AL. 1988. Lipid peroxidation measured as TBARS in tissue slices: characterization and comparison with homogenates and microsomes. Free Radical Biol Med. 4:155–161. doi: https://doi.org/10.1016/0891-5849(88)90023-8
- Friedewald WT, Levy RI, Fredrickson DS. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 18:499–502. doi: https://doi.org/10.1093/clinchem/18.6.499
- Ghosh S, Suryawanshi SA. 2001. Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. Indian J Exp Biol Aug. 39(8):748–759.
- Gillespie KM. 2006. Type 1 diabetes: pathogenesis and prevention. CMAJ. 2(165e):70.
- Gupta R, Saxena AM. 2011. Hypoglycemic and anti hyperglycemic activities of Syzygium cumini (Linn.) skeels whole fruit, in normal and streptozotocin induced diabetic rats. Asian J Phar Biol Res. 1:267–272.
- Gupta GS, Sharma DP. 1974. Triterpenoid and other constituents of Eugenia jambolana leaves. Phytochemistry. 13:2013–2014. doi: https://doi.org/10.1016/0031-9422(74)85151-4
- Habig WH, Pabst MJ, Jakpoby WB. 1974. Glutathione transferase, a first enzymatic step in mercapturic acid formation. J Biol Chem. 249:7130–7139.
- Hassan SA, Barthwal R, Nair MS, Haque SS. 2012. Aqueous bark extract of Cinnamomum Zeylanicum: a potential therapeutic agent for streptozotocin induced type 1 diabetes mellitus (T1DM) rats. Trop J Pharm Res. 11:429–435.
- Horn HD, Burns FH. 1978. Assay of glutathione reductase activity. In: H.V. Bergemeyer, editor. Methods of enzymatic analysis. New York, USA: Academic Press; p. 142–146.
- Imparl-Radosevich J, Deas S, Polansky MM, Baedke DA, Ingebritsen TS, Anderson RA, et al. 1998. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: implications for cinnamon regulation of insulin signalling. Horm Res. 50:177–182.
- Jarvill-Taylor KJ, Anderson RA, Graves DJ. 2001. A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. J Am Coll Nutr. 20:327–336. doi: https://doi.org/10.1080/07315724.2001.10719053
- Jiang ZY, Hunt JV, Wolff SP. 1992. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 202:384–389. doi: https://doi.org/10.1016/0003-2697(92)90122-N
- Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J. 1998. Increased oxidative stress in rat liver and pancreas during progression of streptozotocin induced diabetes. Clin Sci. 94:623–632. doi: https://doi.org/10.1042/cs0940623
- Kanetkar P, Singhal R, Kamat M. 2007. Gymnema sylvestre: a memoir. J Clin Biochem Nutr. 41:77–81. doi: https://doi.org/10.3164/jcbn.2007010
- Karadeniz F, Burdurulu HS, Koca N, Soyer Y. 2005. Antioxidant activity of selected fruits and vegetables grown in Turkey. J Agric Food Chem. 29:297–303.
- Kaviarasan S, Naik GH, Gangabhagirathi R, Anuradha CV, Priyadarsini KI. 2007. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum-graecum) seeds. Food Chem. 103:31–37. doi: https://doi.org/10.1016/j.foodchem.2006.05.064
- Kennedy AL, Lyons TJ. 1997. Glycation, oxidation, and lipoxidation in the development of diabetic complications. Metabolism. 46:14–21. doi: https://doi.org/10.1016/S0026-0495(97)90311-5
- Kirtika KR, Basu BD. 1975. Indian medicinal plants. Vol. 3. New Delhi, India: Periodical Experts.
- Kirubananthan G, Vijayan SG, Thangaraj A, Sundaram R. 2019. Antioxidant potential of theaflavin ameliorates the activities of key enzymes of glucose metabolism in high fat diet and streptozotocin-induced diabetic rats. Redox Rep. 24(1):41–50. doi: https://doi.org/10.1080/13510002.2019.1624085
- Kumar RS, Ponmozhi M, Viswanathan P, Nalini N. 2002. Effect of Cassia auriculata leaf extract on lipids in rats with alcoholic liver injury. Asia Pac J Clin Nutr. 11:157–163. doi: https://doi.org/10.1046/j.1440-6047.2002.00286.x
- Kumar Rajagopal S, Manickam P, Periyasamy V, Namasivayam N. 2003. Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J Nutr Biochem. 14:452–458. doi: https://doi.org/10.1016/S0955-2863(03)00053-6
- Latha M, Pari L. 2003. Preventive effects of Cassia auriculata L. flowers on brain lipid peroxidation in rats treated with streptozotocin. Mol Cell Biochem. 243:23–28. doi: https://doi.org/10.1023/A:1021697311150
- Mandal S, Barik B, Mallick C, De D, Ghosh D. 2008. Therapeutic effect of ferulic acid, an ethereal fraction of ethanolic extract of seed of Syzygium cumini against streptozotocin-induced diabetes in male rat. Methods Finds Exp Clin Pharmacol. 30:121–128. doi: https://doi.org/10.1358/mf.2008.30.2.1143090
- Ministry of Health and Family Welfare. 1996. Ayurvedic pharmacopoeia. Vol. 1. New Delhi: Ministry of Health and Family Welfare, Govt. of India.
- Murray GL, Bao WG, Fukuhara H, Zuo XM, Clark-Walker GD, Chen XJ. 2000. Disruption of the MRP-L23 gene encoding the mitochondrial ribosomal protein L23 is lethal for Kluyveromyces lactis but not for Saccharomyces cerevisiae. Curr Genet. 37(2):87–93. doi: https://doi.org/10.1007/s002940050014
- Nampurath GK, Mathew SP, Khanna V, Zachariah RT, Kanji S, Chamallamudi MR, et al. 2008. Assessment of hypolipidaemic activity of three thiazolidin-4-ones in mice given high-fat diet and fructose. Chem-Biol Interact. 171:363–368. doi: https://doi.org/10.1016/j.cbi.2007.10.006
- Nandhakumar E, Purushothaman A, Sachdanandam P. 2013. Acute and sub-acute toxicity studies using shemamruthaa, a modified indigenous siddha preparation, in Sprague-Dawley rats. Food Sci Biotechnol. 23(1):299–306. doi: https://doi.org/10.1007/s10068-014-0042-3
- Navarro C, Wu LF, Mandrand-Berthelot MA. 1993. The nik operon of Escherichia coli encodes a periplasmic binding-protein-dependent transport system for nickel. Mol Microbiol. 9:1181–1191. doi: https://doi.org/10.1111/j.1365-2958.1993.tb01247.x
- Noomrio MH, Dahot MU. 1996. Nutritive value of Eugenia jambosa fruit. J Islam Acad Sci. 9:9–12.
- Omaye ST, Turbull TD, Sauberlich HC. 1979. Selected method for the determination of ascorbic acid in animal cells, tissues and fluids. In: D.B. McCormic, D.L. Wrighti, editor. Methods enzymology, Vol. 62. New York, USA: Academic Press; p. 3–11.
- Pandey M, Khan A. 2002. Hypoglycaemic effect of defatted seeds and water soluble fibre from the seeds of Syzygium cumini (Linn.) skeels in alloxan diabetic rats. Indian J Exp Biol. 40:1178–1182.
- Pari L, Satheesh MA. 2004. Antidiabetic activity of Boerhaavia diffusa L on hepatic key enzymes in experimental diabetes. J Ethnopharmacol. 91:109–113. doi: https://doi.org/10.1016/j.jep.2003.12.013
- Patel SS, Shah RS, Goyal RK. 2009. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian J Exp Biol. 47:564–570.
- Petit P, Sauvaire Y, Ponsin G, Manteghetti M, Fave A, Ribes G, et al. 1993. Effects of a fenugreek seed extract on feeding behaviour in the rat: metabolic-endocrine correlates. Pharmacol Biochem Behav. 45:369–374. doi: https://doi.org/10.1016/0091-3057(93)90253-P
- Rahul G, Nandhakumar E, Renuka A, Manamalli A. 2019. Antidiabetic potential of polyherbal drug against high-fat diet-induced type 2 diabetes mellitus male Wistar rats. Drug Invention Today. 11(8):1784–1788.
- Raju J, Gupta D, Rao AR, Yadava PK, Baquer NZ. 2001. Trigonella foenum-graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol Cell Biochem. 224:45–51. doi: https://doi.org/10.1023/A:1011974630828
- Reitman S, Frankel S. 1957. Colorimetric method for the determination of serum glutamic oxaloacetic acid and glutamic pyruvic transaminases. Am J Clin Pathol. 28:56–63. doi: https://doi.org/10.1093/ajcp/28.1.56
- Riad S, El-Baradie AA. 1959. Fenugreek mucilage and its relation to the reputed laxative action of this seed. Egypt J Chem. 2:163–168.
- Rotruck JT, Pope AL, Ganther HE. 1973. Selenium biochemical role as a component of glutathione peroxidase purification assay. Science. 179:588–590. doi: https://doi.org/10.1126/science.179.4073.588
- Shanmugasundaram KR, Panneerselvam C, Samudram P, Shanmugasundaram ERB. 1983. Enzyme changes and glucose utilisation in diabetic rabbits: the effect of Gymnema sylvestre. J Ethnopharmacol. 7:205–234. doi: https://doi.org/10.1016/0378-8741(83)90021-1
- Sharafeldin K, Rizvi MR. 2015. Effect of traditional plant medicines (Cinnamomum zeylanicum and Syzygium cumini) on oxidative stress and insulin resistance in streptozotocin-induced diabetic rats. J Basic Appl Zool. 72(October):126–134. doi: https://doi.org/10.1016/j.jobaz.2015.09.002
- Sharma RD, Raghuram TC, Rao NS. 1990. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr. 44:301–306.
- Sheela CG, Augusti KT. 1992. Antidiabetic effects of S-allyl cysteine sulphoxide isolated from garlic Allium sativum Linn. Indian J Exp Biol. 30(6):523–526.
- Sinha AK. 1952. Colorimetric assay of catalase. Anal Biochem. 47:389–394. doi: https://doi.org/10.1016/0003-2697(72)90132-7
- Slot C. 1965. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 17:381–387. doi: https://doi.org/10.3109/00365516509077065
- Sudhakar NS, Pattabiraman TN. 1981. A new colorimetric method for the estimation of glycosylated haemoglobin. Clin Chim Acta. 109:267–274. doi: https://doi.org/10.1016/0009-8981(81)90312-0
- Sultana B, Anwar F, Przybylski R. 2007. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica and Eugenia jambolana Lam. trees. Food Chem. 104:1106–1114. doi: https://doi.org/10.1016/j.foodchem.2007.01.019
- Sundara R, Naresh R, Shanthi P, Sachdanandam P. 2013. Modulatory effect of green tea extract on hepatic key enzymes of glucose metabolism in streptozotocin and high fat diet induced diabetic rats. Phytomedicine. 20:577–584. doi: https://doi.org/10.1016/j.phymed.2013.01.006
- Taylor WG, Zaman MS. 1997. Analysis of steroidal sapogenins from amber fenugreek (Trigonella foenum-graecum) by capillary gas chromatography and combined gas chromatography/mass spectrometry. J Agric Food Chem. 45:753–759. doi: https://doi.org/10.1021/jf960200n
- Trinder P. 1969. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annal Clin Biochem: Int J Biochem Lab Med. 6:24–27. doi: https://doi.org/10.1177/000456326900600108
- Wang Y, Campbell T, Perry B, Beaurepaire C, Qin L. 2011. Hypoglycemic and insulin-sensitizing effects of berberine in high-fat diet- and streptozotocin-induced diabetic rats. Metabolism. 60:298–305. doi: https://doi.org/10.1016/j.metabol.2010.02.005
- Wei M, Ong L, Smith MT, Ross FB, Schmid K, Hoey AJ, Burstow D, Brown L. 2003. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart, Lung Circ. 12:44–50. doi: https://doi.org/10.1046/j.1444-2892.2003.00160.x
- Wybenga DR, Di Giorgio J, Pileggi VJ. 1971. Manual and automated methods for urea nitrogen measurement in whole serum. Clin Chem. 17:891–895. doi: https://doi.org/10.1093/clinchem/17.9.891
- Xue WL, Li XS, Zhang J, Liu YH, Wang ZL, Zhang RJ. 2007. Effect of Trigonella foenum graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin induced diabetic rats. Asia Pac J Clin Nutr. 16(Suppl. 1):422–426.
- Zhang L, Yang J, Chen X, Zan K, Wen X, Chen H, Wang Q, Lai MX. 2010. Antidiabetic and antioxidant effects of extracts from Potentilla discolor Bunge on diabetic rats induced by high fat diet and streptozotocin. J Ethnopharmacol. 132:518–524. doi: https://doi.org/10.1016/j.jep.2010.08.053
- Zlatkis A, Zak B, Boyle GJ. 1953. A simple method for determination of serum cholesterol. J Clin Med. 41:486–492.
